Liver PEComas are extremely rare and differential diagnosis is complex. The treatment of choice is surgical removal. We present our experience (4 cases in 6 years) and a literature review.
Case 1A 46-year-old female with a history of right hepatectomy in 1995 due to a hepatocellular carcinoma (HCC).
After 10 years, damage to segment II was found. The biopsy showed non-tumour hepatocytes. Four years later, the magnetic resonance imaging (MRI) scan showed a growth in size, hypervascular behaviour and a biopsy consistent with HCC.
There was 2.4cm of damage in segment II on the intraoperative ultrasound, and a limited resection was performed.
The final outcome was PEComa.
The histological preparations from the prior resection were requested and they were confirmed as a match with a liver PEComa (company unknown in 1995).
Case 2A 45-year-old female with a malignant melanoma previously resected in her lower left limb, Clark IV. After 8 years, the follow-up CT scan found a solid 25mm lesion on the MRI described as: hypervascular, hyperintense in T1 and hypointense in T2.
Biopsy consistent with PEComa.
On a morphologically normal liver, the intraoperative ultrasound identified a 2.5cm hyperechoic lesion, which was well defined in segment VIII. A limited resection was performed.
Case 3A 48-year-old female diagnosed with ocular choroidal melanoma. The extension study identified a 7cm mass in the liver hilum with a liver PEComa biopsy.
During surgery, a lump in the caudate lobe was observed which compressed the vena cava and the left and middle suprahepatic veins (SHV). The caudate lobe was resected.
The eye lesion was subsequently treated using brachytherapy.
Case 4A 53-year-old male with a history of frequent alcohol consumption examined due to general symptoms.
In the CT scan a 3cm nodule was identified, adjacent to the left SHV, hypervascular with uniform uptake in the arterial phase.
Biopsy: angiomyolipoma.
In the intraoperative ultrasound, a 3cm hyperechoic lesion was seen, which reached the left and middle SHV. A left hepatectomy was performed.
Images, pathological descriptions, immunohistochemical markers and the follow-up time are shown in Table 1.
Liver PEComas described in the literature review.
| Author, year | Age and sex | Location | Size (cm) | Metastatic disease | Follow-up | |
|---|---|---|---|---|---|---|
| Tsui, 1999 | 56 | M | RLL | 6.5 | No | 2 years |
| Tsui, 1999 | 37 | F | RLL | 3 | – | – |
| Tsui, 1999 | 41 | F | RLL | 9 | No | 4 years |
| Tsui, 1999 | 46 | F | RLL | 12.5 | No | 12 years |
| Tsui, 1999 | 41 | M | – | 6 | – | – |
| Yamasaki, 2000 | 30 | F | RLL | 3 | No | 1 year |
| Dalle, 2000 | 70 | F | RLL | 26 | Hepatic | 5 years |
| Yukichi, 2000 | 13 | F | Lig. Teres | 9 | No | 22 months |
| Xu, 2001 | 35 | F | RLL | 2×2 | – | |
| Lin, 2002 | 30 | F | RLL | 3.6×3 | – | |
| Parfitt, 2006 | 60 | F | RLL | 14 | Multiple | 9 years |
| Svajdler, 2007 | 55 | F | LLL | 3.5 | – | – |
| Rouquie, 2007 | 67 | F | LLL | 9 | No | 2 years |
| Song Hua, 2007 | 67 | F | Caudate | 6 | No | 1 year |
