Biological therapies used for the treatment of inflammatory bowel disease (IBD) have shown to be effective and safe, although these results were obtained from studies involving mostly a young population, who are generally included in clinical trials. The aim of our study was to determine the efficacy and safety of the different biological treatments in the elderly population.
MethodsMulticenter study was carried out in the GETECCU group. Patients diagnosed with IBD and aged over 65 years at the time of initiating biological therapy (infliximab, adalimumab, golimumab, ustekinumab or vedolizumab) were retrospectively included.
Among the patients included, clinical response was assessed after drug induction (12 weeks of treatment) and at 52 weeks. Patients’ colonoscopy data in week 52 were assessment, where available. Regarding complications, development of oncological events during follow-up and infectious processes occurring during biological treatment were collected (excluding bowel infection by cytomegalovirus).
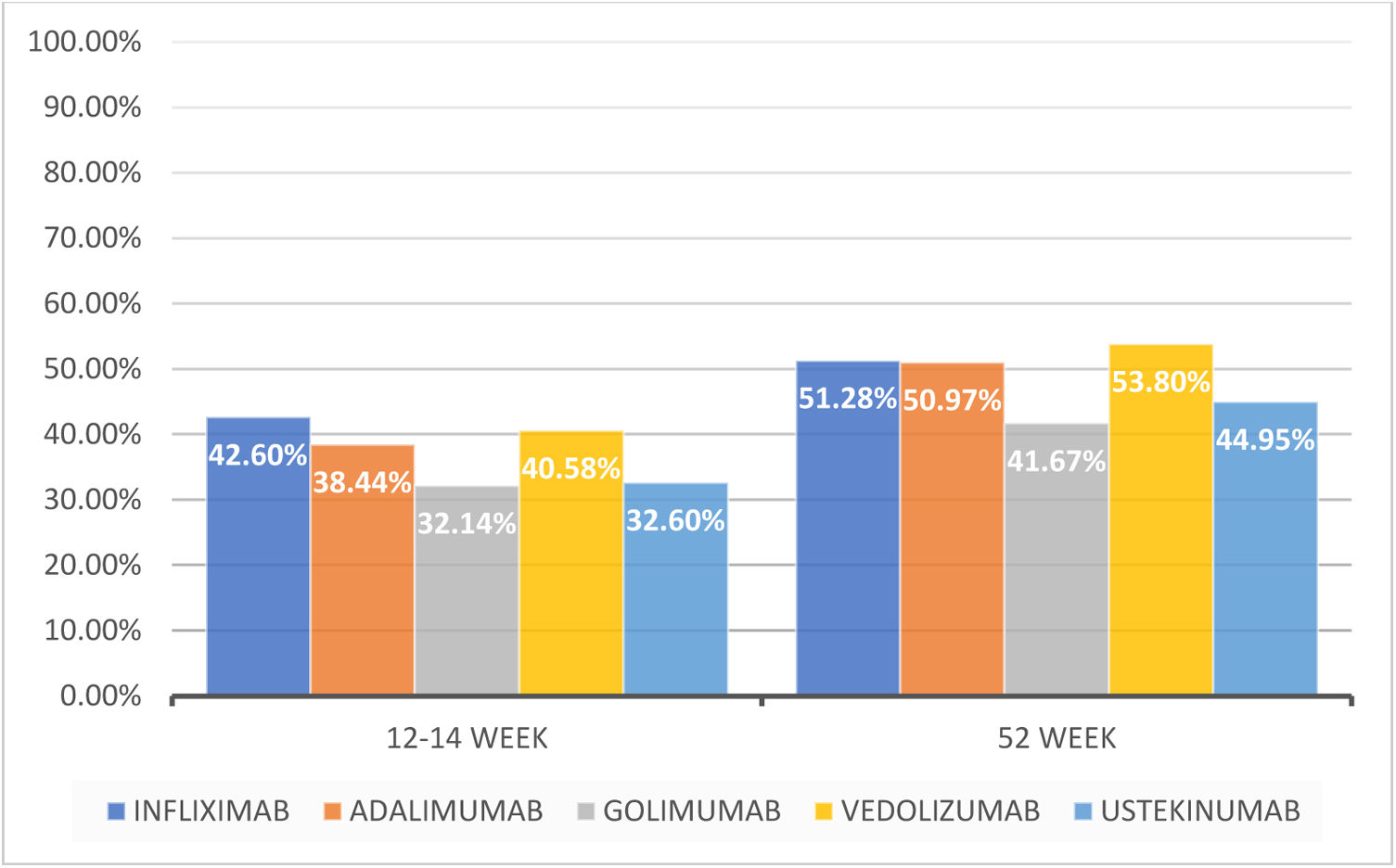
ResultsA total of 1090 patients were included. After induction, at approximately 12–14 weeks of treatment, 419 patients (39.6%) were in clinical remission, 502 patients (47.4%) had responded without remission and 137 patients (12.9%) had no response.
