Hepatocellular adenoma is a rare liver tumour (3–5 cases per million inhabitants).1–10 Malignant transformation, which occurs in 5% of adenomas, is one of the possible complications of these tumours, and only around 70 cases have been reported in the literature.2,3,5–9 We present 2 new cases of malignant hepatocellular adenoma (MHA).
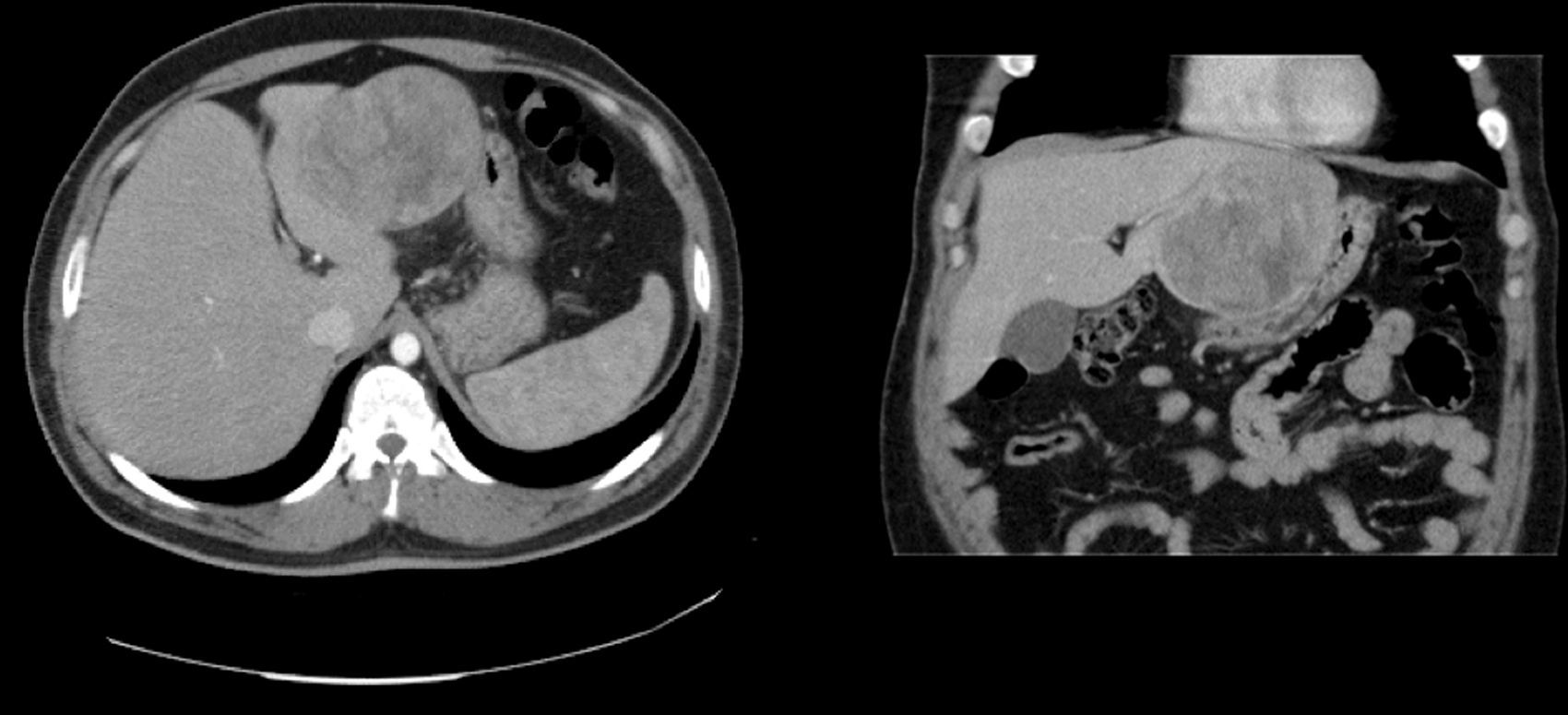
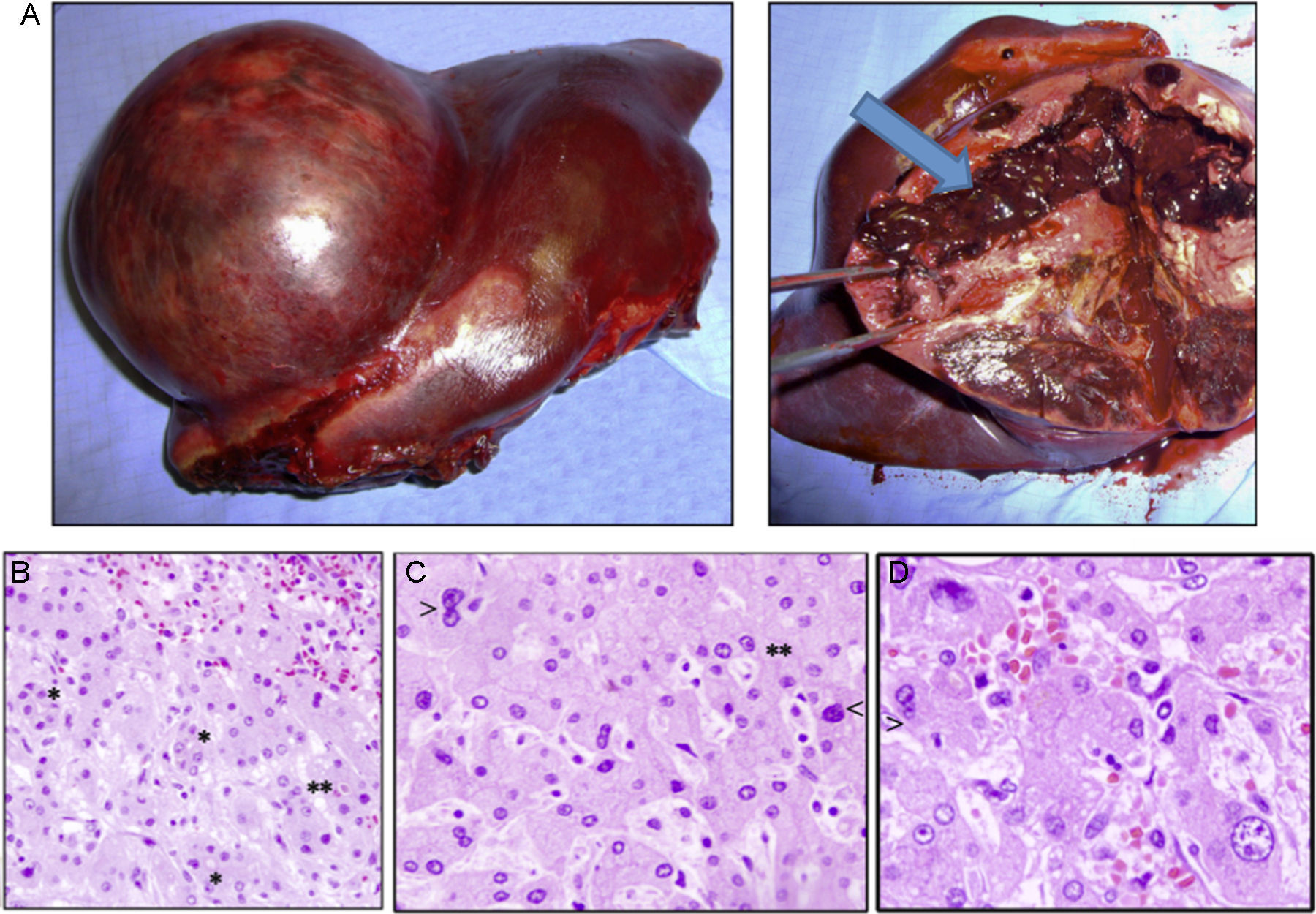
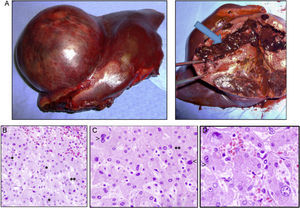
Case 1The patient is a 31-year-old man, ECOG 0, smoker of 10 cigarettes/day, BMI: 31, no medical history of note and no diseases related to the presence of hepatocellular adenomas. Denies use of anabolic steroids. Presents with severe sudden-onset abdominal pain in the epigastric region and right upper quadrant. On examination, the patient is haemodynamically stable (temp. 37.0°C BP: 100/60 and HR: 90bpm). Palpation revealed an abdominal mass in the epigastrium with no signs of peritoneal irritation. Laboratory tests showed ALT: 165U/L, AST: 100U/L, GGT: 107U/L. No previous lab results were available. The remaining parameters, including amylase, bilirubin and coagulation studies, were normal. An abdominal CT scan was performed, showing a 10cm×9cm heterogeneous lesion located on the left lateral segment with hyperdense foci corresponding to areas of bleeding; intravenous contrast showed early arterial enhancement, isoattenuating relative to the hepatic parenchyma in the late and venous phases. No