Real-world studies about the effectiveness and safety of vedolizumab (VDZ) in the treatment of inflammatory bowel disease (IBD) in Latin America are scarce. Our study describes the effectiveness and safety of VDZ in Colombian patients with IBD.
MethodsEXVEDOCOL (EXperience of VEDOlizumab in COLombia) was a retrospective, multicenter, observational study. Adults with IBD receiving a first dose of VDZ between July 2016 and October 2018 were included. The co-primary outcomes clinical response, and remission, were determined at week 14 and last visit during the maintenance phase (LVMP). The secondary outcomes, deep remission and loss of response were recorded at LVMP.
ResultsThirty-one patients (25 ulcerative colitis (UC), 6 Crohn's disease (CD)) were included. At week 14, clinical response was achieved by 87.1% (27/31) of the patients treated with VDZ, while loss of response was reported in 6.7% (2/30). The remission rate at week 14 was 65.5% (19/29) and 75.9% (22/29) at LVMP. Prior anti-TNF exposure was reported in 61.3% (19 patients) of whom 84.2% (16/19) achieved clinical response at week 14 and 89.5% (17/19) at LVMP. For anti-TNF naïve patients, clinical response was recorded in 91.7% (11/12) at week 14 and 100% (12/12) at LVMP.
ConclusionsHigh clinical remission rates and safety profile highlight VDZ as a valuable treatment option for IBD patients. Anti-TNF naïve patients may derive greater benefit from therapy. Studies with larger cohorts could confirm these findings.
Los estudios del mundo real sobre la efectividad y seguridad de vedolizumab (VDZ) en el tratamiento de la enfermedad inflamatoria intestinal (EII) en Latinoamérica son escasos. Nuestro estudio describe la efectividad y seguridad de VDZ en pacientes colombianos con EII.
MétodosEXperiencia de VEDOlizumab en COLombia (EXVEDOCOL) fue un estudio retrospectivo, multicéntrico y observacional. Se incluyeron adultos con EII recibiendo primera dosis de VDZ entre julio de 2016 y octubre de 2018. Los objetivos coprimarios, respuesta clínica, y remisión, se determinaron en la semana 14 y en la última visita durante la fase de mantenimiento (UVFM). Los resultados secundarios, remisión profunda y pérdida de respuesta, se registraron en la UVFM.
ResultadosSe incluyeron 31 pacientes (25 colitis ulcerativa [CU], 6 enfermedad de Crohn [EC]). En la semana 14, el 87,1% (27/31) de los pacientes tratados con VDZ presentaron respuesta clínica, mientras que el 6,7% (2/30) presentaron pérdida de respuesta. La tasa de remisión en la semana 14 fue del 65,5% (19/29) y del 75,9% (22/29) en la UVFM. El 61,3% (19 pacientes) tenían exposición previa a anti-TNF, de los cuales el 84,2% (16/19) alcanzaron una respuesta clínica en la semana 14 y el 89,5% (17/19) en la UVFM. En los pacientes naïve a anti-TNF, se registró respuesta clínica en el 91,7% (11/12) en la semana 14 y en el 100% (12/12) en la UVFM.
ConclusionesLas elevadas tasas de remisión clínica y perfil de seguridad resaltan el potencial de VDZ como adecuada opción terapéutica en EII. Los pacientes naïve al anti-TNF pueden obtener un mayor beneficio de la terapia. Estudios con cohortes más grandes podrían confirmar estos hallazgos.
Inflammatory bowel disease (IBD) encompasses conditions characterized by chronic inflammation resulting in mucosal erosion and tissue damage, including ulcerative colitis (UC), Crohn's disease (CD), and indeterminate colitis.1 The pathogenesis for IBD is not fully understood, but it is believed to result from a combination of immunological, genetic, and environmental factors.2 Global prevalence estimates indicate that nearly 7 million people were affected by IBD in 2017,3 with a significant increase in cases observed in newly industrialized nations, including Latin America.4 A recent study in Colombia examined epidemiological patterns using a national database of 33 million adults and a regional IBD clinical cohort in the city of Medellin between 2001 and 2017.5 The incidence of UC increased from 5.59/100,000 in 2010 to 6.3/100,000 in 2017, and while the incidence of CD did not increase, its prevalence did. A higher risk of IBD was found in women and in patients aged 40–59 years. Compared to Western countries, UC was estimated to be more frequent in Colombia, increasing in urban areas.