| Song Hua, 2007 | 56 | F | LLL | 5 | No | 1 year |
| Labcharoensub, 2007 | 31 | F | RLL | 1.8 | No | 6 months |
| Li, 2007 | 56 | F | LLL | 5×4 | – | – |
| Wang, 2007 | 46 | F | RLL | 4×4 | – | – |
| Fang, 2007 | 56 | F | LLL | 5.1 | No | 24 months |
| Fang, 2007 | 56 | F | LLL | 5.1 | No | 24 months |
| Paiva, 2008 | 51 | F | LLL | 0.8 | No | 2 years |
| Sánchez, 2008 | 32 | F | RLL | 4 | No | 6 months |
| Qiu, 2008 | 67 | F | RLL | 15×12 | – | – |
| Liu, 2008 | 31 | F | RLL | 8×6 | – | – |
| Han, 2008 | 44 | M | RLL | 2×1.6 | – | – |
| Zimmermann, 2008 | 53 | M | – | 8 | No | 17 months |
| Chen, 2009 | 36 | F | RLL | 7×5 | – | – |
| Chen, 2009 | 45 | F | RLL | 5.5×4 | – | – |
| Chen, Li, 2009 | 37 | F | RLL | 5×4 | – | – |
| Strzelczyk, 2009 | 57 | F | RLL | 20 | No | – |
| Akitake, 2009 | 36 | F | LLL | 3.5 | No | 18 months |
| Priola, 2009 | 36 | F | LLL | 11 | No | 34 months |
| Liu, 2010 | 32 | F | RLL | 5.5×5.5 | – | – |
| Zhu, 2010 | 26 | F | RLL | 5×3 | – | – |
| Shi, 2010 | 41 | F | LLL | 5.5×4 | – | – |
| Shi, 2010 | 48 | F | RLL | 8×6 | – | – |
| Deng, 2011 | 66 | M | LLL | 3×3.5 | – | – |
| He, 2011 | 35 | F | RLL | 3.5×3 | – | – |
| Zou, 2011 | 54 | M | RLL | 6×5 | – | – |
| Selvaggi, 2011 | 42 | M | RLL | 7 | Yes | 1 month |
| Ahn, Hur, 2011 | 36 | F | LLL | 6.5 | No | 3 months |
| Zhang, Wang, 2012 | 55 | F | RLL | 3×3 | – | – |
| Gao, 2012 | 59 | F | RLL | 6×5 | – | – |
| Yan, Tan, 2012 | 38 | F | RLL | 4 | No | 48 |
| Yan, Tan, 2012 | 34 | F | RLL | 4.3 | Yes | 14 |
| Yan, Tan, 2012 | 49 | F | LLL | 2.5 | No | 32 |
| Yan, Tan, 2012 | 75 | F | RLL | 8 | No | 36 |
| Yan, Tan, 2012 | 33 | F | RLL | 2.5 | No | 27 |
| Yan, Tan, 2012 | 71 | F | RLL | – | No | 29 |
| Yan, Tan, 2012 | 41 | F | LLL | Multiple | Yes | 12 |
| Liu, 2014 | 25 | F | LLL | 2.5×1 | – | – |
| Otegi, 2015 | 46 | F | LLL | 2.4 | No | 6 years |
| Otegi, 2015 | 45 | F | RLL | 2.9 | No | 4 years |
| Otegi, 2015 | 48 | F | Caudate | 8 | No | 18 months |
| Otegi, 2015 | 53 | M | LLL | 2.5 | No | 11 months |
RLL, right lobe of liver; LLL, left lobe of liver; F, female; M, male; –, not found.
There were no deaths and no post-operative complications during the post-operative follow-up.
In 1991, Pea et al.1 described the presence of a cell type with specific cytoplasmic characteristics and strong positivity for HMB-45 in the angiomyolipomas (AML). One year later, these cells were given the name of perivascular epithelioid cell (PEC).2
In 2001, the World Health Organisation (WHO) defined PEComas as a group of rare mesenchymal tumours comprised of perivascular epithelioid cells with well-defined histologic and immunohistochemical characteristics.3
Currently the following are included in the PEComas group: angiomyolipoma (AML), pulmonary lymphangioleiomyomatosis, clear cell “sugar” tumours and clear cell myomelanocytic tumours of the falciform ligament.4
In a systematic review in 2012, Bleeker et al.5 compiled 234 cases. The uterus is the most common location, and it is rare in the liver. In our literature search, we found 51 described cases of liver PEComas (Table 2).