intra-abdominal free fluid (Fig. 1). We considered performing selective embolisation of the lesion, but as this technique would not be available for at least 48h, and in view of active parenchymal bleeding and the risk of tumour rupture, we decided to perform emergency surgery. Emergency laparotomy was performed; complete abdominal exploration showed no other pathological findings, and a left lateral sectionectomy was performed. (Fig. 2A). The patient was discharged after 5 days. The histological study showed non-encapsulated hepatocellular adenomatoid tumour with foci of high-grade dysplasia (focal carcinoma). Immunohistochemistry: Hep Par 1, CD34+ in trabeculae, p53+ (focal), inconclusive for glypican-3 (Fig. 2B–D). Follow-up (54 months) was performed every 3 months during the first 2 years and then every 6 months, and consisted of abdominal ultrasound and determination of alpha-fetoprotein, CEA and liver function panel. No recurrence was observed.
(A) Macroscopic image of the tumour, well-delimited from the normal parenchyma, with abundant bleeding (arrow). (B) Histology shows sinusoidal congestion (top right) and pseudo-acinar formations in areas of dysplasia (*), sometime with bile (**). (C and D) Detail of the nuclear pleomorphism of hepatocytes with large nuclei (<) and a disproportionate nuclear-cytoplasmic ratio, binucleation (>) and pseudo-acinar formations (*). (B to D: haematoxylin and eosin).
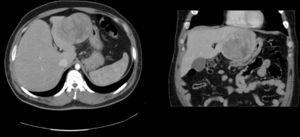