Over the past two decades, the availability of biological medications has changed the therapeutic landscape for IBD and significantly improved clinical outcomes in patients with UC and CD,6 different treatment guidelines have emerged to help make better decisions regarding medication choice using a patient-centred approach.7 Vedolizumab (VDZ) was the first approved biological molecule for IBD treatment after the introduction of anti-TNFs.8 VDZ selectively targets the integrin alpha-4-beta-7, a protein involved in the migration of immune cells to the gastrointestinal tract. VDZ has a gut-selective mechanism of action, which may reduce the risk of systemic side effects compared to other medications that target the immune system.9 VDZ has demonstrated efficacy in inducing and maintaining clinical remission, improving endoscopic and histologic outcomes, and reducing the need for corticosteroid use,10 in addition to having a favourable safety profile.11–17
There is an increasing need to better understand IBD dynamics and treatment in the Latin American region.18 Real-world evidence studies become critical in contributing knowledge related to this condition in a real-life setting. Suboptimal response to TNF inhibitors in patients with IBD has been documented in the region, including Colombia, occurring in more than one-third of UC patients (36.4%) and nearly half of CD patients (46.5%) at 24 months, respectively.19 This evidences an unmet need for new IBD therapies to improve long-term outcomes in these patients. Despite various studies conducted in different parts of the world, there is a scarcity of data on VDZ usage in Latin America for treating IBD.20 Within this context, in this study, we aimed to describe the effectiveness and safety of VDZ in Colombian patients with CD and UC.
Materials and methodsEXVEDOCOL (EXperience of VEDOlizumab in COLombia) is an observational, retrospective, longitudinal study conducted by a group of IBD experts across nine IBD centres in five cities in Colombia (study preliminary results have been previously published as an abstract).21 The study aimed to investigate the clinical outcomes of VDZ treatment for moderate to severe CD and UC in the country. Eligible patients were ≥18 years old, had active moderate to severe IBD at the initiation of VDZ, and received their first dose of VDZ (index date) between July 2016 and July 2018. Active disease at baseline was defined by the treating physician at each participating centre using validated UC and CD activity scores. Patient medical charts were reviewed, and relevant demographic and clinical variables were captured in a structured database. Patients with no information required to establish clinical response or remission at week 14 and last visit during maintenance phase were excluded. VDZ was administered as prescribed by the treating physician, according to the approved label (local summary of product characteristics). The standard dosing of VDZ is 300mg intravenously at weeks 0, 2 and 6 (induction phase), followed by a maintenance dose every eight weeks or as medically indicated. Data were collected at baseline, at 14 weeks after the first VDZ dose (visit based on clinical practice), and at the last visit in the maintenance phase. The study recorded the following variables: sex, age, disease duration, disease extension (Montreal classification),22 disease severity (Harvey–Bradshaw index (HBI) in CD patients,23 and partial Mayo Clinic score in UC patients),24 extraintestinal manifestations, previous anti-TNF exposure, concomitant medications, steroid use, biochemical measures (haemoglobin, platelet count, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), endoscopic activity (Mayo endoscopic subscore in UC patients),25 and Simple Endoscopic Score for CD (SES-CD)),26 adverse events and reasons for VDZ discontinuation.
The co-primary outcomes, clinical response, and remission were measured at week 14 and the last visit during the maintenance phase. Clinical response was defined as a reduction of at least 2 points in the Mayo score and ≥30% from baseline in UC patients,24 and at least 3 points in the HBI in CD patients.23 Remission was defined as a Mayo score ≤2 in UC patients and HBI <5 in CD patients, associated with steroid discontinuation. Secondary outcomes included endoscopic response, deep remission, loss of response and adverse events (AE) during treatment. The endoscopic response was defined as a Mayo sub-score ≤127 for UC and SES-CD ≤226 in CD and it was determined at week 14 and the last visit during the maintenance phase. Deep remission was defined as clinical and endoscopic response with normal CRP, ESR and platelet values at the last visit during the maintenance phase.28 Loss of response was defined as clinical exacerbation after the initial clinical response achieved at week 14.
