The introduction of direct-acting antivirals (DAAs) has revolutionised the treatment of hepatitis C virus (HCV) infection. With short-term treatments applicable to almost all infected patients and excellent tolerance, cure rates above 95% have been achieved.
Cure of the infection or sustained virologic response (SVR) is defined as aviraemia at week 12 after treatment. In patients without cirrhosis, an SVR is associated with normalisation of liver function tests and improvement in or disappearance of necroinflammation and liver fibrosis. In patients with cirrhosis, SVR is associated with a decrease in the risk of clinical events related to chronic liver disease, but the risk is not eliminated completely.
Pre-treatment investigations should include non-invasive assessment of liver fibrosis. This is important for determining the duration of treatment for certain combinations of antivirals and it is essential to identify patients with advanced fibrosis, who will still require long-term follow-up after being cured. In most cases, if pan-genotypic combinations are used, identifying the genotype is not necessary for selecting treatment. However, knowing the genotype can help to differentiate re-infection from recurrence and determine the epidemiology of the infection in specific scenarios; for example, in flare-ups of acute hepatitis.
Medical follow-up during the treatment is not necessary, except for patients at risk of complications due to advanced liver disease or patients in whom therapeutic compliance may be in doubt. Cured patients without fibrosis or with only mild fibrosis (F0–F2) can be discharged once SVR is confirmed. Although probably not necessary in many cases, patients with advanced fibrosis should be followed up indefinitely until we have more data on factors which are predictors of progression.
Since 2014, over 100,000 patients have been treated with these new combinations in Spain, with cure being achieved in most cases. We now need to take a step further in order to diagnose and give access to treatment to patients who are outside the system. This will require a national strategy for the elimination of HCV. Such a strategy should include population screening and facilitate access to assessment for antiviral therapy. Treatment guidelines should be made as simple as possible.
The purpose of this document is to help doctors treating infected patients to select the most effective, safest and simplest treatment. In 2018, treatment for any patient with HCV should be based on DAAs without either interferon (IFN) or ribavirin (RBV).
MethodsThese treatment guidelines were developed by first defining a set of objectives and then conducting a systematic review of the literature aimed at responding to the questions raised. We followed the Appraisal of Guidelines for Research and Evaluation (AGREE) methodology, which is described in detail on the website: www.agreecollaboration.org
The following points were defined as primary objectives:
- •
Provide management recommendations for IFN-naive patients and patients previously treated with IFN, according to the different virus genotypes.
- •
Provide recommendations for DAA treatment failure.
- •
Base treatment decisions on whether or not special clinical situations apply: acute infection, liver cirrhosis and clinical decompensation, transplant patients with recurrence of HCV infection, coinfection with human immunodeficiency virus (HIV) and renal failure.
Recommendations are graded with a letter, indicating their strength (A: should always be offered; B: in general should be offered; C: should be offered optionally), and a number, which describes the evidence supporting the recommendation (I: results obtained from one or more clinical trials, a meta-analysis or an integrated analysis of clinical trials; II: observational cohort data; III: expert opinion).
The regimens are listed in alphabetical order and not by order of preference or recommendation. A summary of the treatment recommendations is shown in Tables 1 and 2.
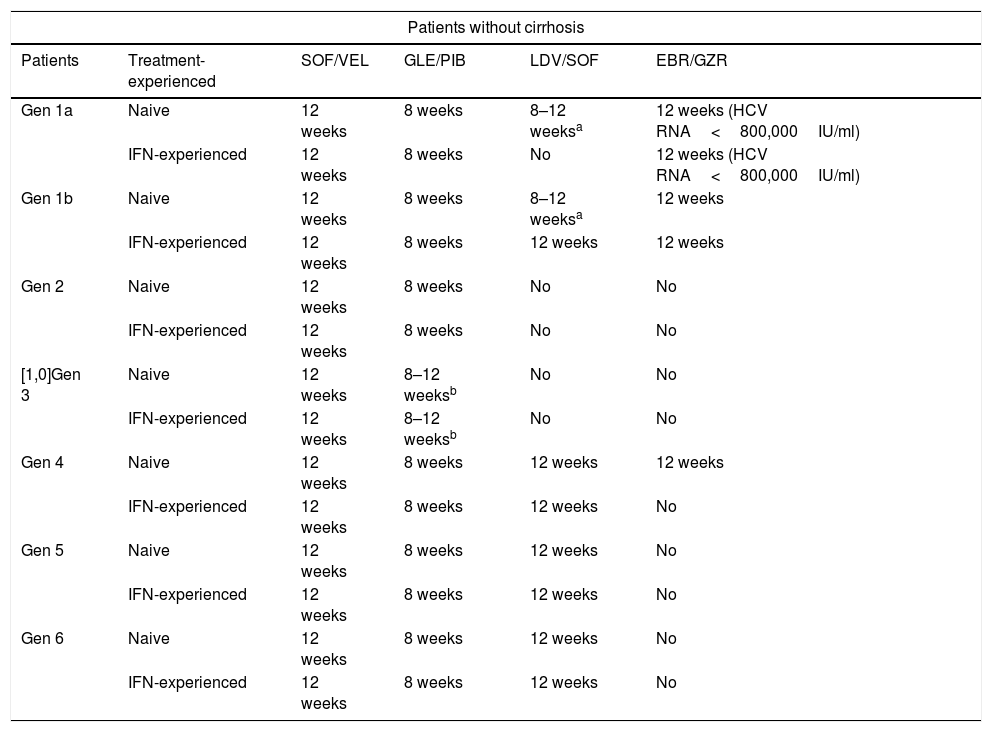
Treatment recommendations for chronic infection with hepatitis C virus in interferon-naive and interferon-experienced patients without cirrhosis.