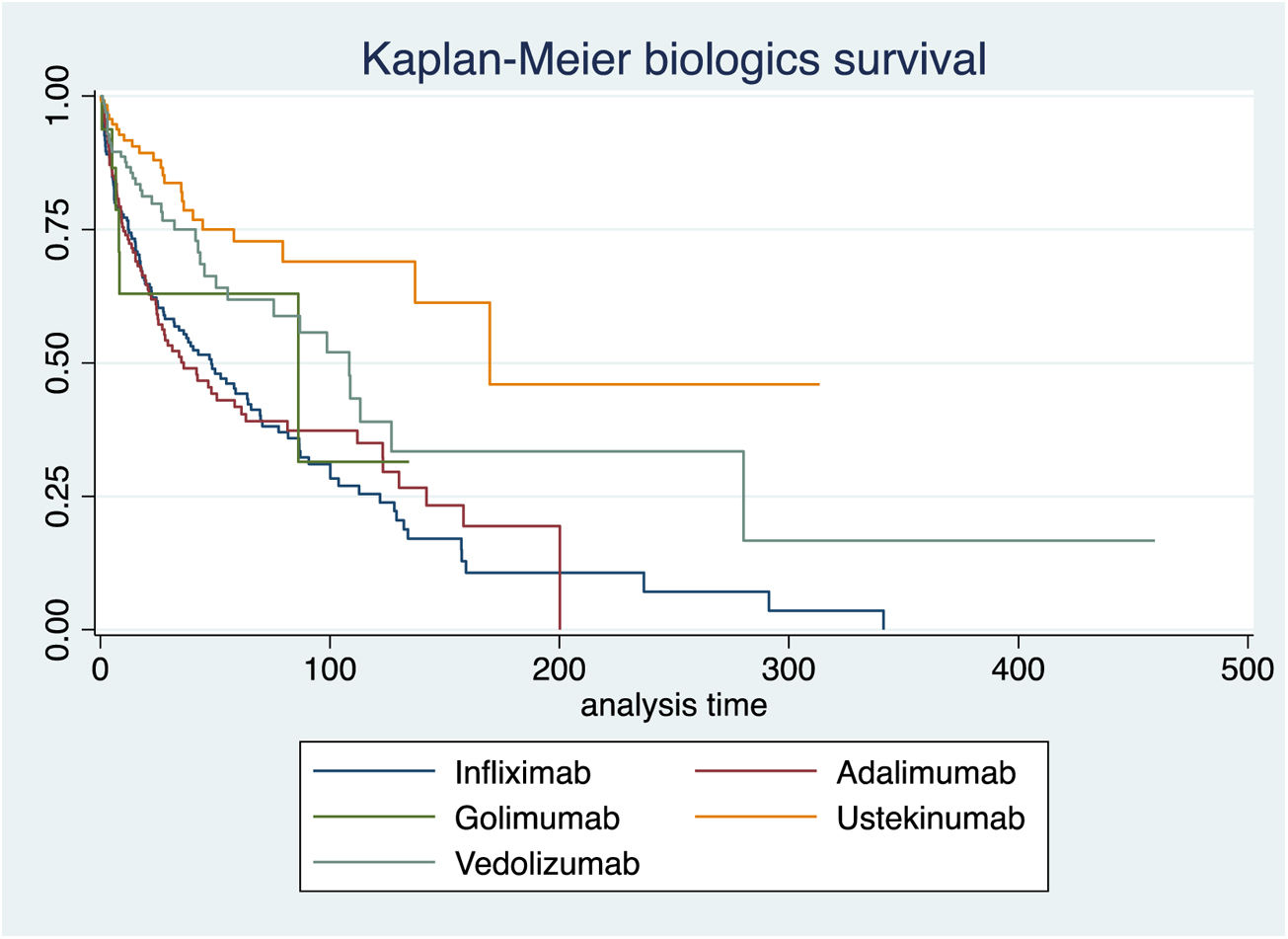
At 52 weeks of treatment 442 patients (57.1%) had achieved clinical remission, 249 patients had responded without remission (32.2%) and 53 patients had no response to the treatment (6.8%). Before 52 weeks, 129 patients (14.8%) had discontinued treatment due to inefficacy, this being significantly higher (p<0.0001) for Golimumab – 9 patients (37.5%) – compared to the other biological treatments analyzed.