A descriptive analysis was performed presenting data as proportions for categorical variables and measures of central tendency for numerical variables according to their distribution. All analyses were performed using IBM SPSS version 25.0 statistical software (IBM Corp., Armonk, NY, USA: 2017). This study was conducted following the ethical guidelines of the Declaration of Helsinki, and the local ethics committee of each participating centre approved it. This research was deemed to carry minimal risk due to its utilization of retrospective data, thus obviating the need for patient informed consent in accordance with national legislation.29
ResultsPopulation characteristicsThis study included 31 patients with IBD, of whom 25 had UC and 6 had CD. Table 1 summarizes the demographic and clinical characteristics of the patients according to the type of IBD. The median time from drug initiation to the last follow-up visit was 42 weeks (IQR 47); the median follow-up time of UC and CD patients was 42 weeks (IQR 45) and 44 weeks (IQR 78.5) respectively.
Demographic, clinical and biochemical characteristics of Colombian IBD patients included in the EXVEDOCOL study.
| Ulcerative colitisn=25 | Crohn's diseasen=6 | Overalln=31 | |
|---|---|---|---|
| Sex, female, n (%) | 18 (72.0) | 3 (50.0) | 21 (67.7) |
| Age, years. Median (IQR) | 37 (17) | 57 (15) | 41 (24) |
| Disease duration, years. Median (IQR) | 6 (7) | 9 (13) | 7 (7) |
| Montreal classification (UC extension), n (%)* | |||
| Ulcerative proctitis | 1 (4.0) | – | |
| Left-sided UC (distal) | 6 (24.0) | – | |
| Extensive UC (pancolitis) | 9 (36.0) | – | |
| Data not available+ | 9 (36.0) | ||
| Montreal classification (UC severity), n (%)* | |||
| Moderate UC | 11 (44.0) | – | |
| Severe UC | 7 (28.0) | – | |
| Data not available+ | 7 (28.0) | ||
| Montreal classification (CD location), n (%) | |||
| Isolated ileal disease | – | 2 (33.3) | |
| Isolated colonic disease | – | 1 (16.7) | |
| Ileocolonic disease | – | 3 (50.0) | |
| Montreal classification (CD behaviour), n (%) | |||
| Non-stricturing, non-penetrating | – | 2 (33.3) | |
| Stricturing | – | 2 (33.3) | |
| Penetrating | – | 1 (16.7) | |
| Penetrating and perianal disease | – | 1 (16.7) | |
| Extraintestinal manifestations. n. patient (%) | 7 (28.0) | 2* (33.3) | 9* (29.03) |
| EIM by typea, n (%) | |||
| Musculoskeletal | 6 (24.0) | 1 (16.7) | 7 (22.6) |
| Dermatologic | 0 (0.0) | 1 (16.7) | 1 (3.2) |
| Ocular | 1 (4.0) | 0 (0.0) | 1 (3.2) |
| Hepatic | 0 (0.0) | 1 (16.7) | 1 (3.2) |
| Previous anti-TNF exposure, n (%) | 15 (60.0) | 4 (66.7) | 19 (61.3) |
| One previous anti-TNF, n (%) | 9 (60.0) | 2 (50.0) | 11 (57.9) |
| Two previous anti-TNF, n (%) | 6 (40.0) | 2 (50.0) | 8 (42.1) |
| Reason for VDZ initiation, n (%) | |||
| Primary failure to anti-TNF | 4 (16.0) | 0 (0.0) | 4 (12.9) |
| Secondary failure to anti-TNF | 10 (40.0) | 3 (50.0) | 13 (41.9) |
| VDZ in first-line treatment | 10 (40.0) | 2 (33.3) | 12 (38.7) |
| Other reason/no data | 1 (4.0) | 1 (16.7) | 2 (6.5) |
| Immunomodulator, n (%) | 17 (68.0) | 2 (33.3) | 19 (61.3) |
| Before VDZ | 12 (70.6) | 2 (100.0) | 14 (73.7) |
| Concomitant with VDZ | 2 (11.8) | 0 (0.0) | 2 (10.5) |
| Data not available | 3 (17.6) | 0 (0.0) | 3 (15.8) |
| Steroids in induction therapyb,c, n (%) | 5/18 (27.8) | 2/5 (40.0) | 7/23 (30.4) |
| Steroids in maintenance therapyb,d, n (%) | 8/25 (32.0) | 2/5 (40.0) | 10/30 (33.3) |
| Elevated CRP, n (%) | 21 (84.0) | 6 (100.0) | 27 (87.1) |
| Elevated ESR, n (%)d | 11/13 (84.6) | 4/4 (100.0) | 15/17 (88.2) |
| Anaemia, n (%) | 13 (52.0) | 2 (33.3) | 15 (48.4) |
| Thrombocytosis, n (%)d | 3/22 (13.6) | 2/5 (40.0) | 5/27 (18.5) |
CD, Crohn's disease; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HBI, Harvey–Bradshaw index; IBD, inflammatory bowel disease; IQR, interquartile range; SESCD, simple endoscopic score for Crohn's disease; TNF, tumour necrosis factor; UC, ulcerative colitis.