| Patients without cirrhosis | |||||
|---|---|---|---|---|---|
| Patients | Treatment-experienced | SOF/VEL | GLE/PIB | LDV/SOF | EBR/GZR |
| Gen 1a | Naive | 12 weeks | 8 weeks | 8–12 weeksa | 12 weeks (HCV RNA<800,000IU/ml) |
| IFN-experienced | 12 weeks | 8 weeks | No | 12 weeks (HCV RNA<800,000IU/ml) | |
| Gen 1b | Naive | 12 weeks | 8 weeks | 8–12 weeksa | 12 weeks |
| IFN-experienced | 12 weeks | 8 weeks | 12 weeks | 12 weeks | |
| Gen 2 | Naive | 12 weeks | 8 weeks | No | No |
| IFN-experienced | 12 weeks | 8 weeks | No | No | |
| [1,0]Gen 3 | Naive | 12 weeks | 8–12 weeksb | No | No |
| IFN-experienced | 12 weeks | 8–12 weeksb | No | No | |
| Gen 4 | Naive | 12 weeks | 8 weeks | 12 weeks | 12 weeks |
| IFN-experienced | 12 weeks | 8 weeks | 12 weeks | No | |
| Gen 5 | Naive | 12 weeks | 8 weeks | 12 weeks | No |
| IFN-experienced | 12 weeks | 8 weeks | 12 weeks | No | |
| Gen 6 | Naive | 12 weeks | 8 weeks | 12 weeks | No |
| IFN-experienced | 12 weeks | 8 weeks | 12 weeks | No | |
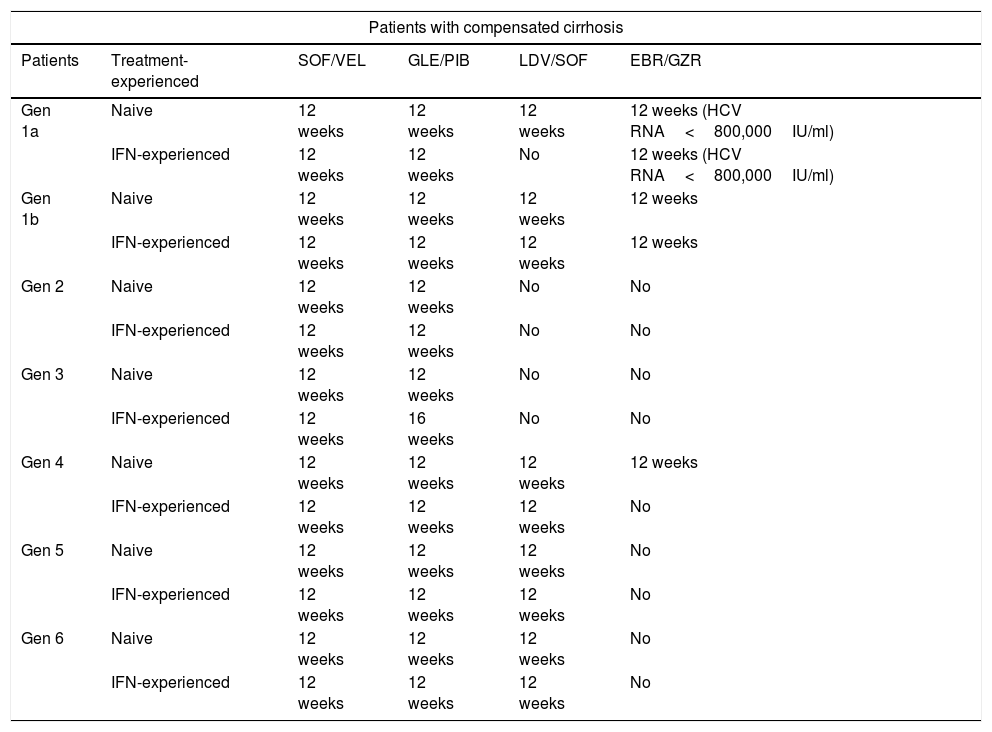
Treatment recommendations for chronic infection with hepatitis C virus in interferon-naive and interferon-experienced patients with cirrhosis.
| Patients with compensated cirrhosis | |||||
|---|---|---|---|---|---|
| Patients | Treatment-experienced | SOF/VEL | GLE/PIB | LDV/SOF | EBR/GZR |
| Gen 1a | Naive | 12 weeks | 12 weeks | 12 weeks | 12 weeks (HCV RNA<800,000IU/ml) |
| IFN-experienced | 12 weeks | 12 weeks | No | 12 weeks (HCV RNA<800,000IU/ml) | |
| Gen 1b | Naive | 12 weeks | 12 weeks | 12 weeks | 12 weeks |
| IFN-experienced | 12 weeks | 12 weeks | 12 weeks | 12 weeks | |
| Gen 2 | Naive | 12 weeks | 12 weeks | No | No |
| IFN-experienced | 12 weeks | 12 weeks | No | No | |
| Gen 3 | Naive | 12 weeks | 12 weeks | No | No |
| IFN-experienced | 12 weeks | 16 weeks | No | No | |
| Gen 4 | Naive | 12 weeks | 12 weeks | 12 weeks | 12 weeks |
| IFN-experienced | 12 weeks | 12 weeks | 12 weeks | No | |
| Gen 5 | Naive | 12 weeks | 12 weeks | 12 weeks | No |
| IFN-experienced | 12 weeks | 12 weeks | 12 weeks | No | |
| Gen 6 | Naive | 12 weeks | 12 weeks | 12 weeks | No |
| IFN-experienced | 12 weeks | 12 weeks | 12 weeks | No | |
The following combinations are recommended for the treatment of HCV genotype 1a infection: elbasvir/grazoprevir, glecaprevir/pibrentasvir, ledipasvir/sofosbuvir and sofosbuvir/velpatasvir.