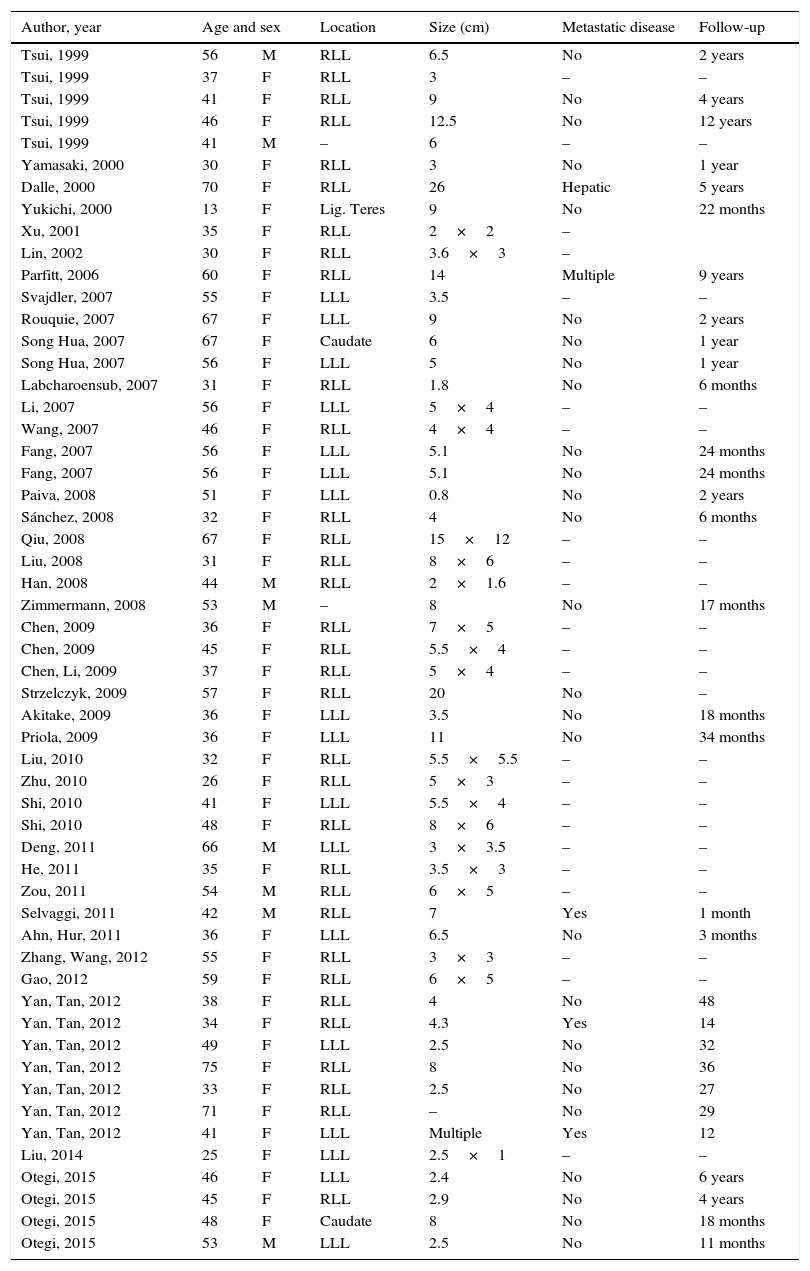
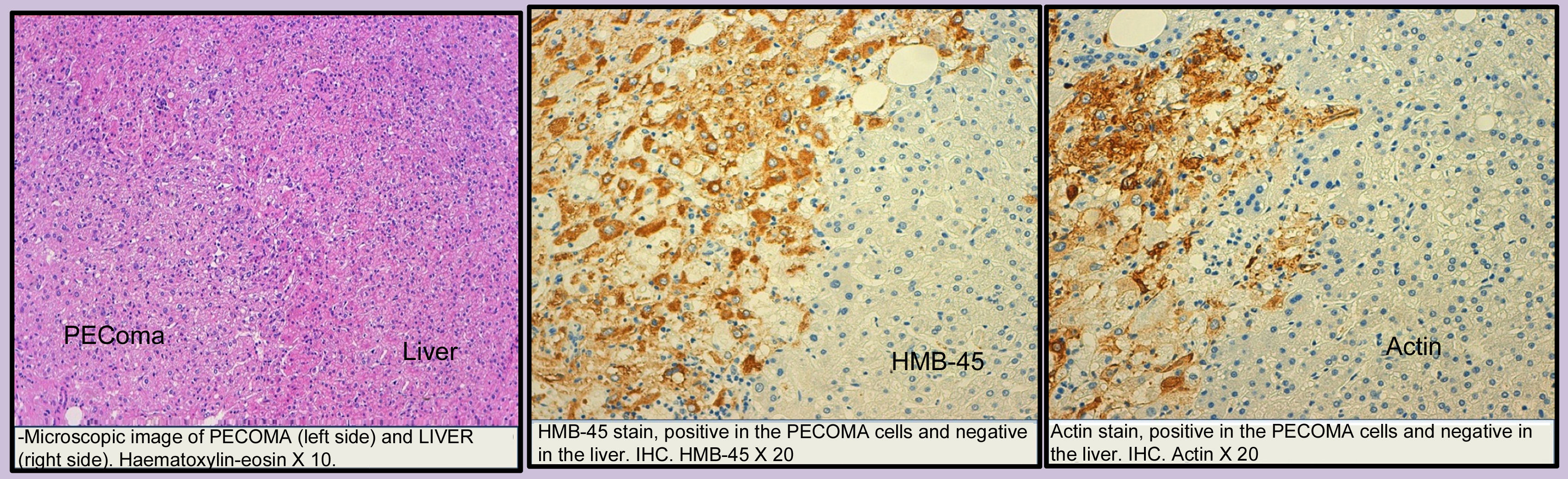
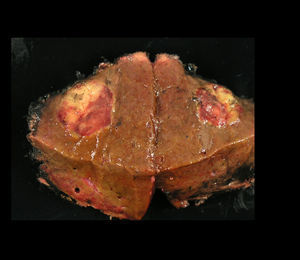
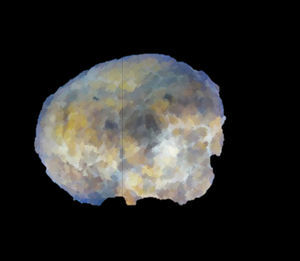
Images and anatomopathological characteristics and immunohistochemistry.