In the UC group, pancolitis (36%, 9 patients) and distal UC (24%, 6 patients) were the predominant types, with 36% of cases (9 patients) categorized as unclassified UC. In the CD group, ileocolonic disease was more common (50%, 3 patients), and 66.7% (4 patients) of cases had a stricturing or penetrating behaviour.
Prior anti-TNF exposure was reported in 61.3% (19 patients) of the patients, 60% in the UC group (15 patients) and 66.7% (4 patients) in the CD group. Among the patients, 61.3% used immunomodulators (azathioprine) as concomitant treatment.
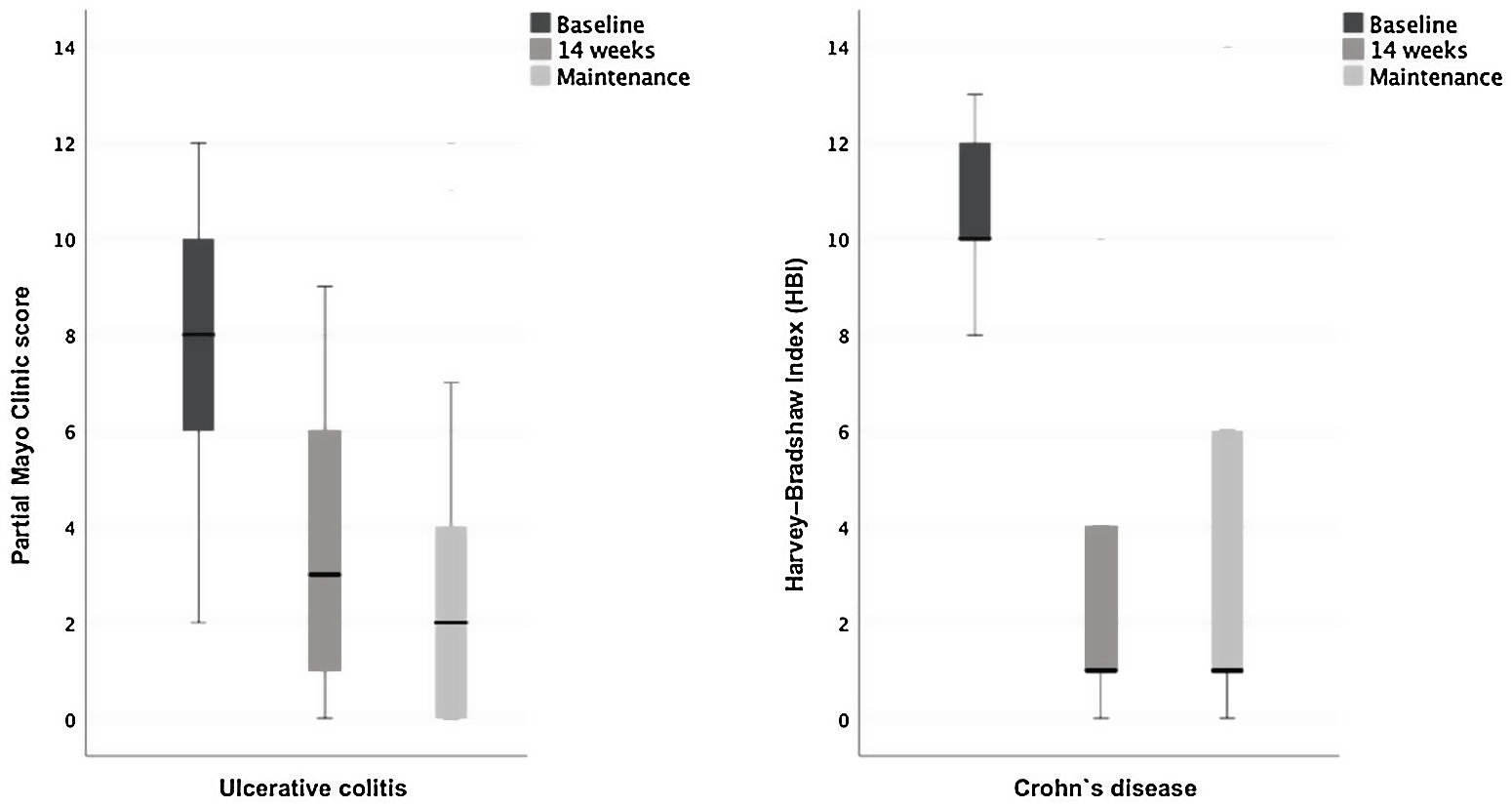
Clinical response and remissionThe response and remission rates at week 14 and maintenance therapy with VDZ are presented in Table 2. At week 14, clinical response was achieved by 87.1% (27/31) of the patients treated with VDZ. During the maintenance therapy visit, 93.5% (29/31) of patients achieved clinical response. The analysis of UC and CD scores showed a change in disease activity and severity following VDZ treatment (Fig. 1). Loss of response was reported in two subjects (2/27 responders at week 14, 7.4%), one with severe UC and the other with penetrating and perianal CD.