Elbasvir/grazoprevir. The combination elbasvir/grazoprevir is recommended for 12 weeks in IFN-naive and patients previously treated with IFN without cirrhosis or with compensated cirrhosis (Child A) with baseline viraemia below 800,000IU/ml. This recommendation is based on the C-EDGE Treatment-Naive (TN) and C-EDGE Treatment-Experienced (TE)1,2 studies, in which an SVR rate of 92% was observed for IFN-naive (144/157) and previously treated patients (55/60). In an integrated analysis of all patients with genotype 1a (including those in phase ii and phase iii studies with this combination) an SVR rate of 99% (121/122) was found in patients with a viral load of less than 800,000IU/ml.3 However, the SVR rate was found to be lower in patients with a viral load greater than 800,000IU/ml who had baseline resistance to NS5a inhibitors. As our guidelines do not propose determining baseline resistance before starting treatment and there are other therapeutic options, use of this combination is not recommended in patients with genotype 1a and baseline viral load greater than 800,000 copies.3
This combination is contraindicated in patients with decompensated cirrhosis or previous history of decompensation (see section dedicated to decompensated patients).
Glecaprevir/pibrentasvir. The combination glecaprevir/pibrentasvir is recommended in IFN-naive and previously treated patients for eight weeks in patients without cirrhosis and 12 weeks in patients with cirrhosis. This recommendation is based on the studies ENDURANCE-1, where the SVR rate was 98% (150/152) in patients without cirrhosis after eight weeks of treatment,4 and EXPEDITION-1,5 with an SVR rate of 98% (47/48) in patients with cirrhosis treated for 12 weeks.
This combination is contraindicated in patients with decompensated cirrhosis or previous history of decompensation (see section dedicated to decompensated patients).
Ledipasvir/sofosbuvir. The combination ledipasvir/sofosbuvir is recommended for 12 weeks in naive patients without cirrhosis or with compensated cirrhosis. Naive patients without cirrhosis with a viral load of less than 6,000,000IU/ml can be treated for eight weeks. This recommendation is based on the results of the ION-1, ION-3 and ION-4 studies,6–8 as well as numerous published real-practice studies. The SVR rate in naive patients with and without cirrhosis in the ION-1 study was 98% (141/144).6 The SVR rate in the real-life study in Spain was 95.8%.9
Administration of this regimen in patients previously treated with IFN is not recommended as it requires the addition of RBV.3 This combination can be administered in patients with decompensated cirrhosis (see section dedicated to decompensated patients).
Sofosbuvir/velpatasvir. The combination sofosbuvir/velpatasvir is recommended for 12 weeks in naive or previously treated patients with or without compensated cirrhosis. This recommendation is based on the ASTRAL-110 study, in which an SVR rate of 98% (206/210) was obtained, including patients with and without cirrhosis with all genotypes. In the POLARIS-2 study, an SVR rate of 98% was obtained in naive patients.11 These results have been confirmed in real-life studies.12,13
This combination can be administered in patients with decompensated cirrhosis (see section dedicated to decompensated patients).
Genotype 1a recommendations. Any of the following combinations may be used- -
Elbasvir/grazoprevir for 12 weeks in patients with baseline viral load of less than 800,000IU/ml (BI).
- -
Glecaprevir/pibrentasvir for eight weeks in patients without cirrhosis and 12 weeks in patients with cirrhosis (AI).
- -
Ledipasvir/sofosbuvir for 12 weeks in naive patients (AI). In naive patients without cirrhosis with a viral load of less than 6,000,000IU/ml treatment can be shortened to eight weeks (BII).
- -
Sofosbuvir/velpatasvir for 12 weeks in all patients (AI).
The following combinations are recommended for the treatment of HCV genotype 1b: elbasvir/grazoprevir, glecaprevir/pibrentasvir, ledipasvir/sofosbuvir and sofosbuvir/velpatasvir.
Elbasvir/grazoprevir. The combination elbasvir/grazoprevir is recommended for 12 weeks in IFN-naive and previously treated patients without cirrhosis or with compensated cirrhosis (Child A). This recommendation is based on the results of the studies C-EDGE-TN1 (treatment-naive) and C-EDGE-TE2 (treatment-experienced), in which SVR rates of 99% (129/131) and 100% (34/34), respectively, were obtained. An integrated analysis of all phase ii and iii studies showed an SVR rate of 97% (1040/1070).14
This combination is contraindicated in patients with decompensated cirrhosis or previous history of decompensation (see section dedicated to decompensated patients).
Glecaprevir/pibrentasvir. The combination glecaprevir/pibrentasvir is recommended in IFN-naive and previously treated patients for eight weeks in patients without cirrhosis and 12 weeks in patients with cirrhosis. This recommendation is based on the studies ENDURANCE-1,4 where the SVR rate was 100% (198/198) in patients without cirrhosis after eight weeks of treatment, and EXPEDITION-1,5 with an SVR rate of 100% (39/39) in patients with cirrhosis treated for 12 weeks.
This combination is contraindicated in patients with decompensated cirrhosis or previous history of decompensation (see section dedicated to decompensated patients).
Ledipasvir/sofosbuvir. The combination ledipasvir/sofosbuvir is recommended for 12 weeks in IFN-naive and previously treated patients without cirrhosis or with compensated cirrhosis. Naive patients with a viral load of less than 6,000,000IU/ml can be treated for eight weeks. This recommendation is based on the results of the ION-1, ION-3 and ION-4 studies,6–8 as well as numerous published real-practice studies.12,13 The SVR rate in naive patients with and without cirrhosis in the ION-1 study was 100% (66/66).6 An integrated analysis of the phase II and III studies in patients with cirrhosis showed an SVR rate of 96%6 in IFN-naive patients and 96% (124/129) in IFN-experienced patients. The SVR rate in the real-life study in Spain was 97%.9
This combination can be administered in patients with decompensated cirrhosis (see section dedicated to decompensated patients).
Sofosbuvir/velpatasvir. The combination sofosbuvir/velpatasvir is recommended for 12 weeks in IFN-naive or previously treated patients with or without compensated cirrhosis. This recommendation is based on the ASTRAL-1 study,10 in which an SVR rate of 99% (117/118) was obtained, including all types of patients. These results have been confirmed in real-life studies.12,13
This combination can be administered in patients with decompensated cirrhosis (see section dedicated to decompensated patients).