A 74-year-old man. ECOG 0. Personal history: BMI: 30, atrial fibrillation, prostate cancer resected in 2000 and treated with radiotherapy. No diseases related to hepatocellular adenomas. A liver lesion was diagnosed during prostate cancer ultrasound follow-up. All laboratory tests (blood panel and liver function tests) obtained during follow-up were normal. MRI: 8cm×7cm×7cm nodular lesion in segments IV–VIII, hypointense relative to parenchyma in T1 and discretely hypointense and heterogeneous in T2, with areas of necrosis and small areas of fatty infiltration well delimited by a hypointense pseudocapsule, and cholelithiasis (Fig. 3). Laboratory tests were normal. We performed a resection of segment IV and a partial resection of segment VIII+cholecystectomy (Fig. 2). The patient presented low output biliary fistula that was resolved by sphincterotomy and a plastic prosthesis. Histology showed an 8cm, solid, light brown tumour, well-delimited from the surrounding parenchyma by a fibrous capsule. Microscopic examination showed mild low-grade nuclear atypia (mild). The Ki-67 proliferation index is low (5–10%). Immunohistochemistry: alpha-fetoprotein (−), glypican-3 (focally +) and CD34 (+) in intratumoral vasculature. Histologically, the sample shows borderline hepatocellular adenoma and well-differentiated hepatocellular carcinoma. The patient was followed up for 12 months using the same studies as patient 1, with no signs of recurrence.
