To evaluate the results of a hepatitis B and C screening program in hospitalized COVID-19 patients.
MethodTransversal prospective study conducted in two Spanish hospitals. Patients admitted from March 1st to December 31st 2020 with a diagnosis of COVID-19 were tested for markers of hepatitis B (HBsAg, anti-HBc) and C (anti-HCV, HCV RNA) infection.
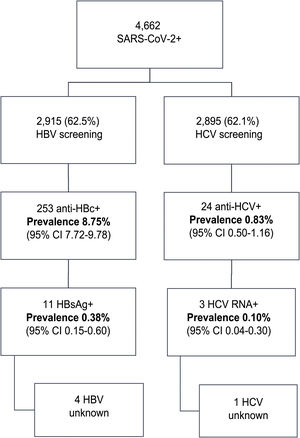
ResultsIn this period, 4662 patients with COVID-19 were admitted to our centers: 56.3% were male, median age was 76 (0–104) years. Data regarding HBV infection was available in 2915 (62.5%) patients; 253 (8.75%) were anti-HBc + and 11 (0.38%) HBsAg+. From these, 4 patients did not have a previous diagnosis of hepatitis B, 7 received corticosteroids and one received prophylaxis. There was one HBV reactivation. Anti-HCV were available in 2895 (62%) patients; 24 (0.83%) were positive. From these, 13 patients had a previous hepatitis C diagnosis: 10 patients had been treated with SVR, one achieved spontaneous cure and 2 did not receive treatment. From the 11 previously unknown anti-VHC + patients, 10 had a negative HCV RNA. Overall, only 3 (0.10%) patients tested RNA HCV + . However, none received HCV treatment (2 older than 90 years with comorbidities, 1 died from COVID-19).
ConclusionScreening of hepatitis C infection in hospitalized COVID-19 patients seems less useful than expected. The low prevalence of active infection after antiviral treatments and the high age of our population limit the detection of potential candidates for treatment. HBV screening should be aimed to prevent reactivation under immunosuppressive treatments.
Evaluar el resultado del cribado de hepatitis B y C en pacientes ingresados con COVID-19.
Pacientes y métodosEstudio transversal, prospectivo, realizado en dos hospitales españoles de tercer nivel. Se estudiaron marcadores de hepatitis B (HBsAg, anti-HBc) y C (anti-VHC, ARN VHC) a todos los pacientes hospitalizados con COVID-19 del 1 de marzo al 31 de diciembre de 2020.
ResultadosEn este periodo ingresaron 4662 pacientes con COVID-19: 56,3% fueron varones, la edad mediana fue 76 (0–104) años. Se realizó serología de hepatitis B a 2915 (62,5%) pacientes; 253 (8,75%) presentaban anti-HBc + y 11 (0,38%) HBsAg+. De los 11 pacientes, 4 desconocían el diagnóstico, 7 recibieron esteroides y uno recibió profilaxis. Hubo un caso de reactivación del VHB. Se determinaron anticuerpos anti-VHC a 2895 (62%) pacientes; 24 (0,83%) fueron positivos. De ellos, 13 pacientes estaban diagnosticados: 10 habían recibido tratamiento, uno se había curado espontáneamente y dos no habían sido tratados. De los 11 restantes, 10 tenían ARN VHC indetectable. En total, sólo 3 (0,10%) pacientes tenían carga viral detectable. Sin embargo, ninguno recibió tratamiento (2 > 90 años con comorbilidades, 1 falleció por COVID-19).
ConclusionesEl cribado de hepatitis C en pacientes ingresados por COVID-19 en nuestro medio ha mostrado menor utilidad de la esperada. La baja prevalencia de infección activa tras los tratamientos antivirales y la alta edad mediana de nuestra población limitan la detección de potenciales candidatos a tratamiento. El cribado de hepatitis B debería dirigirse a prevenir la reactivación en pacientes que precisen tratamientos inmunosupresores.
Viral hepatitis remains a global public health problem due to its high prevalence and high rates of morbidity and mortality. According to calculations, in 2015, around 257 million people worldwide had chronic hepatitis B virus (HBV) infection, and around 71 million had chronic hepatitis C virus (HCV) infection, causing 1.34 million deaths in that year, most due to chronic liver disease (720,000 deaths) or primary liver cancer (470,000 deaths). Consequently, the World Health Organization (WHO) has set as a goal of elimination of viral hepatitis by 2030, with a 90% reduction in new infections and a 65% reduction in mortality.1
Following the introduction of the HBV vaccine in the 1990s, Spain is considered a low-endemicity country, with a prevalence of HBsAg of 0.2%-0.5% and of anti-hepatitis B core (HBc) antibodies of 4%–6%.2
Regarding HCV infection, the development of the new direct-acting antivirals (DAAs) in 2015 and the preparation of the Plan Estratégico Nacional [Spanish National Strategic Plan] to address HCV in the Sistema Nacional de Salud [Spanish National Health System] have enabled more than 143,000 people with HCV in Spain to be treated.3 However, a nationwide study of seroprevalence by the Spanish Ministry of Health in 2018 estimated a prevalence of anti-HCV antibodies of 0.85% and of individuals with detectable HCV RNA of 0.22%; based on this, the number of people in Spain with untreated HCV can be calculated at around 76,500.4 A 2019 position statement by the Asociación Española para el Estudio del Hígado [Spanish Association for the Study of the Liver] (AEEH) recommended various strategies for eliminating HCV in Spain, including screening in higher-prevalence age groups and in groups at risk of contracting HCV, as well as identification of patients previously diagnosed and not treated or not cured with prior treatments.5 All these efforts combined position Spain among the countries most likely to achieve the WHO's HCV elimination targets.6
In 2020, the COVID-19 pandemic paralysed HBV and HCV screening as it prevented patients from making in-person visits and posed multiple obstacles to the diagnosis and care of these diseases. On the one hand, patients with advanced liver disease had a worse prognosis following SARS-CoV-2 infection.7 On the other hand, healthcare system reorganisation has led to delays in most HCV elimination programmes, resulting in decreased rates of initiation of new treatments.3 With regard to HBV, as hospitalised patients with COVID-19 pneumonia often receive high doses of corticosteroids and other immunosuppressant drugs, determining prior infection status may be essential to preventing HBV reactivation by administering prophylaxis to these patients.