Genotype 1b recommendations. Any of the following combinations may be used- –
Elbasvir/grazoprevir for 12 weeks (AI).
- –
Glecaprevir/pibrentasvir for eight weeks in patients without cirrhosis and 12 weeks in patients with cirrhosis (AI).
- –
Ledipasvir/sofosbuvir for 12 weeks (AI). In naive patients without cirrhosis with a viral load of less than 6,000,000IU/ml treatment can be shortened to eight weeks (BII).
- –
Sofosbuvir/velpatasvir for 12 weeks in all patients (AI).
The following combinations are recommended for the treatment of HCV genotype 2 infection: glecaprevir/pibrentasvir and sofosbuvir/velpatasvir.
Glecaprevir/pibrentasvir. The combination glecaprevir/pibrentasvir is recommended in treatment-naive patients and patients previously treated with IFN or sofosbuvir plus RBV, for eight weeks in patients without cirrhosis and 12 weeks in patients with cirrhosis. This recommendation is based on the results of the phase ii SURVEYOR-2 study,15 in which an SVR rate of 98% (53/54) was obtained in patients without cirrhosis treated for eight weeks. These results were confirmed in the CERTAIN-2 study16 (127/129) on Japanese patients. In patients with cirrhosis treated for 12 weeks, SVR rates of 100% (31/31) were obtained in the EXPEDITION-1 study5 and in the CERTAIN-2 study16 on Japanese patients.
This combination is contraindicated in patients with decompensated cirrhosis or previous history of decompensation (see section dedicated to decompensated patients).
Sofosbuvir/velpatasvir. The combination sofosbuvir/velpatasvir is recommended for 12 weeks in naive or previously treated patients with or without compensated cirrhosis. This recommendation is based on the ASTRAL-2 study,17 in which an SVR rate of 99% (133/134) was obtained, including all types of patients with genotype 2. These results have been confirmed in real-life studies.13
This combination can be administered in patients with decompensated cirrhosis (see section dedicated to decompensated patients).
Genotype 2 recommendations. Any of the following combinations may be used- –
Glecaprevir/pibrentasvir for eight weeks in patients without cirrhosis and 12 weeks in patients with cirrhosis (AI).
- –
Sofosbuvir/velpatasvir for 12 weeks in all patients (AI).
The following combinations are available for the treatment of HCV genotype 3 infection: sofosbuvir/velpatasvir and glecaprevir/pibrentasvir.
Sofosbuvir/velpatasvir. In patients without cirrhosis, treatment for 12 weeks is recommended. This recommendation is based on the ASTRAL-3 study,17 in which an SVR rate of 98% (160/163) was obtained. Treatment for 12 weeks without RBV is also recommended in patients with cirrhosis. This recommendation is based on the ASTRAL-3 study,17 in which a response rate without RBV of 91% (73/80) was obtained, and on the POLARIS-3 study,11 in which the rate was 96% (105/109), also without RBV. A recent study obtained an SVR rate of 96% (99/103) in patients with cirrhosis treated with RBV, and 91% (92/101) without RBV.18 The benefit from RBV was found mainly in patients with baseline resistance in the NS5a gene. The recommendation to use RBV in patients in whom there is evidence of resistance in NS5a was therefore debated by the panel, with 42% of the members voting in favour, and 58% supporting the option of using sofosbuvir/velpatasvir without RBV in all patients.
Glecaprevir/pibrentasvir. In patients without cirrhosis, eight weeks of treatment with glecaprevir/pibrentasvir is recommended. This recommendation is based on the ENDURANCE-3 study,19 in which the overall SVR rate was 95% (149/157) for the eight-week treatment and 96% (223/233) for 12 weeks. After eight weeks of treatment, the SVR rate was 86% (19/22) in patients with baseline resistance in NS3 and 76% (12/16) in patients with resistance in NS5a (A30K). In patients with F3, the SVR rate was 89% (24/27). Therefore, when there is evidence of these poor prognostic factors (F3 fibrosis or any of the types of resistance cited), it is recommended that treatment be extended to 12 weeks.
Based on the SURVEYOR-2 study,15 where the SVR rate was 100% (48/48), 12 weeks of treatment is recommended in naive patients with cirrhosis. In patients with cirrhosis who previously did not respond to IFN, based on the same study, treatment for 16 weeks is recommended.
This combination is contraindicated in patients with decompensated cirrhosis or previous history of decompensation (see section dedicated to decompensated patients).
Genotype 3 recommendations. Any of the following combinations may be used- –
Glecaprevir/pibrentasvir for eight weeks for patients without cirrhosis. In the case of poor prognostic factors or compensated cirrhosis in naive patients, extend the treatment to 12 weeks, and in patients with failure to IFN and compensated cirrhosis, extend to 16 weeks (AI).
- –
Sofosbuvir/velpatasvir for 12 weeks for patients with or without cirrhosis (AI).
The following combinations are recommended: elbasvir/grazoprevir, glecaprevir/pibrentasvir, ledipasvir/sofosbuvir and sofosbuvir/velpatasvir.
Elbasvir/grazoprevir. Elbasvir/grazoprevir is recommended for 12 weeks in naive patients. In a joint analysis of clinical trials with elbasvir/grazoprevir, 97 out of 101 (96%) naive patients with genotype 4 who were treated for 12 weeks, without RBV, achieved SVR12. However, as only 39 out of 44 (89%) previously treated patients responded,20 this regimen is not advisable in that scenario.
This combination is contraindicated in patients with decompensated cirrhosis or previous history of decompensation (see section dedicated to decompensated patients).