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Pieces | ||||
| Macroscopic description | White nodule that has a maximum diameter of 1.6×1.7cm | Well-defined nodule, 2.9cm in diameter with yellowish and dark brown coloured areas | Subserosal nodule 9cm in diameter | Nodular lesion 2.5cm in diameter with a liver-like colour and granular consistency |
| Microscopic description | Proliferation of epithelioid and spindle cells with eosinophil cytoplasm. Thick vascular structures were noticed, which break up both spindle and epithelioid cells. In one of the slices, adipose tissue was found interspersed with the cellular proliferation | Lumps that show different sized mature adipocytes, interspersed with epithelioid-like cells with large, clear, eosinophilic cytoplasm in groups and around veins with uneven walls | Proliferation of eosinophilic polygonal cells associated with veins which are dilated in appearance and showing a proliferation of elongated, myoid-like cells, epithelioid and adipocyte cells | Proliferation of polygonal cells with a cytoplasm that oscillates between clear and eosinophilic and a diffuse or trabecular growth with abundant medium-sized veins with surrounding epithelial cells. Approximately 30% of fat globules were observed interspersed with this proliferation |
| IHC positive | Melan-A HMB-45 Actin MITF, c-Kit | Melan-A HMB-45 Actin Calponin | Melan-A HMB-45 Actin | Melan-A HMB-45 Actin |
| IHC negative | Hepatocyte S-100 | S-100 | S-100 | S-100 |
| Ki-67 index | 1% | <1% | 1% | 1% |
| Follow-up | 6 years | 4 years | 18 months | 11 months |
The clinical presentation of the liver PEComa is variable and nonspecific, usually diagnosed by chance.6–8
The differential diagnosis of liver PEComa includes HCC, haemangioma, focal nodular hyperplasia and liver adenoma.8
In 2012, Tan and Xiao6 published 7 cases of liver PEComas. Radiologically they were well-defined masses with uniform density. In both the CT scan and the intravenous contrast-enhanced MRI, there was varied and premature enhancement in the arterial phase with uniform clearance in venous and late phases. The radiological characteristics are similar to the HCCs. We must consider the PEComas in light of radiological patterns described in patients without elevated alpha-fetoprotein (AFP) and no hepatitis or cirrhosis.
The PEComas are made up of epithelioid cells, linked with vascular structures, eosinophil cytoplasm, abundant glycogen and the characteristic presence in the ultrastructure of premelanosomes. They show immunoreactivity against melanocytic markers HMB-45, microphthalmia transcription factor (MITF), Melan-A and PNL2 melanoma-associated antigen, which indicates melanocytic differentiation between the tumours. They are generally negative for the S-100 which makes the differential diagnosis with melanoma less difficult.4,8
Two of the cases have a history of a melanoma, which makes the differential diagnosis with metastases of this tumour more difficult. But in both cases, in addition to the negativity for the S-100, vascular and adipose proliferation were recognised, which is typical of AML.
The co-expression of actin and HMB45/Melan-A is indispensable for diagnosing PEComa and, together with the negativity for the S-100, it allows for a differential diagnosis with a metastatic melanoma.
They can show variable expression for other melanocytic markers, including the S-100 protein, tyrosinase and the microphthalmia transcription factor (MTF) and for other muscle markers such as smooth muscle myosin (SMMS) and calponin.
c-Kit expression in PEComa cases is also described.
The majority of published PEComa cases seem to have a benign behaviour, although cases of tumour recurrence or distant metastasis have been described.5 The tumour size (greater than 7cm) and the pathological characteristics (high mitotic index and cellular necrosis) are considered a threat for recurrence.6
The most effective currently accepted treatment is surgery.5,9 Adjuvant therapy is advised along with chemotherapy in PEComas with risk indicators.5,10
Despite PEComas being extremely rare, they are part of an emerging group of tumours.
Please cite this article as: Otegi Altolagirre I, de Miguel Valencia M, Sánchez Acedo P, Zazpe Ripa C, Tarifa Castilla A, Herrera Cabezón J, et al. Nuestra experiencia en el tratamiento del PEComa hepático. Gastroenterol Hepatol. 2017;40:24–28.