However, the high number of hospital admissions due to SARS-CoV-2 infection represents an opportunity to continue HBV and HCV screening by taking advantage of these patients' contact with the healthcare system and thus to continue to advance towards the WHO goal of eliminating viral hepatitis by 2030.
The primary objective of this study was to evaluate the results of an HCV and HBV screening strategy in hospitalised patients with COVID-19. The secondary objectives consisted of investigation of the percentage of patients diagnosed with HCV through screening who could access treatment with DAAs and whether SARS-CoV-2 infection and its treatment had any impact on the course and treatment of HBV infection.
Patients and methodsA cross-sectional, multi-centre study with prospective inclusion was conducted at two Spanish tertiary hospitals: Hospital Universitario de Burgos [Burgos University Hospital] and Complejo Asistencial Universitario de León [León University Healthcare Complex]. In accordance with the recommendations of the Spanish Ministry of Health on the management of COVID-19 in hospital care,8 all patients admitted to our hospitals between 1 March 2020 and 31 December 2020 with SARS-CoV-2 infection were screened by means of testing for markers of HBV infection (HBsAg and anti-HBc antibodies) and HCV infection (anti-HCV antibodies and HCV RNA) hence, universal opportunistic screening was performed. Diagnosis of HCV infection was one-step. The prevalence of anti-HCV and anti-HBc antibodies, the proportion of patients with active and past infection, and the proportion of patients previously diagnosed and/or treated for HBV and HCV at our hospitals were studied. The percentage of patients diagnosed with HCV through screening who could access treatment with DAAs and whether treatment for SARS-CoV-2 infection influenced management of HBV infection detected in screening were also investigated. Operational decisions on performing serology, starting treatment or prophylaxis and consulting with the hepatology unit fell to each COVID team, which functioned in an autonomous and independent manner.