Glecaprevir/pibrentasvir. Use of glecaprevir/pibrentasvir is recommended for eight weeks in patients without cirrhosis and 12 weeks in patients with cirrhosis. The ENDURANCE-4 phase iii trial included 76 patients without liver cirrhosis, either treatment-naive or previously treated with Peg-IFN and/or RBV and/or sofosbuvir, who received glecaprevir/pibrentasvir for 12 weeks21; 76 (99%) achieved SVR12. In an integrated analysis of clinical trials with glecaprevir/pibrentasvir involving 62 genotype 4 patients without cirrhosis treated for eight weeks, 59 (95%) achieved SVR12 in the intention-to-treat analysis. However, as none of the three who did not achieve SVR12 had virological failure, the modified rate of SVR by intention-to-treat was 100%.22
In the phase 3 EXPEDITION-1 trial, 16 patients with genotype 4 and compensated cirrhosis were selected and treated for 12 weeks with glecaprevir/pibrentasvir; all achieved SVR12.5 The real-life data reported to date (German registry and NAVIGATOR-II study23), although still limited, are consistent with those of EXPEDITION-1.
This combination is contraindicated in patients with decompensated cirrhosis or previous history of decompensation (see section dedicated to decompensated patients).
Ledipasvir/sofosbuvir. Use of ledipasvir/sofosbuvir for 12 weeks is recommended. Of the 21 patients (33% with cirrhosis) in the SYNERGY study,24 20 (95%) achieved SVR12. In a French multicentre study,25 31 of the 34 (91%) patients without cirrhosis and all 10 with cirrhosis (100%) achieved SVR12. The data reported from clinical practice with a greater number of patients than in clinical trials have also shown SVR rates above 95%.
Sofosbuvir/velpatasvir. Use of sofosbuvir/velpatasvir for 12 weeks is recommended. In the ASTRAL-1 trial, which overall included 19% patients with cirrhosis, 116 patients with genotype 4 were treated with sofosbuvir/velpatasvir for 12 weeks, all of whom achieved SVR12.10 In the POLARIS-2 trial,11 57 patients with genotype 4 were treated with sofosbuvir/velpatasvir and 98% achieved SVR12. Additionally, the 60 patients with genotype 4 included in an integrated analysis of patients with advanced fibrosis or cirrhosis who were participants in clinical trials of sofosbuvir/velpatasvir all achieved SVR12.26
Genotype 4 recommendations. Any of the following combinations may be used- –
Elbasvir/grazoprevir for 12 weeks (BI) in naive patients.
- –
Glecaprevir/pibrentasvir for eight weeks in patients without cirrhosis and 12 weeks in patients with cirrhosis (AI).
- –
Ledipasvir/sofosbuvir for 12 weeks (BI).
- –
Sofosbuvir/velpatasvir for 12 weeks (AI).
The following combinations can be used in these patients: glecaprevir/pibrentasvir, ledipasvir/sofosbuvir and sofosbuvir/velpatasvir.
Glecaprevir/pibrentasvir. Glecaprevir/pibrentasvir is recommended for eight weeks in patients without cirrhosis and 12 weeks in patients with cirrhosis. Preliminary reports from the ENDURANCE-5,6 study27 suggest that 22 out of 23 (96%) patients with genotype 5 and 46 out of 47 (98%) with genotype 6 achieved SVR12, with no differences between patients with or without cirrhosis. In an integrated analysis of clinical trials with glecaprevir/pibrentasvir which included 30 patients with genotype 5 (2 treated for 8 weeks) and 43 with genotype 6 (12 treated for 8 weeks), 100% achieved SVR12.21
This combination is contraindicated in patients with decompensated cirrhosis or previous history of decompensation (see section dedicated to decompensated patients).
Ledipasvir/sofosbuvir. Ledipasvir/sofosbuvir for 12 weeks is recommended. In a French multicentre study,28 39 out of 41 (95%) patients with genotype 5 (9 with cirrhosis) achieved SVR12 after taking ledipasvir/sofosbuvir for 12 weeks. In a New Zealand study,29 the same regimen achieved SVR12 in 24 out of 25 (96%) treatment-naive and treatment-experienced patients with genotype 6.
Sofosbuvir/velpatasvir. Sofosbuvir/velpatasvir for 12 weeks is recommended. Twenty-three of the 24 (96%) patients with genotype 5 and the 38 (100%) with genotype 6 treated for 12 weeks with sofosbuvir/velpatasvir in the ASTRAL-1 trial achieved SVR12.10 The nine patients with genotype 6 in the sofosbuvir/velpatasvir arm of POLARIS-2 also achieved SVR.11 In an integrated analysis of selected patients with advanced fibrosis or cirrhosis in clinical trials with sofosbuvir/velpatasvir, the 13 (100%) with genotype 5 and 20 (100%) with genotype 6 achieved SVR12.26
Genotype 5 and 6 recommendations. Any of the following regimens may be used- –
Glecaprevir/pibrentasvir for eight weeks in patients without cirrhosis and 12 weeks in patients with cirrhosis (BI).
- –
Ledipasvir/sofosbuvir for 12 weeks (BI).
- –
Sofosbuvir/velpatasvir for 12 weeks (BI).
The rate of SVR is increasingly higher, but, in a limited number of patients, the previous antiviral treatment has failed. In these cases, it is important to assess treatment compliance, and to check whether or not they received the optimal regimen for the right amount of time.
Re-treatment should be guided by the likelihood of response according to the individual's resistance profile or, if the determination of resistance is not available, by the previous treatment received.
The sofosbuvir/velpatasvir/voxilaprevir combination for 12 weeks is recommended in patients without cirrhosis or with compensated cirrhosis following failure to any DAA regimen. This recommendation is based on the POLARIS-1 and POLARIS-4 studies.30 In the POLARIS-1 study, which included patients (46% with cirrhosis) who had prior failure with regimens containing NS5a inhibitors, an SVR rate of 96% (253/263) was obtained. In the POLARIS-4 study, which included patients (46% with cirrhosis) who had prior failure with regimens not containing NS5a inhibitors, an SVR rate of 98% (178/182) was obtained after 12 weeks of treatment. Neither the genotype nor baseline resistance were found to have any effect on the SVR rate in these studies. There are no data to recommend the use of RBV.