Clinical and biochemical remission and response rates in IBD patients treated with VDZ.
| Ulcerative colitis (n=25) | Crohn's disease (n=6) | Overall (n=31) | ||||
|---|---|---|---|---|---|---|
| Induction therapy, week 14 (n, %) | Maintenance therapy, last follow-up visit (med 42 weeks IQR 45)(n, %) | Induction therapy, week 14 (n, %) | Maintenance therapy, last follow-up visit (med 44 weeks IQR 78.5)(n, %) | Induction therapy, week 14 (n, %) | Maintenance therapy, last follow-up visit (med 42 weeks IQR 47)(n, %) | |
| Clinical response | 22/25 (88.0) | 24/25 (96.0) | 5/6 (83.3) | 5/6 (83.3) | 27/31 (87.1) | 29/31 (93.5) |
| Clinical remission | 15/24 (62.5) | 18/24 (75.0) | 4/5 (80.0) | 4/5 (80.0) | 19/29 (65.5) | 22/29 (75.9) |
| Deep remission | 13/21 (61.9) | 3/5 (60.0) | 16/26 (61.5) | |||
| Endoscopic response | 13/21 (61.9) | 17/20 (85) | 2/3 (66.7) | 2/3 (66.7) | 15/24 (62.5) | 19/23 (78.9) |
| Biochemical response | 20/24 (83.3) | 19/24 (79.2) | 3/5 (60.0) | 3/5 (60.0) | 23/29 (79.3) | 22/29 (79.3) |
| Normal CRP | 15/24 (62.5) | 21/24 (87.5) | 3/5 (60.0) | 3/5 (60.0) | 18/29 (62.1) | 24/29 (82.8) |
| Normal ESR | 11/17 (64.7) | 12/16 (75.0) | 2/5 (40.0) | 2/5 (40.0) | 13/22 (59.1) | 14/21 (66.7) |
| Normal Hb | 23/24 (95.8) | 24/24 (100.0) | 4/5 (80.0) | 4/5 (80.0) | 27/29 (93.1) | 28/29 (96.6) |
| Normal platelets | 18/22 (81.8) | 18/22 (81.8) | 3/5 (60.0) | 3/5 (60.0) | 21/27 (77.8) | 21/27 (77.8) |
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, haemoglobin; med, median; IQR, interquartile range.
Sample size variations are due to missing data for some individuals.
Clinical response was defined as a reduction of at least 2 points in the Mayo score and ≥30% from baseline in UC patients 22, and at least 3 points in the HBI in CD patients. Endoscopic response was defined as a Mayo sub-score ≤1 25 for UC and SES-CD ≤2 24 in CD. Remission was defined as a Mayo score ≤2 in UC patients and HBI <5 in CD patients, associated with steroid discontinuation. Deep remission was defined as clinical and endoscopic response with normal CRP, ESR and platelet values. Laboratory normality was defined by normal ranges reported by each laboratory report.
In the group of patients who received anti-TNF previously, 84.2% (16/19) achieved clinical response at week 14 (induction phase) and 89.5% (17/19) at the last visit during the maintenance phase. For anti-TNF naïve patients, clinical response was recorded in 91.7% (11/12) at week 14 and in 100% (12/12) at the last visit during maintenance. The response and remission results in these patients are presented in Table 3.