Demographic and epidemiological variables in relation to liver disease (age, sex, prior diagnosis of HBV or HCV and prior treatment) were collected, as were laboratory variables (complete blood count; coagulation; basic and liver-related clinical chemistry; markers of systemic inflammation such as C-reactive protein, interleukin 6 [IL-6] and ferritin; and polymerase chain reaction [PCR] for SARS-CoV-2). Liver fibrosis was measured using non-invasive tests (aspartate aminotransferase-to-platelet ratio index [APRI] and Fibrosis-4 [FIB-4]) in patients diagnosed with HBV or HCV.
The study was approved by the independent ethics committee at the two hospitals: Hospital Universitario de Burgos and Complejo Asistencial Universitario de León. The data were analysed using the statistics software programme SPSS 20.0. Categorical variables were expressed in terms of percentage, and continuous (quantitative) variables were expressed in terms of median and range. Comparisons between quantitative variables were made using the Mann–Whitney U test. p value < 0.05 was considered statistically significant.
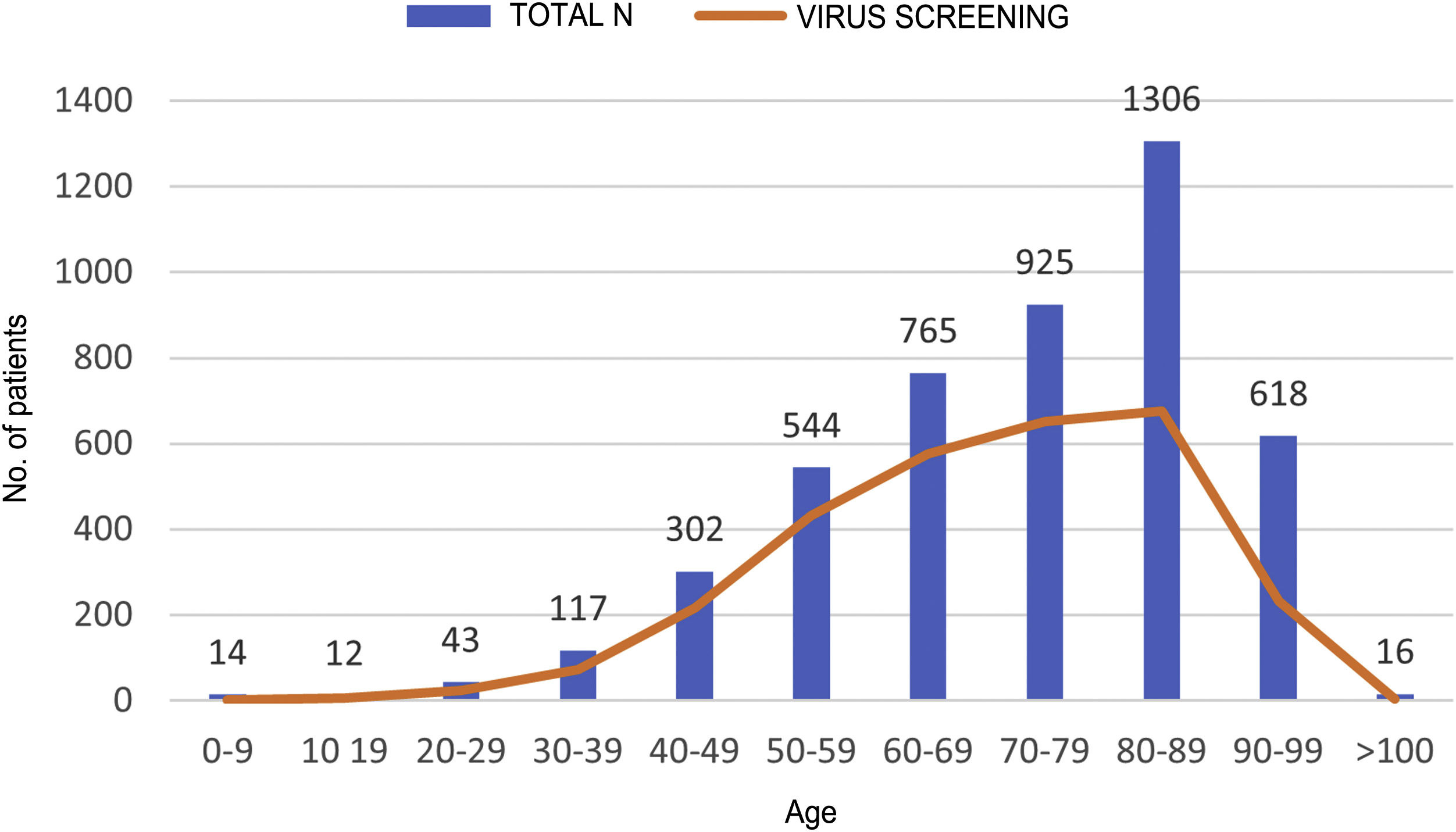
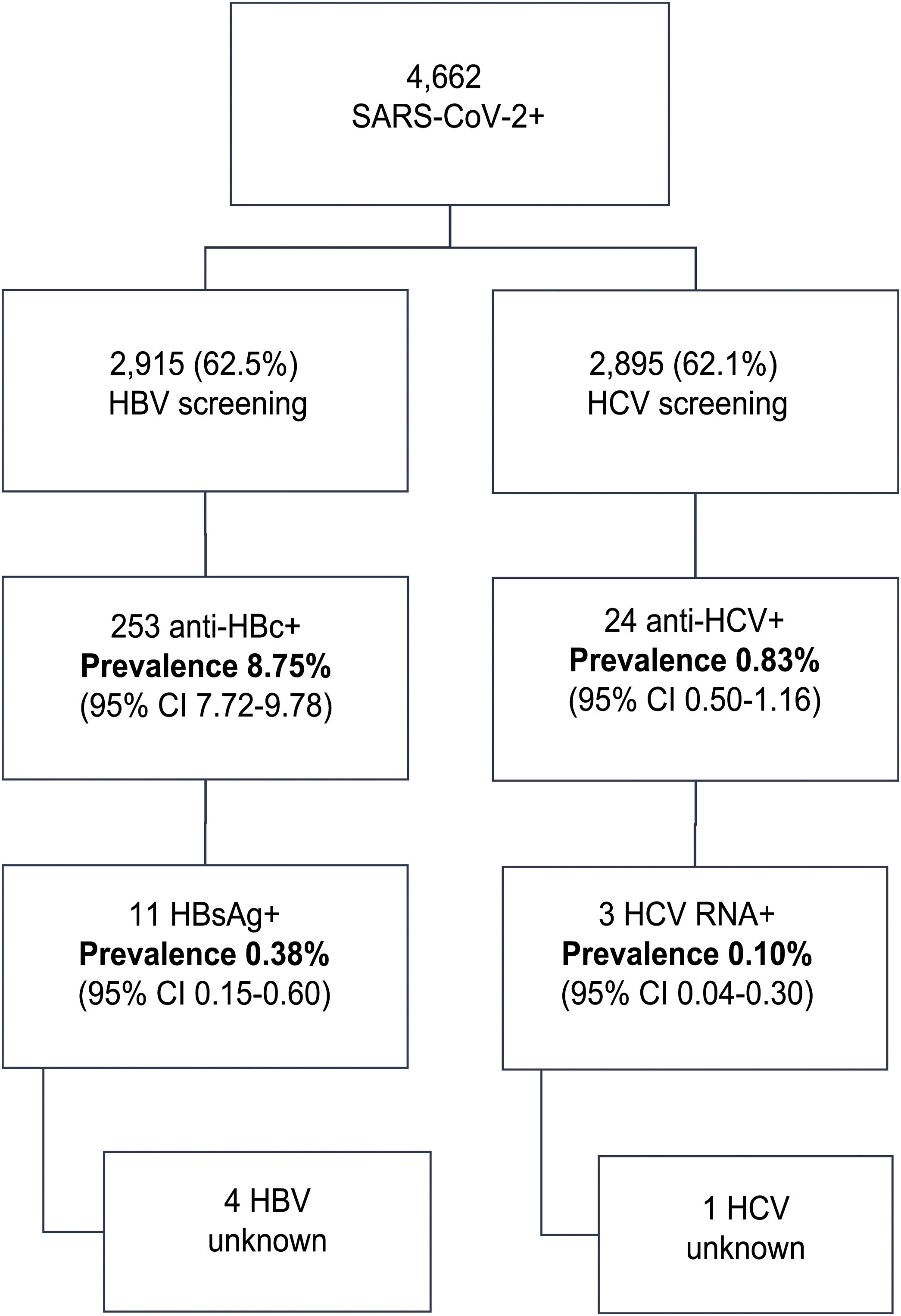
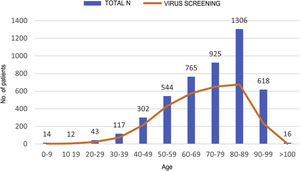
ResultsDuring the study period, 4,662 patients with COVID-19 were hospitalised at our centres. Males comprised 56.3% of the patients. The median age was 76 (0–104) years. The distribution of the admitted patients by age group was as follows: 0–9 years 0.3%, 10–19 years 0.3%, 20–29 years 0.9%, 30–39 years 2.5%, 40–49 years 6.5%, 50–59 years 11.7%, 60–69 years 16.4%, 70–79 years 19.8%, 80–89 years 28%, 90–99 years 13.3% and >100 years 0.3% (Fig. 1). HBV serology was done in 2,915 (62.5%) patients and HCV serology was done in 2,895 (62%) patients (Fig. 2). The median age of the patients who underwent HBV and HCV screening was significantly younger compared to the patients who did not undergo such screening. As for HBV screening, screened patients had a median age of 71.8 (0–102) years whereas unscreened patients had a median age of 83.5 (0–104) years (p < 0.05). The same was seen in patients who underwent HCV serology, with a median age of 72 (0–102) years, versus those who did not undergo such serology, with a median age of 83 (0–104) years (p < 0.05).