In patients with decompensated cirrhosis who cannot be given a regimen containing a protease inhibitor, sofosbuvir/velpatasvir with RBV for 24 weeks is recommended.
The use of glecaprevir/pibrentasvir as rescue therapy is not recommended, based on data from the MAGELLAN-1 study.31 The combination of sofosbuvir/glecaprevir/pibrentasvir can be used as a last rescue in patients with poor response criteria. This recommendation is based on the more potent in vitro inhibition achieved by pibrentasvir compared to all the other NS5a inhibitors and on preliminary data from a study where it achieved 100% SVR. In the MAGELLAN-3 study,32 this combination effectively rescued 22/23 patients who had prior failure with glecaprevir/pibrentasvir. Although there is no published evidence, the combination sofosbuvir/velpatasvir/voxilaprevir could be considered for rescue therapy, which in many situations does not achieve 100% response.
Recommendations in patients with direct-acting antiviral treatment failure- -
Knowing the resistance profile of the individual patient before re-treatment is useful, but not essential (BI).
- -
The combination sofosbuvir/velpatasvir/voxilaprevir for 12 weeks is recommended as rescue therapy in all patients without cirrhosis or with compensated cirrhosis who have failed previous treatment, regardless of the type of treatment or genotype (AI).
- -
In patients with decompensated cirrhosis, 24 weeks of the combination sofosbuvir/velpatasvir with RBV can be used (BII).
- -
The combination sofosbuvir/glecaprevir/pibrentasvir should be reserved for exceptional cases, such as rescue of patients in whom the combination sofosbuvir/velpatasvir/voxilaprevir (CIII) or of glecaprevir/pibrentasvir (BII) fails.
Generally speaking, patients with acute hepatitis due to HCV should be started on treatment with DAA. The regimens recommended are ledipasvir/sofosbuvir and elbasvir/grazoprevir.
SVR rates of 100% are achieved with six weeks of ledipasvir/sofosbuvir in genotype 1.33 The SVR to this same treatment in patients infected with HIV is 77%. Ledipasvir/sofosbuvir34 and elbasvir/grazoprevir,35 both taken for eight weeks, produce high SVR rates in genotypes 1 and 4 in HIV infection (100 and 98%, respectively).
In subjects with a high likelihood of spontaneous clearance (age≤35, peak bilirubin ≥6mg/dl, IL28B CC and fall in HCV-RNA≥1log10 four weeks after infection or ≥2log10 in patients coinfected by HIV) it may be suitable to simply observe the patient and postpone the decision to treat the acute infection. There are insufficient data to support specific recommendations in patients with genotypes other than 1 and 4. Treatment should therefore be the same as for chronic infection.
Recommendations in acute HCV infection- –
Acute infection by genotype 1 or 4 should be treated in all patients with little chance of spontaneous cure (AII).
- –
Patients not infected with HIV with acute hepatitis due to genotype 1 or 4 can be treated with ledipasvir/sofosbuvir for six weeks (AI).
- –
Patients infected with HIV with acute hepatitis genotype 1 or 4 can be treated with elbasvir/grazoprevir for eight weeks (AI) or with ledipasvir/sofosbuvir for eight weeks (AI).
- –
Acute hepatitis due to genotypes other than 1 and 4 should be treated in the same way as the corresponding chronic infections (AIII).
Antiviral treatment is indicated in all patients with decompensated cirrhosis, regardless of the indication for liver transplantation. However, treatment would obviously not be indicated in patients without indication for transplant in the event of serious comorbidities affecting their short-term survival.
In patients with indication for transplant, it is important to remember that clearance of the virus is associated with improvement in liver function in a significant proportion of cases; in fact, some 15–20% of the patients who obtain SVR are able to come off the transplant waiting list.36,37 From the available data, it would seem that the greatest benefit is obtained in individuals with a MELD score below 18–20. In patients with a very high MELD score, antiviral treatment does not seem to provide any clear benefit and can therefore be delayed until after the patient has been transplanted.3
It is also important to remember that the use of protease inhibitors is contraindicated in this group of patients and that the treatment will therefore be based on the combination of sofosbuvir and an NS5a protein inhibitor (sofosbuvir/ledipasvir, sofosbuvir/velpatasvir).
In patients with indication for liver transplantation due to hepatocellular carcinoma, antiviral treatment needs to be individualised. Studies have shown a temporal association between the administration of DAAs and the recurrence of hepatocellular carcinoma.38,39 However, these findings are subject to debate and no such association has been confirmed by other retrospective, cohort and meta-analysis studies.
The recommended therapeutic regimens in patients with decompensated cirrhosis are ledipasvir/sofosbuvir and sofosbuvir/velpatasvir.
Ledipasvir/sofosbuvirThe SOLAR-1 and SOLAR-2 studies evaluated the efficacy of ledipasvir/sofosbuvir with RBV, for 12 or 24 weeks, in mostly genotype 1 patients with Child–Pugh B7 to C12.39,40 They included more than 300 patients, 70% treatment-experienced and 58% genotype 1a. SVR rates at 12 weeks were in the range 83–94% with no differences in the rates for patients treated for 24 weeks. The efficacy in genotype 4 was 57% (SOLAR-1) and 86% after 12 or 24 weeks of treatment (SOLAR-2).
In the British real-life cohort study, in which 280 mostly Child–Pugh B and C patients were included, the ledipasvir/sofosbuvir combination for 12 weeks was effective in genotype 1 in 86% with RBV and 81% without RBV. This combination (with and without RBV) was also assessed in patients infected by genotype 3, with SVR rates of 59% and 43% respectively, which is why it is considered suboptimal.41
Sofosbuvir/velpatasvirThe ASTRAL-4 study evaluated the efficacy of sofosbuvir/velpatasvir, with or without RBV for 12 or 24 weeks in patients with decompensated cirrhosis (Child–Pugh B only).42 The study included 267 patients and the overall rate of SVR in patients treated for 12 weeks, without RBV, was 83%. By subgroup according to genotype: 1a: 88%; 1b: 89%; 2: 100%; 3: 50%; and 4: 100%. With RBV, the SVR was 94% overall and according to genotype: 1a: 94%; 1b: 100%; 2: 100%; 3: 85%; and 4: 100%.
