Life-long hepatocellular carcinoma (HCC) surveillance is recommended after sustained virological response (SVR) in patients with advanced hepatitis C. Since the identification of patients who could be safely discontinued for surveillance is essential, we aimed to identify subsets of patients with low-risk HCC.
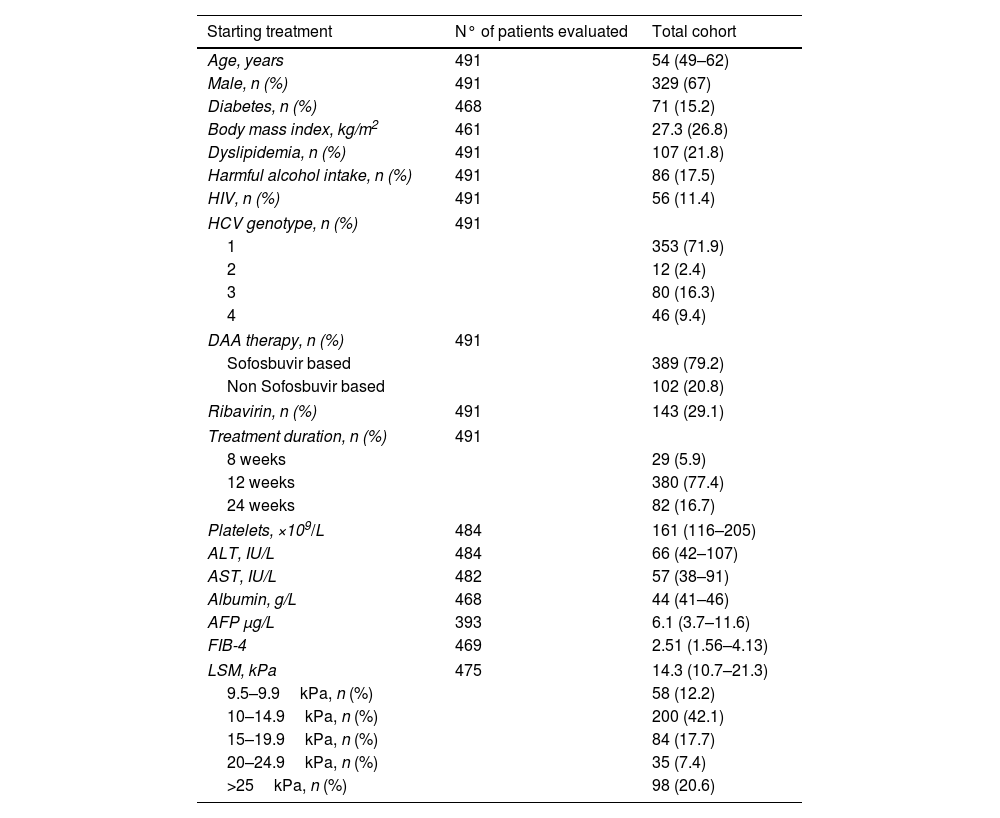
Methods491 patients with advanced and compensated fibrosis (≥F3) were prospectively followed after achieving SVR with interferon-free therapies. Clinical–biological parameters and liver stiffness measurement (LSM) were performed before starting treatment (ST) and at SVR, and HCC surveillance was carried out.