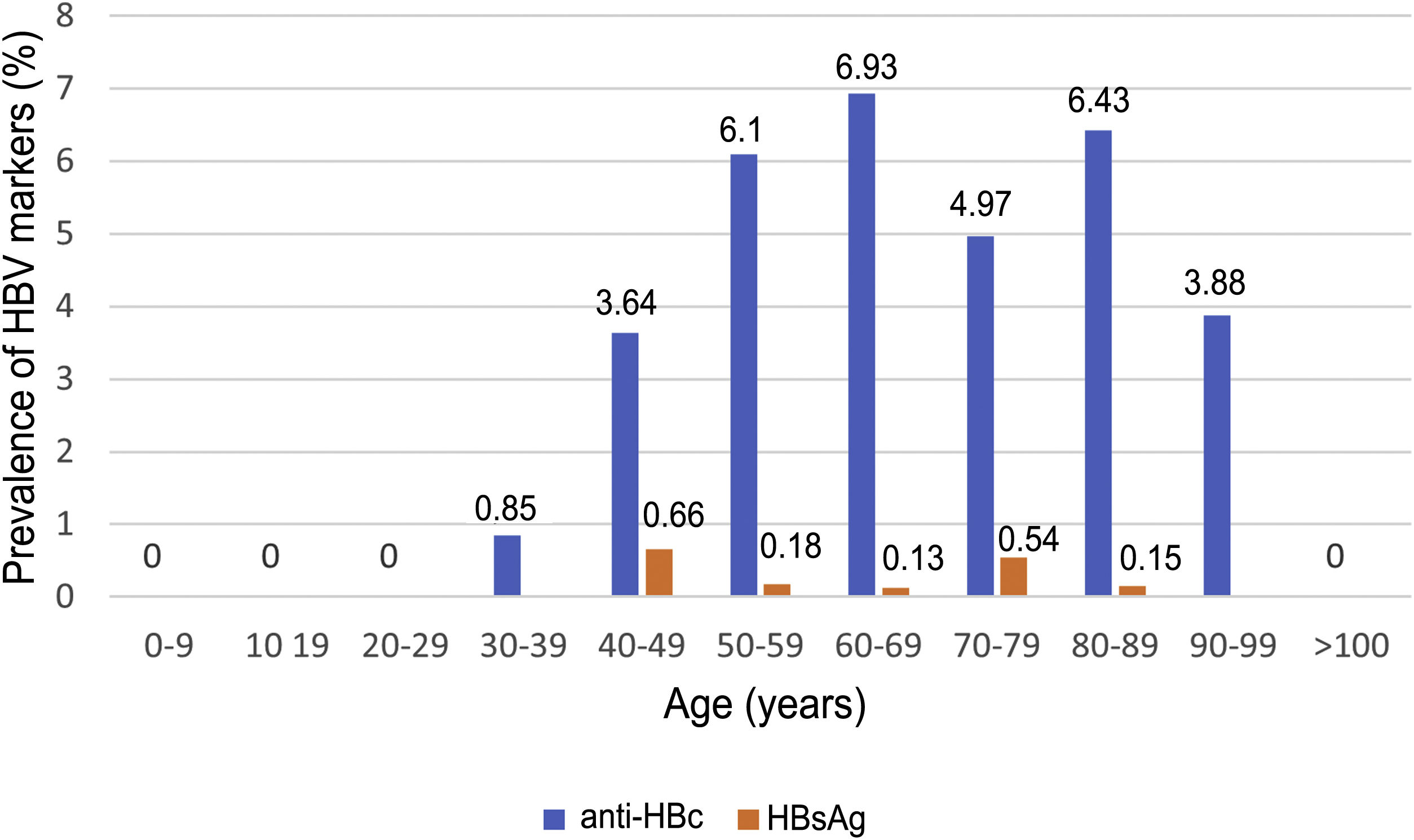
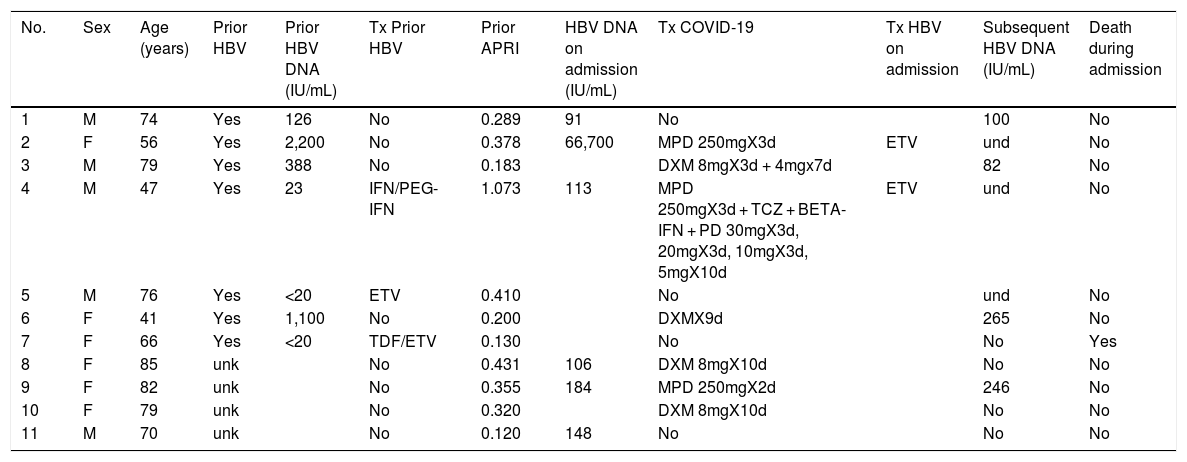
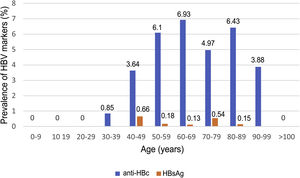
Of the patients with HBV infection data, 253 patients tested positive for anti-HBc antibodies and 11 patients tested positive for HBsAg, indicating a prevalence of anti-HBc antibodies of 8.75% (95% CI 7.72–9.78) and of HBsAg of 0.38% (95% CI 0.15−0.60). Fig. 3 illustrates the prevalence of positive results for anti-HBc antibodies and positive results for HBsAg by age group. Of the 11 patients with active HBV infection, just four (36.4% of patients with HBV and 0.14% of the entire cohort) lacked a prior diagnosis of HBV. The median age of these patients was 80 years, and none of them showed significant fibrosis (all had an APRI < 0.5). Table 1 shows the characteristics of the HBsAg-positive patients. Seven of the 11 HBsAg-positive patients (63.6%) received high-dose corticosteroids during admission; of them, just one received prophylaxis with entecavir (14.2%) and another (14.2%) subsequently started treatment with entecavir due to mild reactivation (HBV DNA 69,900 IU/mL).
Characteristics of HBsAg-positive patients.