Recommendations in liver cirrhosis due to HCV and clinical decompensation- –
In general, antiviral treatment is indicated in all patients with decompensated cirrhosis who do not have serious comorbidities that threaten patient survival (AI).
- –
In patients on the transplant waiting list, antiviral treatment could prevent graft infection and, in a considerable proportion of cases (15–20%), can avoid the need for liver transplantation by improving liver function (AII).
- –
The improvement in liver function has been observed particularly in patients with MELD<20. For that reason, in patients with severe decompensated cirrhosis (MELD≥20) it is recommended to postpone treatment until after liver transplantation (BII).
- –
The recommended therapeutic regimen in these patients is the combination of velpatasvir and sofosbuvir for 12 weeks. Whenever possible, the use of RBV is recommended; if not, the treatment should be extended to 24 weeks (AI).
- –
In patients on the transplant waiting list due to hepatocellular carcinoma, the priority should be treatment of the cancer. The indication of antiviral treatment should be considered on an individual basis, as a possible effect of DAAs on tumour progression cannot be ruled out (BII).
In our area, cirrhosis due to HCV was until recently the main indication for liver transplantation. Treatment of patients with cirrhosis who are infected with HCV has led to a significant reduction in the indication for transplant due to decompensation in this group of patients.43 In addition, the majority of patients with hepatocellular carcinoma in the context of HCV cirrhosis and indication for transplant have already received treatment with DAAs and do not have active HCV infection. If we add the fact that most liver transplant patients with HCV infection have already received treatment over recent years, few patients now require treatment in the post-transplant period.
Despite all that, we do have effective and safe therapeutic regimens to eradicate the infection in this group.
The treatments recommended in patients receiving liver transplant are ledipasvir/sofosbuvir, glecaprevir/pibrentasvir and sofosbuvir/velpatasvir.
Ledipasvir/sofosbuvirThe combination ledipasvir/sofosbuvir+RBV for 12 (vs 24) weeks is one option studied in two clinical trials,44,45 with cure rates close to 100% in F0–F4 patients and over 80% in patients with decompensated cirrhosis (Child–Turcotte–Pugh [CTP] B and C), regardless of the duration of treatment. In these studies, which mainly included patients infected with genotype 1, the SVR rate was 96–98% in patients without cirrhosis (108/111) or with compensated cirrhosis (49/51), 85–88% (45/52) in patients with moderate dysfunction and 60–75% (6/9) in patients with severe liver failure. The safety profile was very good.
Glecaprevir/pibrentasvirThe MAGELLAN-2 trial evaluated the safety and efficacy of glecaprevir/pibrentasvir treatment in 100 liver transplant patients (80) and kidney transplant patients (20).46 Patients infected with any genotype (1, 2, 4–6), naive to or experienced with IFN and RBV treatment (with or without sofosbuvir), were included from three months after transplant. The majority of the patients had mild fibrosis (individuals with cirrhosis were not included). Treatment duration was 12 weeks. The SVR rate was 98% and only one virological failure was found in a liver transplant patient infected with G3 and with mild fibrosis. No serious medication-related adverse effects were detected. It should be noted that glecaprevir can mildly interact with commonly used immunosuppressants, such as ciclosporin A and tacrolimus, and monitoring levels during treatment is therefore recommended.
Sofosbuvir/velpatasvirThis therapeutic regimen was tested in 79 liver transplant patients infected by different genotypes (47% genotype 1, 4% genotype 2, 44% genotype 3 and 5% genotype 4).47 The patients received treatment with sofosbuvir/velpatasvir, without RBV, for 12 weeks; 18% had compensated cirrhosis and 60% were treatment-experienced. The median time from transplant to start of treatment was seven years. The SVR rate was 96%. Only two virological failures were recorded: one treatment-naive patient without cirrhosis with genotype 1; and one treatment-experienced patient without cirrhosis with genotype 3. No adverse effects attributable to the treatment were reported.
Recommendations in transplant patients with recurrence of HCV infection- –
Treatment of post-transplant hepatitis C is indicated in all patients (AI).
- –
The use of pan-genotypic treatment regimens is the most recommended option at present. Suitable regimens for the treatment of hepatitis C in transplant patients (AI) are either glecaprevir/pibrentasvir or sofosbuvir/velpatasvir, without RBV, for a period of 12 weeks.
- –
In the case of glecaprevir/pibrentasvir, it is advisable to monitor the levels of immunosuppressants, especially ciclosporin (BII).
- –
In patients with compensated graft cirrhosis post-transplantation and genotype 3 infection, the recommendations for the use of these two treatment regimens are the same as for immunocompetent patients (BII).
- –
In patients with decompensated liver cirrhosis, the regimens should be the same as those used in non-transplant patients (BII).
There are data suggesting that SVR rates may be slightly lower in HIV/HCV co-infection. In acute hepatitis, six-week regimens which are highly effective in individuals without HIV lead to poorer SVR rates in those with HIV. In chronic hepatitis, most studies report the SVR rate to be slightly lower in individuals with HIV/HCV,48–50 as there is a higher recurrence rate among these patients. Before considering treatment with DAAs in HIV/HCV co-infection, the potential interactions between antiretroviral treatment and DAAs need to be reviewed. For this purpose, it is very helpful to consult the University of Liverpool viral hepatitis drug interactions website (https://www.hep-druginteractions.org).
Recommendations in HIV co-infection- –
Patients with chronic HCV and HIV infection should be treated with the same regimen as those without HIV (AI).
- –
Short-term regimens not tested in clinical trials in HIV/HCV coinfection should not be used. They may be considered on an individual basis in patients with a high likelihood of response (BII).