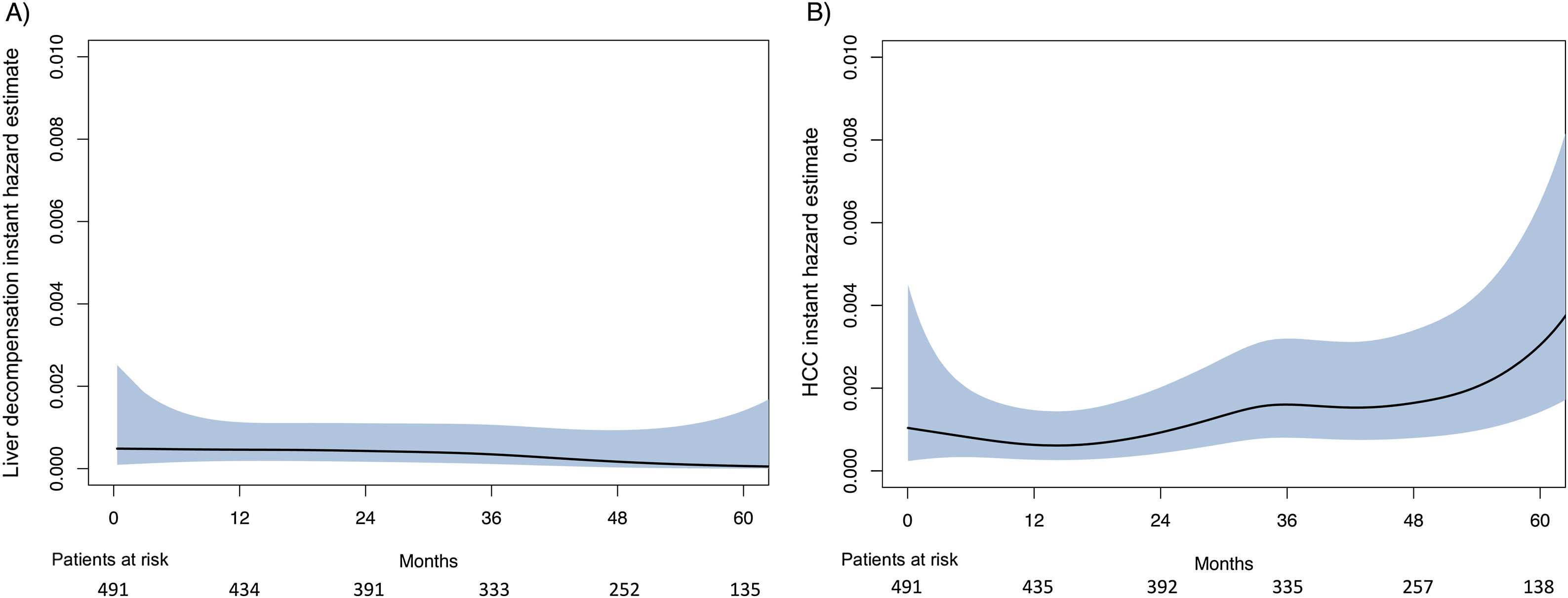
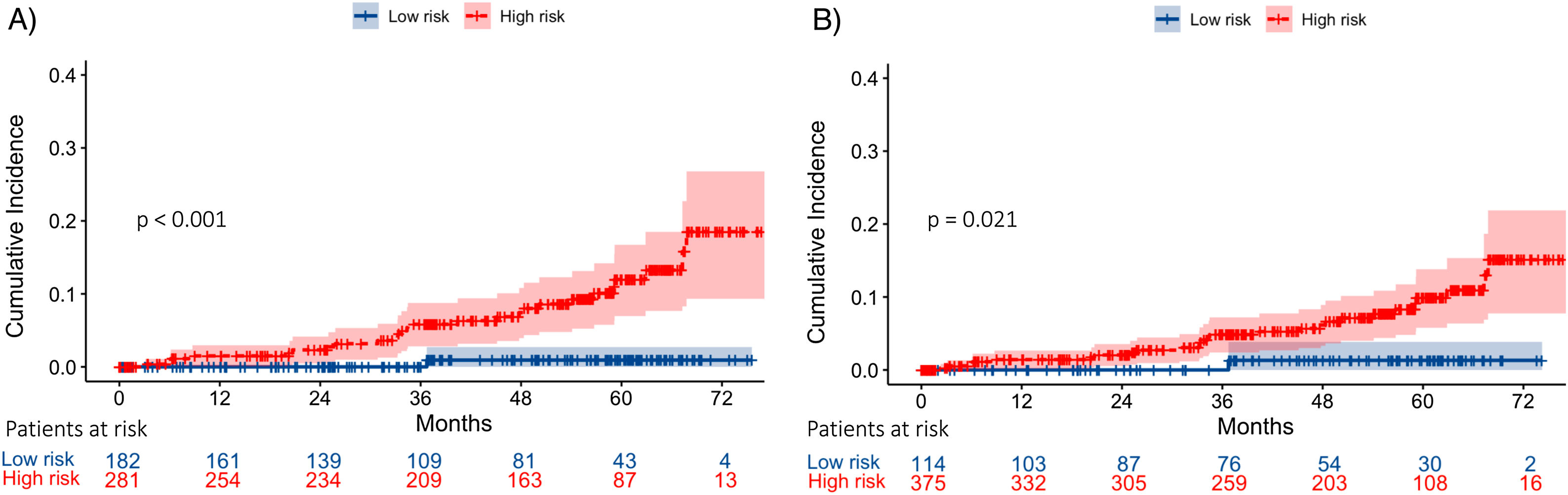
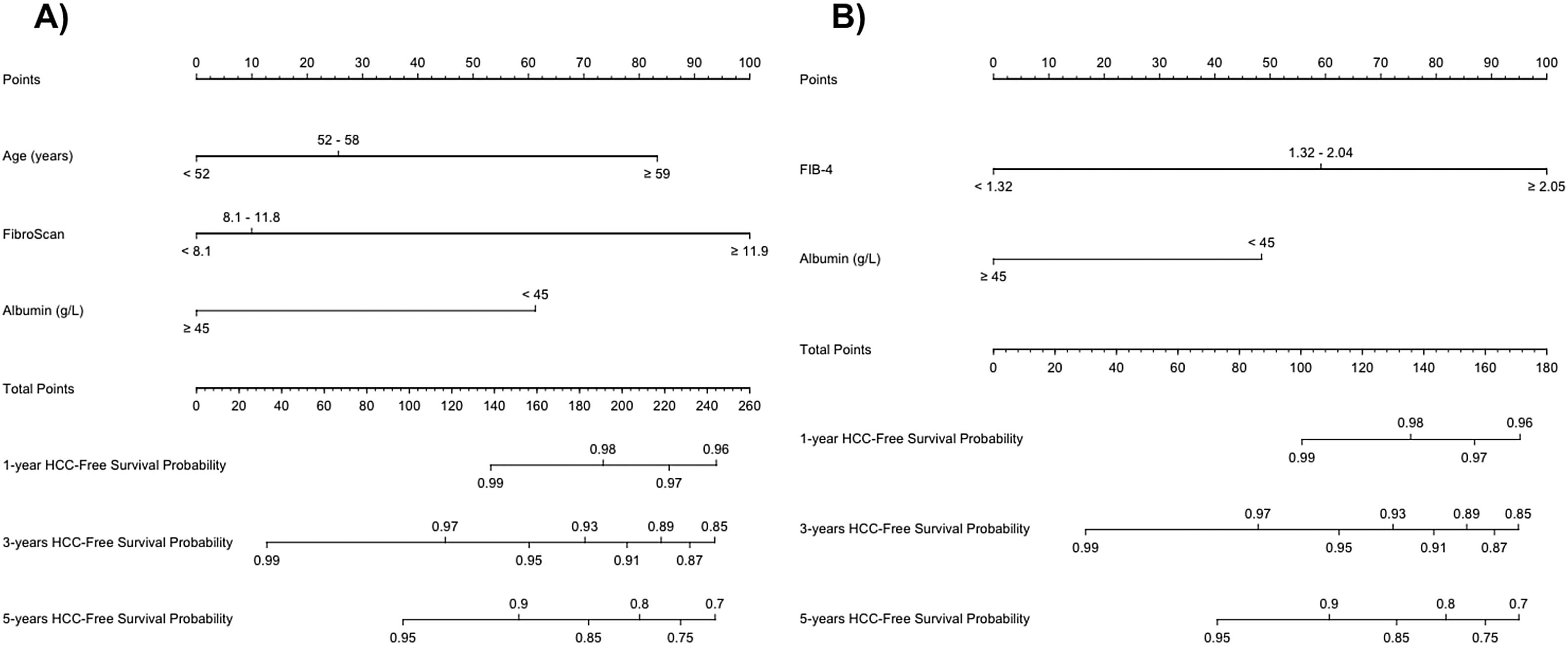
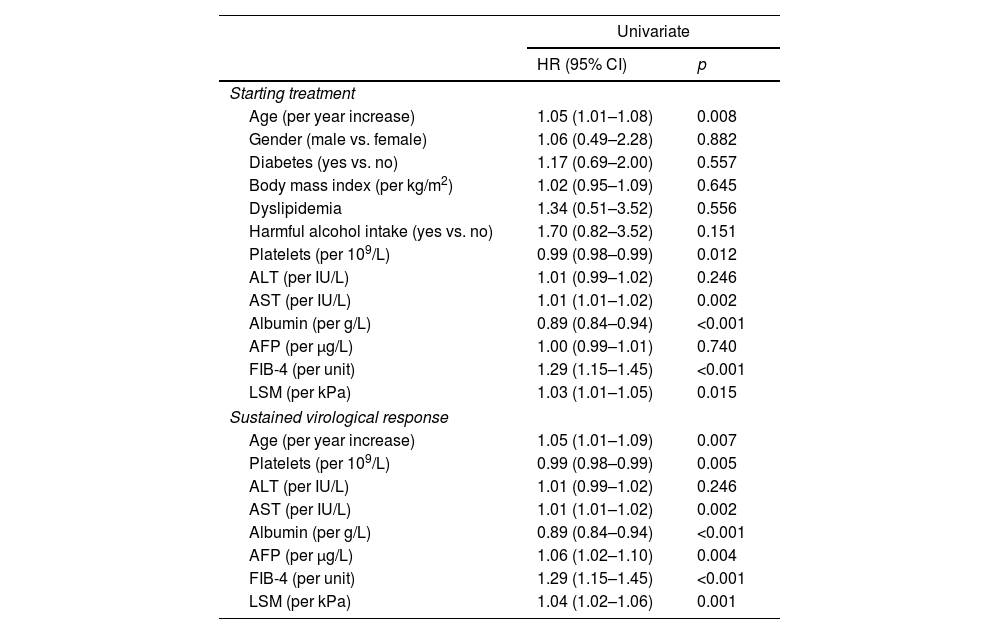
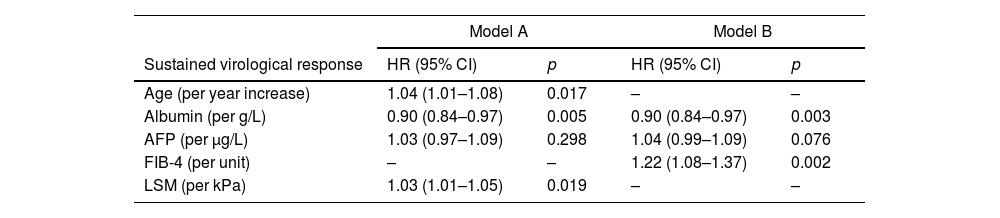
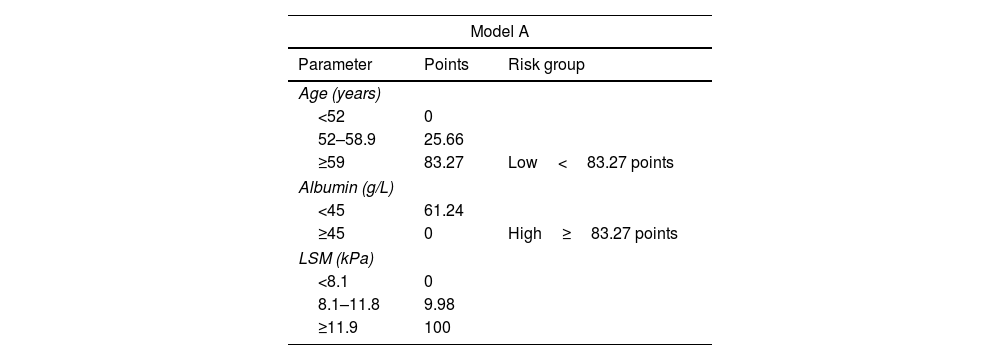
ResultsDuring a median follow-up of 49.8 months, 29 (5.9%) patients developed HCC [incidence rate: 1.6/100 patient-years (PYs)]. Two predictive models based on LSM (Model-A) or FIB-4 score (Model-B) were proposed. Only SVR parameters were included in the models, because they showed a higher accuracy for predicting HCC than ST measurements. Variables independently associated with HCC were LSM (HR, 1.03; 95% CI, 1.01–1.05), age (HR, 1.04; 95% CI, 1.01–1.08) and albumin levels (HR, 0.90; 95% CI, 0.84–0.97) in Model-A, and FIB-4 (HR, 1.22; 95% CI, 1.08–1.37) and albumin (HR, 