Hepatocellular adenoma is diagnosed predominantly in young women (15–45 years) taking oral contraceptives.3,5 Only 4.3% of cases occur in men,6 usually in individuals who have consumed anabolic steroids.6 Other risk factors in men are: alcoholism, smoking and high body mass index.4
The aetiology and pathogenesis of hepatocellular adenoma is not fully understood, although the relationship between oral contraceptives or anabolic steroids is evident, and also dose-dependent.2,3,5,6 Certain diseases are associated with hepatocellular adenoma: type I and III glycogenosis, tyrosinaemia, ¿-thelassaemia, haemochromatosis, familial polyposis and Fanconi anaemia.1,2,4–6,8,10
Hepatocellular adenoma is usually asymptomatic, although it can cause abdominal pain or complications, such as bleeding or rupture,1,4–6 and malignant transformation can occur in rare cases.3,6,10 Malignancy is practically impossible to diagnose on the basis of radiological images, and serum alpha-fetoprotein levels are usually normal.5,7 Malignancy occurs by an adenoma-carcinoma progression sequence that has been confirmed by genetic studies.1,2,7 MHAs are usually well-differentiated tumours.5
Very few studies have been published on MHAs.3,5,9 A systematic literature review performed by Stoot et al. in 2010 retrieved 68 references to MHA out of 1635 studies in hepatocellular adenoma. This represents 4.2% of the total, and 4.5% of resected tumours.6 The MHAs were included in case reports (19 patients) or series. More detailed epidemiological information was provided in case reports than in series.1,3,4,6 In total, 26% (4/19) patients were men, 26% (4/19) had not previously used steroids or contraceptives (2 men and 2 women), 39% (6/19) showed signs of bleeding, 19% presented multiple HCAs, with an average size of 10.5cm (range: 4.5–18cm).6 These data confirm that our patients were exceptional cases: men with no history of anabolic steroid use, one of whom presented intratumoral haemorrhage.
Following Stoot's review, Farges et al. published a series of 218 adenomas with 23 cases of MHA (10.5%), 16 of whom were men and 7 women. Malignant transformation was far more frequent in men (47%) than in women (4%), leading the authors to suggest an association between MHA and metabolic syndrome, which is more common in men than women.5 Mean tumour size was 10cm, and only 3/23 measured less than 5cm.5 The authors define two histological types of malignant transformation: nodules or malignant microscopic foci. This latter is more common in men, and was the type observed in our cases.5
Suggested risk factors of malignant transformation are: use of anabolic steroids or androgens, male sex, glycogenosis, polyposis, metabolic syndrome, presence of dysplasia, mutation of the beta-catenin gene, non-steatotic and large hepatocellular adenomas.3–6,9,11 The correlation between tumour size and malignant transformation could not be confirmed statistically; however, MHAs are usually large (mean: 10.5cm).6 The smallest malignant tumour measured only 3cm, and only 5% of MHAs measure less than 5cm.5,6 Currently, the standard treatment for all adenomas greater than 5cm is surgical resection,5,9 since the risk of malignant transformation or bleeding in adenomas measuring less than 5cm is extremely low.3,5,6,8
The prognosis for MHA is unclear. Details of oncological follow-up are only available in 13 of the 19 patients mentioned above: 1 case of postoperative death, 2 tumour-related deaths at 5 and 7 months, and 1 case of recurrence 6 years after resection.6 Based on these data, overall disease-free survival at 5 years is 83%. It is unclear whether these patients received adjuvant treatment.6
In conclusion, MHAs are very rare. A number of factors are associated with malignant transformation, although not enough cases have been published to allow us to reach scientifically solid conclusions.
Conflicts of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: Ramia JM, Baquedano Rodríguez J, Alonso S, Fernandez-Bueno F, de la Plaza R. Adenoma hepático malignizado en varones: presentación de 2 casos. Gastroenterol Hepatol. 2017;40:91–93.