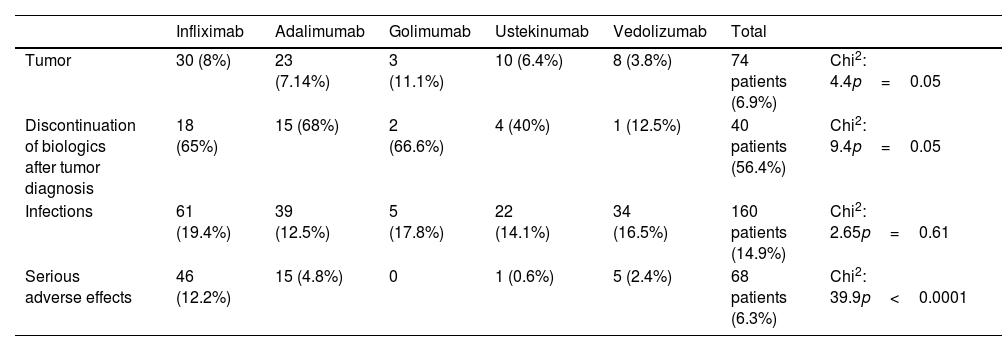
With respect to tumor development, an oncological event was observed in 74 patients (6.9%): 30 patients (8%) on infliximab, 23 (7.14%) on adalimumab, 3 (11.1%) on golimumab, 10 (6.4%) on ustekinumab, and 8 (3.8%) on vedolizumab. The incidence was significantly lower (p=0.04) for the vedolizumab group compared to other treatments.
As regards infections, these occurred in 160 patients during treatment (14.9%), with no differences between the different biologicals used (p=0.61): 61 patients (19.4%) on infliximab, 39 (12.5%) on adalimumab, 5 (17.8%) on golimumab, 22 (14.1%) on ustekinumab, and 34 (16.5%) on vedolizumab.
ConclusionsBiological drug therapies have response rates in elderly patients similar to those described in the general population, Golimumab was the drug that was discontinued most frequently due to inefficacy.
In our experience, tumor development was more frequent in patients who used anti-TNF therapies compared to other targets, although its incidence was generally low and that this is in line with younger patients based on previous literature.
Las terapias biológicas utilizadas para el tratamiento de la enfermedad inflamatoria intestinal (EII) han demostrado ser efectivas y seguras, aunque estos resultados se obtuvieron de estudios que involucran, mayoritariamente, a una población joven, que generalmente es incluida en ensayos clínicos. El objetivo de nuestro estudio fue determinar la eficacia y seguridad de los diferentes tratamientos biológicos en la población de adultos mayores.
MétodosSe realizó un estudio multicéntrico en el Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU). Se incluyeron retrospectivamente pacientes diagnosticados de EII y mayores de 65 años en el momento de iniciar la terapia biológica (infliximab, adalimumab, golimumab, ustekinumab o vedolizumab). Entre los pacientes incluidos, se evaluó la respuesta clínica después de la inducción del fármaco (12 semanas de tratamiento) y a las 52 semanas.
Se evaluaron los datos de la colonoscopia de los pacientes en la semana 52, cuando estaban disponibles. Respecto a las complicaciones, se recogió la evolución de eventos oncológicos hasta el fin del seguimiento y los procesos infecciosos ocurridos durante el tratamiento biológico (excluyendo infección intestinal por citomegalovirus).
ResultadosSe incluyeron un total de 1.090 pacientes. Después de la inducción, aproximadamente a las 12-14 semanas de tratamiento, 419 pacientes (39,6%) estaban en remisión clínica, 502 (47,4%) habían respondido sin remisión y 137 (12,9%) no tuvieron respuesta.
A las 52 semanas de tratamiento, 442 pacientes (57,1%) habían alcanzado la remisión clínica, 249 habían respondido sin remisión (32,2%) y 53 no tuvieron respuesta al tratamiento (6,8%). Antes de las 52 semanas, 129 pacientes (14,8%) habían suspendido el tratamiento por ineficacia, siendo significativamente mayor (p < 0,0001) para golimumab —nueve pacientes (37,5%)—, respecto al resto de tratamientos biológicos analizados.
Con respecto al desarrollo del tumor, se observó un evento oncológico en 74 pacientes (6,9%): 30 (8%) con infliximab, 23 (7,14%) con adalimumab, tres (11,1%) con golimumab, 10 (6,4%) con ustekinumab y ocho (3,8%) con vedolizumab. La incidencia fue significativamente menor (p=0,04) para el grupo de vedolizumab en comparación con otros tratamientos.
En cuanto a las infecciones, se produjeron en 160 pacientes durante el tratamiento (14,9%), sin diferencias entre los distintos biológicos utilizados (p=0,61): 61 pacientes (19,4%) con infliximab, 39 (12,5%) con adalimumab, 5 (17,8%) %) con golimumab, 22 (14,1%) con ustekinumab y 34 (16,5%) con vedolizumab.
ConclusionesLas terapias biológicas tienen tasas de respuesta en pacientes de edad avanzada similares a las descritas en la población general, golimumab fue el fármaco que con mayor frecuencia se suspendió por ineficacia. En nuestra experiencia, el desarrollo de tumores fue más frecuente en pacientes que utilizaron terapias anti-factor de necrosis tumoral (anti-TNF) en comparación con otras dianas, aunque su incidencia fue en general baja, en línea con lo publicado en la literatura previa en pacientes más jóvenes.