| No. | Sex | Age (years) | Prior HBV | Prior HBV DNA (IU/mL) | Tx Prior HBV | Prior APRI | HBV DNA on admission (IU/mL) | Tx COVID-19 | Tx HBV on admission | Subsequent HBV DNA (IU/mL) | Death during admission |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 74 | Yes | 126 | No | 0.289 | 91 | No | 100 | No | |
| 2 | F | 56 | Yes | 2,200 | No | 0.378 | 66,700 | MPD 250mgX3d | ETV | und | No |
| 3 | M | 79 | Yes | 388 | No | 0.183 | DXM 8mgX3d + 4mgx7d | 82 | No | ||
| 4 | M | 47 | Yes | 23 | IFN/PEG-IFN | 1.073 | 113 | MPD 250mgX3d + TCZ + BETA-IFN + PD 30mgX3d, 20mgX3d, 10mgX3d, 5mgX10d | ETV | und | No |
| 5 | M | 76 | Yes | <20 | ETV | 0.410 | No | und | No | ||
| 6 | F | 41 | Yes | 1,100 | No | 0.200 | DXMX9d | 265 | No | ||
| 7 | F | 66 | Yes | <20 | TDF/ETV | 0.130 | No | No | Yes | ||
| 8 | F | 85 | unk | No | 0.431 | 106 | DXM 8mgX10d | No | No | ||
| 9 | F | 82 | unk | No | 0.355 | 184 | MPD 250mgX2d | 246 | No | ||
| 10 | F | 79 | unk | No | 0.320 | DXM 8mgX10d | No | No | |||
| 11 | M | 70 | unk | No | 0.120 | 148 | No | No | No |
d, days; DXM, dexamethasone; ETV, entecavir; F, female; IFN, interferon; M, male; MPD, methylprednisolone; PD, prednisone; PEG-IFN, pegylated interferon; TCZ, tocilizumab; TDF, tenofovir; Tx, treatment; und, undetectable; unk, unknown.