0.90; 95% CI, 0.84–0.97) in model-B. Both models allow HCC risk stratification, identifying low-risk groups with an HCC incidence rate of 0.16/100 and 0.25/100 PYs, respectively. An overall increased hazard of HCC was observed over time.
ConclusionSimple models based on non-invasive markers of liver fibrosis, LSM or FIB-4, together with age and albumin levels at SVR permit to identify subsets of patients with HCC risk clearly <1%/year, for whom HCC surveillance might not be cost-effective.
En pacientes con hepatitis C avanzada se recomienda la vigilancia del carcinoma hepatocelular (CHC) de por vida tras la respuesta viral sostenida (RVS). La identificación de pacientes que podrían interrumpir de manera segura el screening es esencial, por ello nuestro objetivo fue identificar subgrupos de pacientes con bajo riesgo de desarrollo de CHC.
MétodosSe realizó un seguimiento prospectivo de 491 pacientes con fibrosis avanzada y compensada (≥F3) tras la RVS obtenida con terapias libres de interferón. Se registraron parámetros clínico-biológicos y se midió la rigidez hepática mediante elastografía de transición (ET) antes del inicio del tratamiento y en la respuesta viral sostenida y se realizó screening para el desarrollo de CHC.
ResultadosDurante una mediana de seguimiento de 49,8 meses, 29 (5,9%) pacientes desarrollaron CHC. (Tasa de incidencia: 1,6/100 pacientes-año [PA]). Se propusieron dos modelos predictivos basados en la puntuación de ET (Modelo-A) o FIB-4 (Modelo-B). Se incluyeron los parámetros en RVS en los modelos porque mostraron una mayor precisión para predecir CHC que las mediciones basales. Las variables asociadas de forma independientes con CHC fueron: ET (HR 1,03 IC; IC 95%, 1,01-1,05), edad (HR 1,04; IC 95%, 1,01-1,08) y niveles de albúmina (HR 0,90; IC 95%, 0,84-0,97) en el Modelo-A, y FIB-4 (HR 1,22; IC 95%, 1,08-1,37) y albúmina (HR 0,90; IC 95%, 0,84-0,97) en el Modelo-B. Ambos modelos permiten la estratificación del riesgo de CHC, identificando grupos de bajo riesgo con una tasa de incidencia de CHC de 0,16/100 y 0,25/100 PA, respectivamente. Se observó un aumento general del riesgo de desarrollar CHC con el tiempo.
ConclusiónModelos simples basados en marcadores no invasivos de fibrosis hepática, ET o FIB-4, junto con la edad y los niveles de albúmina en la RVS permiten identificar subgrupos de pacientes con riesgo de CHC claramente < 1%/año, para quienes la vigilancia de CHC podría no resultar coste-efectiva.