- –
Potential interactions between antiretroviral therapy and current DAAs should be reviewed before starting treatment with these drugs (AI).
The treatment options for patients with creatinine clearance <30ml/min/1.73m2 are limited to elbasvir/grazoprevir and glecaprevir/pibrentasvir.
If the use of RBV is necessary, start at low doses (200–400mg every 24–48h) and adjust according to haemoglobin levels. Where possible, patients in stage 4 chronic kidney disease (CKD), i.e. with a filtration rate of 15–29ml/min/1.73m2, should not be given sofosbuvir as this drug and its main metabolite are excreted by the kidneys.51 In the TARGET cohort, sofosbuvir-based treatments in patients in stage 4 or 5 CKD resulted in SVR and tolerability rates similar to those of other patients, although there was a higher risk of deterioration in renal function.52 In patients with chronic kidney failure in stage 3 CKD (filtration rate of 30–59ml/min/1.73m2) the general treatment recommendations should be followed without adjusting the dose.
Elbasvir/grazoprevirElbasvir/grazoprevir is a safe and effective regimen in patients with genotype 1 infection with creatinine clearance <30ml/min/1.73m2. In the C-SURFER study,53,54 212 of the 221 (96%) patients (6% with cirrhosis) treated with elbasvir/grazoprevir 50/100mg/24h achieved SVR, with a similar rate of adverse effects in both the placebo and treated groups. These results have been confirmed in an observational study,55 with SVR rates of 96% in 747 patients in CKD stage 4 or 5.
Glecaprevir/pibrentasvirGlecaprevir/pibrentasvir was tested in 104 patients in CKD stage 4 or 5 with different genotypes, including 54 with genotype 1, in the single-arm, open-label EXPEDITION-4 study.56 The overall SVR rate was 98%. The serious adverse effects observed were not attributable to treatment.
If kidney transplantation is being proposed in subjects infected with HCV, the best time to treat the infection will need to be discussed. The DAAs available nowadays do not interact to any significant degree with the immunosuppressants used to prevent rejection (https://www.hep-druginteractions.org), which opens up the options to treat HCV both before or after the transplant.
Recommendations in renal failureIn patients with chronic kidney failure in CKD stage 4 or 5, i.e. with creatinine clearance <30ml/min/1.73m2, the recommendations below should be followed:
- –
Genotype 1 or 4:
- ∘
Elbasvir/grazoprevir following the general recommendations (AI for genotype 1, AIII for genotype 4).
- ∘
Glecaprevir/pibrentasvir following the general recommendations (AI).
- –
Genotype 2 or 3:
- ∘
Glecaprevir/pibrentasvir following the general recommendations (AI).
- ∘
Situations in which an HCV-protease-inhibitor-based regimen (elbasvir/grazoprevir or glecaprevir/pibrentasvir) cannot be used: sofosbuvir/velpatasvir, monitoring renal function closely (CIII).
- –
In patients with chronic kidney failure in CKD stage 3, i.e. with creatinine clearance 30–59ml/min/1.73m2, the general treatment recommendations should be followed (AI).
- –
Patients who have had a kidney transplant can be given DAAs following the general recommendations (AII). The decision to treat HCV infection before or after kidney transplantation should be made on an individual basis (AII).
The authors declare the following conflicts of interest:
JLC: consultant for AbbVie, Gilead and MSD.
XF: consultant for AbbVie and Gilead.
JM: adviser for AbbVie, Gilead and MSD. Has received fees for lectures from Janssen, Gilead and MSD and has received grants to attend training activities from Janssen and Gilead.
JAP: adviser for AbbVie, Janssen, Gilead and MSD. Has received support for research from AbbVie, Janssen, Gilead and MSD. Has also received fees for lectures from AbbVie, Janssen, Gilead and MSD and has received grants to attend training activities from Janssen and Gilead.
M. Berenguer: has given presentations for AbbVie, Gilead, MSD, Astellas and Novartis.
M. Buti: consultant for AbbVie, Gilead and MSD.
JAC: has received fees for lectures from Bristol-Myers Squibb, AbbVie, Merck Sharp & Dohme, Roche/Genentech, Intercept and Gilead.
IF: consultant for AbbVie, Janssen, MSD and Gilead.
FG: adviser for ViiV, Gilead, AbbVie, Roche and Hologic. Has received support for research from Gilead and Roche. Has also received fees for lectures from ViiV, Gilead, AbbVie, MSD, Janssen, Roche, bioMérieux, Hologic and Qiagen and has received grants to attend training activities from ViiV, Gilead, AbbVie, MSD, Janssen, Roche, bioMérieux, Hologic and Qiagen.
MG: adviser for AbbVie, Janssen, Gilead and MSD. Has received support for research from AbbVie, Janssen, Gilead and MSD. Has also received fees for lectures from AbbVie, Janssen, Gilead and MSD and has received grants to attend training activities from Janssen and Gilead.
RG: adviser for Janssen and AbbVie. Has received fees for lectures from Janssen, Gilead, MSD, AbbVie and Bristol-Myers Squibb and has received grants to attend training activities from AbbVie, Gilead, MSD and Janssen.
LM: adviser for AbbVie, Janssen and Gilead. Has received fees for lectures from AbbVie, Janssen, Gilead, ViiV and MSD and has received grants to attend training activities from Janssen, ViiV, MSD and Gilead.
PC: declares that he has no conflicts of interest.
José Luis Calleja
Xavier Forns
Juan Macías
Juan Antonio Pineda
Panel of experts:
Marina Berenguer
María Buti
Jose Antonio Carrión
Inmaculada Fernández
Federico García
Miguel García Deltoro
Rafael Granados
Luis Morano
Please cite this article as: Calleja JL, Macias J, Forns X, Garcia F, Berenguer M, Garcia Deltoro M, et al. Guía de tratamiento de la infección por virus de la hepatitis C. Asociación Española para el Estudio del Hígado (AEEH). Gastroenterol Hepatol. 2018;41:597–608.