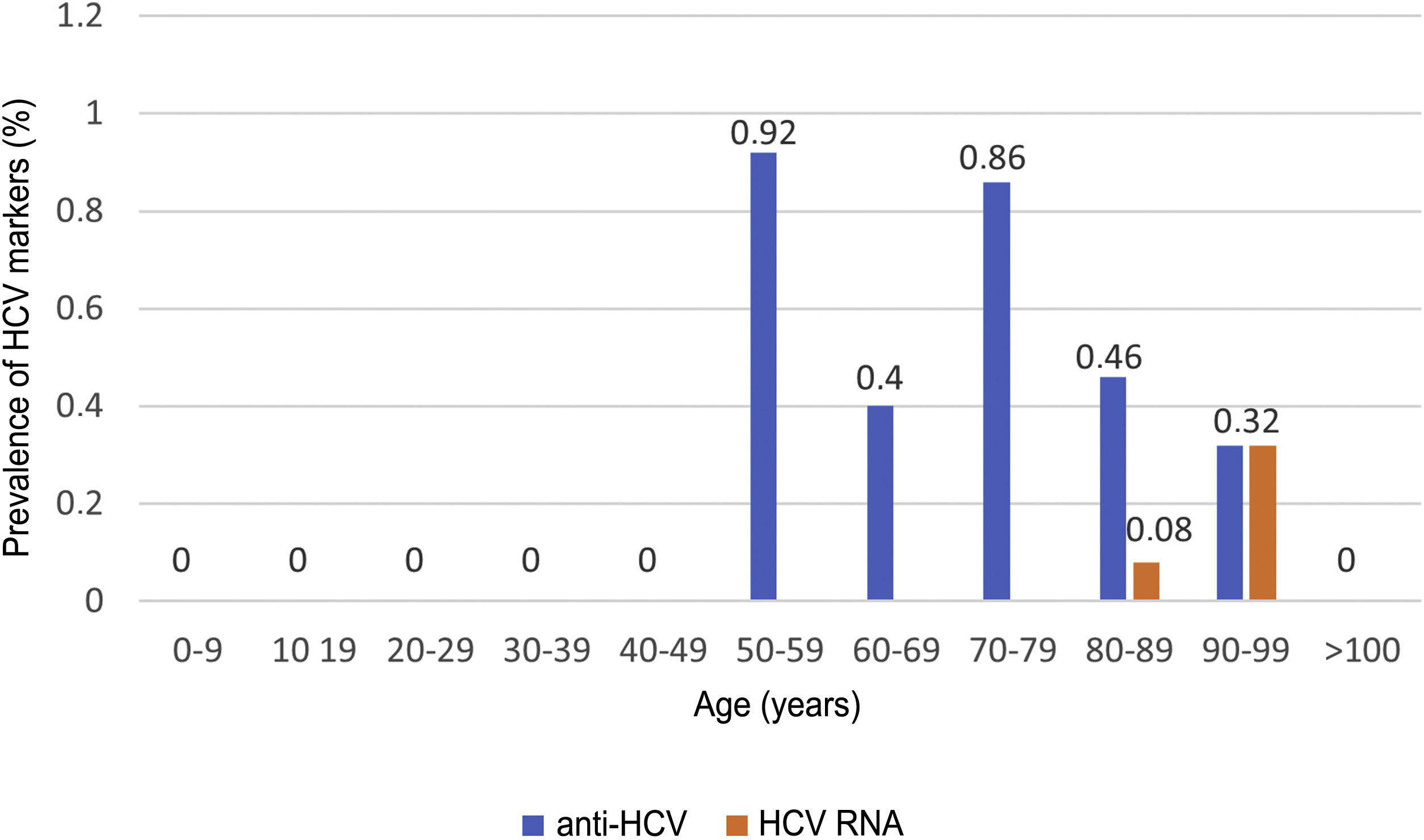
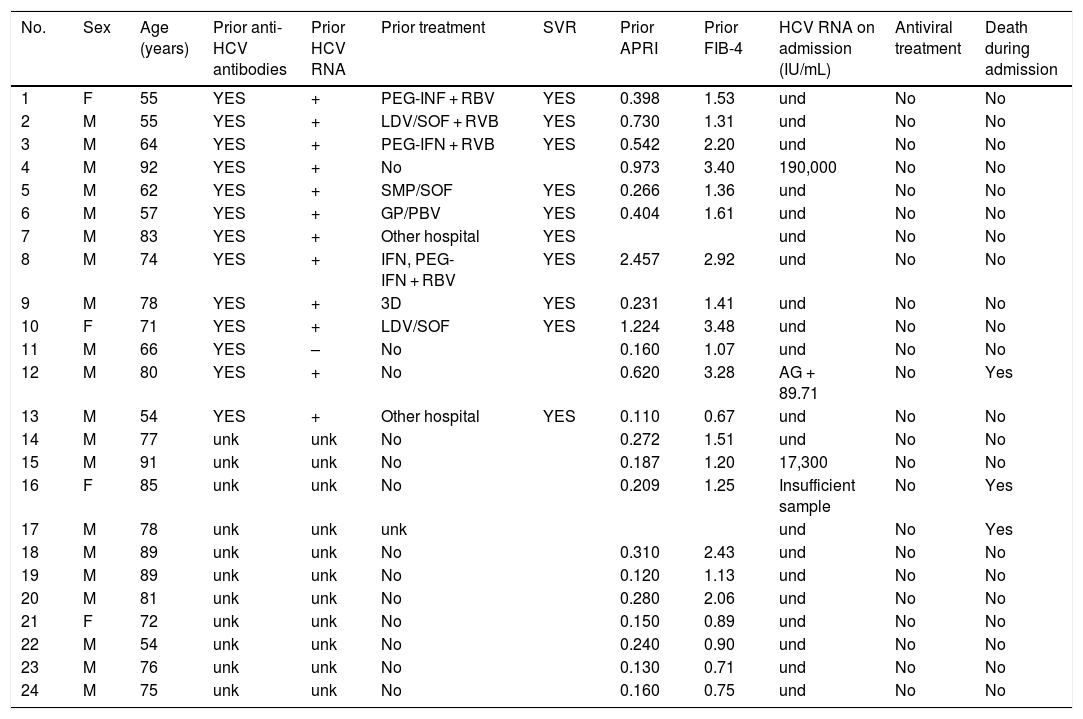
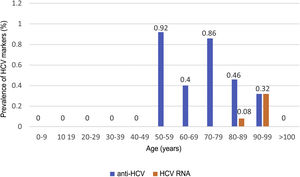
HCV serology was performed in 2,895 (62%) patients; 24 tested positive for anti-HCV antibodies, with a prevalence of 0.83% (95% CI 0.50–1.16). Of them, 13 patients had been previously diagnosed with HCV: 10 patients had received antiviral treatment and showed sustained viral response (SVR), one patient had shown a spontaneous cure, and two patients had not received treatment due to advanced age in the era of interferon-based treatment. Of the 11 patients with positive HCV serology not previously known (neither reported by the patient nor demonstrable in our laboratory), 10 showed undetectable HCV RNA. In total, just three of the patients studied presented positive HCV RNA; therefore, the prevalence of active HCV infection was 0.10% (95% CI 0.04−0.30). The distribution of the prevalence of positive anti-HCV antibodies and positive HCV RNA by age group is shown in Fig. 4. Of the three patients with positive HCV RNA, just one (33.3%) had not been previously diagnosed with HCV. Nevertheless, none of the patients identified in screening received antiviral treatment. Three patients were over 90 years of age: the first, who had dementia and a Charlson Comorbidity Index of 8, died a few weeks following discharge; the second, who had peripheral artery disease and a Charlson Comorbidity Index of 6, declined treatment; and the third died during admission of COVID-19. The data for the patients who tested positive for anti-HCV antibodies, whether or not they tested positive for HCV RNA, are shown in Table 2.
Characteristics of patients positive for anti-HCV antibodies.
| No. | Sex | Age (years) | Prior anti-HCV antibodies | Prior HCV RNA | Prior treatment | SVR | Prior APRI | Prior FIB-4 | HCV RNA on admission (IU/mL) | Antiviral treatment | Death during admission |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 55 | YES | + | PEG-INF + RBV | YES | 0.398 | 1.53 | und | No | No |
| 2 | M | 55 | YES | + | LDV/SOF + RVB | YES | 0.730 | 1.31 | und | No | No |
| 3 | M | 64 | YES | + | PEG-IFN + RVB | YES | 0.542 | 2.20 | und | No | No |
| 4 | M | 92 | YES | + | No | 0.973 | 3.40 | 190,000 | No | No | |
| 5 | M | 62 | YES | + | SMP/SOF | YES | 0.266 | 1.36 | und | No | No |
| 6 | M | 57 | YES | + | GP/PBV | YES | 0.404 | 1.61 | und | No | No |
| 7 | M | 83 | YES | + | Other hospital | YES | und | No | No | ||
| 8 | M | 74 | YES | + | IFN, PEG-IFN + RBV | YES | 2.457 | 2.92 | und | No | No |
| 9 | M | 78 | YES | + | 3D | YES | 0.231 | 1.41 | und | No | No |
| 10 | F | 71 | YES | + | LDV/SOF | YES | 1.224 | 3.48 | und | No | No |
| 11 | M | 66 | YES | – | No | 0.160 | 1.07 | und | No | No | |
| 12 | M | 80 | YES | + | No | 0.620 | 3.28 | AG + 89.71 | No | Yes | |
| 13 | M | 54 | YES | + | Other hospital | YES | 0.110 | 0.67 | und | No | No |
| 14 | M | 77 | unk | unk | No | 0.272 | 1.51 | und | No | No | |
| 15 | M | 91 | unk | unk | No | 0.187 | 1.20 | 17,300 | No | No | |
| 16 | F | 85 | unk | unk | No | 0.209 | 1.25 | Insufficient sample | No | Yes | |
| 17 | M | 78 | unk | unk | unk | und | No | Yes | |||
| 18 | M | 89 | unk | unk | No | 0.310 | 2.43 | und | No | No | |
| 19 | M | 89 | unk | unk | No | 0.120 | 1.13 | und | No | No | |
| 20 | M | 81 | unk | unk | No | 0.280 | 2.06 | und | No | No | |
| 21 | F | 72 | unk | unk | No | 0.150 | 0.89 | und | No | No | |
| 22 | M | 54 | unk | unk | No | 0.240 | 0.90 | und | No | No | |
| 23 | M | 76 | unk | unk | No | 0.130 | 0.71 | und | No | No | |
| 24 | M | 75 | unk | unk | No | 0.160 | 0.75 | und | No | No |
3D, paritaprevir/ritonavir + ombitasvir + dasabuvir; AG, antigen; F, female; GP, glecaprevir; IFN, interferon; LDV, ledipasvir; M, male; PBV, pibrentasvir; PEG-IFN, pegylated interferon; RBV, ribavirin; SMP, simeprevir; SOF, sofosbuvir; SVR, sustained viral response; und, undetectable; unk, unknown.
The AEEH's 2019 position statement on eliminating HCV recommended combining different screening methods, including age-based population screening,5 since various recent studies have shown population screening followed by HCV treatment to be cost-effective in different age groups and in the general adult population.9–11 Since the national survey of seroprevalence by the Spanish Ministry of Health in 2017 and 2018 found that the prevalence of HCV infection was residual in people under 40 years of age,4 and that the maximum prevalence of active infection was concentrated in males 50–69 years old, our society proposed population screening in those 40–70 years of age,5 in addition to other strategies such as screening based on risk factors for contracting HCV.
The COVID-19 pandemic slowed or even halted HCV screening programmes around the world. However, the high number of hospital admissions due to SARS-CoV-2 infection represented an opportunity to make a shift in strategy by carrying out opportunistic screening for HCV infection in patients admitted with COVID-19, since this population could have favourable characteristics for detection of unknown cases of HCV. First, the vast majority of patients admitted for complications of SARS-CoV-2 infection are over 50 years of age.12,13 Second, the COVID-19 pandemic equally affected people with and without risk factors for HCV infection and all types of groups,12 including vulnerable people and people with limited contact with the healthcare system (it could even be hypothesised that it affected the latter more due to deficient healthcare conditions); therefore, COVID-19 could represent a special opportunity to screen these patients with a direct link between evaluation of their disease and treatment during the very same hospital stay.
Although the pathogenesis of SARS-CoV-2 is not completely understood, some patients have been seen to have an exaggerated immune response causing serious lung inflammation; hence, the treatment of this condition has included the use of immunosuppressants intended to limit immune system-mediated damage.14 Immune abnormalities resulting from
SARS-CoV-2 infection itself as well as the use of immunosuppressants (corticosteroids, interleukin [IL]-1 and IL-6 receptors and janus kinase inhibitors) represent a risk of HBV reactivation, which causes significant morbidity and mortality.15 The risk of HBV reactivation depends on both infection status (active or past) and type of immunosuppressants used; it is highest in patients with active or past infection treated with B cell-depletion therapies such as rituximab and in HBsAg-positive patients receiving high-dose corticosteroids, anthracyclines or tumour necrosis factor (TNF)-alpha inhibitors.16–18 Therefore, with the exception of steroids, all immune-modulating drugs used in the treatment of serious COVID-19 are considered to carry a low risk of reactivation, especially in patients who test negative for HBsAg or positive for anti-HBc antibodies.
For these reasons, according to the above-mentioned document of the Spanish Ministry of Health,8 a decision was made to include HBV and HCV serology in the protocol for COVID-19 testing at the time of hospital admission.
Our study yielded some results that we find worthy of highlighting. First, the prevalence of HBV infection was similar to that of other studies conducted in Spain,2 though the prevalence of active HCV was lower than that previously reported.4,9,11 This could be explained by the significant ageing of our study population, in which age groups with the theoretically highest prevalence of HCV infection (50–74 years of age) were under-represented compared to the total admitted population. In addition, the current prevalence of active HCV infection could be linked to the high rate of treatments with DAAs in Spain, especially in people with known HCV infection, as can be gathered from the data collected in this study.
There was, however, a notably low percentage of previously diagnosed patients: just 54.2% of patients with anti-HCV antibodies and 63.6% of HBsAg-positive patients had a prior diagnosis of viral hepatitis or were being followed up at our hospitals for this reason. Nevertheless, in the group of patients with previously unknown positivity for anti-HCV antibodies at our hospitals, rates of viraemic patients were 1/11 (9.1%) as opposed to 12/13 (92.3%) in previously diagnosed patients, suggesting that many of these patients might have been previously tested and even treated at other centres. Our data were consistent with the results of the Spanish national seroprevalence survey, in which 71% of patients with current active HCV infection were aware of their diagnosis5; in our case, this percentage was 66%.
Also of note are the older age and generally limited liver impairment in patients with HBV and HCV detected in the screening programme. This, together with the patients' comorbidities and personal decision-making, ultimately led to none of them receiving antiviral treatment after being diagnosed, since curing their HCV infection would have been of limited benefit to them and since they did not meet the criteria for HBV treatment.
Regarding HBV infection, the limited overall impact of the diagnosis thereof on patients' actual management is worthy of mention, since it had repercussions for management in just two of the 2,915 screened cases. The relative importance of diagnosis increases substantially when HBsAg-positive patients are considered in isolation. Of them, nearly two thirds received treatment with high-dose corticosteroids during admission, and a diagnosis of HBV altered the management of two out of seven patients. Strikingly, prophylaxis for HBV reactivation was indicated in just 14.2% of cases. This explains why there was a case of HBV reactivation that, though mild, required the patient to receive subsequent treatment.
The main limitations of this study were: first, the high rate of non-compliance with the screening protocol, given the COVID-19 teams' full autonomy in decision-making, and second, the population ageing in our setting, with a high median age in patients admitted for complications of COVID-19, which might have led to underestimation of the true prevalence of infection in our area. The strength of our study lies in the high number of screened patients, such that it offers quite a realistic picture of the current situation with respect to the prevalence of viral hepatitis in our setting and supports the suggestions of the 2020 Spanish Ministry of Health HCV infection screening guidelines, which do not recommend screening in people with no risk exposure based on the available evidence.19
ConclusionsAlthough opportunistic screening for HCV in hospitalised patients with COVID-19 may represent a good opportunity as it facilitates continuity between diagnosis and treatment with no need to leverage additional resources, our results suggested a lower-than-expected prevalence of active HCV infection in our setting. The widespread use of antiviral treatments and our population's older mean age limited the available options for detection of potentially treatable patients. Screening for HBV infection in this context should be aimed at preventing HBV reactivation in patients who require immunosuppressant therapy.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Gómez Camarero J, Badia Aranda E, Quiñones Castro R, Saiz Chumillas RM, Alcoba Vega L, Díez Ruiz S, et al., Cribado de infección por virus de la hepatitis B y C en pacientes hospitalizados con infección por SARS-CoV-2, Gastroenterología y Hepatología. 2022;45:256–264.