Clinical response, remission and endoscopic response stratified by previous anti-TNF exposure in IBD patients treated with VDZ.
| Overall, n=31 | Anti-TNF naïve, n=19 | Anti-TNF exposed, n=12 | ||||
|---|---|---|---|---|---|---|
| Induction therapy, week 14 (n, %) | Maintenance therapy, last follow-up visit (med 42 weeks IQR 47) (n, %) | Induction therapy, week 14 (n, %) | Maintenance therapy, last follow-up visit (med 51 weeks IQR 62) (n, %) | Induction therapy, week 14 (n, %) | Maintenance therapy, last follow-up visit (med 38 weeks IQR 35) (n, %) | |
| Clinical response | 27/31 (87.1) | 29/31 (93.5) | 11/12 (91.7) | 12/12 (100) | 16/19 (84.2) | 17/19 (89.5) |
| Clinical remission | 19/29 (65.5) | 22/29 (75.9) | 9/12 (75.0) | 11/12 (91.7) | 10/17 (58.8) | 11/17 (64.7) |
| Deep remission | 16/26 (61.5) | 9/10 (90.0) | 7/16 (43.8) | |||
| Endoscopic response | 15/24 (62.5) | 19/23 (78.9) | 8/12 (66.7) | 10/11 (90.9) | 7/12 (58.3) | 9/12 (75.0) |
Abbreviations: med, median; IQR, interquartile range.
Clinical response was defined as a reduction of at least 2 points in the Mayo score and ≥30% from baseline in UC patients 22, and at least 3 points in the HBI in CD patients. Endoscopic response was defined as a Mayo sub-score ≤1 25 for UC and SES-CD ≤2 24 in CD. Remission was defined as a Mayo score ≤2 in UC patients and HBI<5 in CD patients, associated with steroid discontinuation. Deep remission was defined as clinical and endoscopic remission with normal CRP, ESR and platelet values. Laboratory normality was defined by normal ranges reported by each laboratory report.
For the remission analysis, 29 patients with data at both evaluation time points were included. The remission rate at week 14 was 65.5% (19/29) for the overall cohort and 75.9% (22/29) at the last visit during maintenance Among anti-TNF naïve patients, 75% (9/12) achieved clinical remission at week 14 and 91.7% (11/12) at the last visit during maintenance. Among those who had previously failed to at least one anti-TNF agent, clinical remission was achieved in 58.8% (10/17) after induction and 64.7% (11/17) at the visit in the maintenance phase. Data regarding deep remission information during the maintenance phase was available for 26 patients. The overall cohort had a deep remission rate at last visit during maintenance phase of 61.5% (16/26), with 43.8% (7/16) in anti-TNF experienced and 90.0% (9/10) in anti-TNF naïve patients.
Endoscopy and biochemistryObjective endoscopic evaluations using UC and CD scores evidenced improvement in disease activity and severity following VDZ treatment (Fig. 2). In UC patients, the median Mayo score improved from severe disease (Partial Mayo score 8, IQR 5; Mayo subscore for endoscopy 3, IQR 1) to mild (Partial Mayo score 3, IQR 5; Mayo subscore for endoscopy 1, IQR 2) after induction therapy. Disease scores remained mild at the last visit assessment during maintenance (Partial Mayo score 2, IQR 4; Mayo subscore for endoscopy 1, IQR 2). For CD patients, the median baseline HBI ameliorated from moderate disease (10, IQR 4) to remission at week 14 and at last visit in maintenance, both timepoints with a median HBI score of 1 (IQR 7). Likewise, in SES-CD, the median baseline group improved from moderate disease (4, IQR-) to inactive disease at both induction (1 IQR-) and maintenance (median 0 IQR-). Additionally, high rates of acute phase reactants and haemoglobin normalization were observed in during induction and maintenance, compared to baseline (Table 2).
Corticosteroid therapyInformation on steroid use during therapy was available for 23 patients in the induction phase and 30 patients in the maintenance phase, although data on whether this treatment was initiated before, during or after the onset of VDZ were not recorded. During the induction phase and maintenance therapy with VDZ, 30.4% (7/23) and 33.3% (10/30) of the patients, respectively, received concomitant corticosteroids. Among patients who received steroids at some point during VDZ treatment (n=11), a response was recorded in 63.6% (7/11). Of these, three patients discontinued corticosteroids (all in clinical response), three patients initiated steroid tapering, and one case of moderate pancolitis continued corticosteroid therapy unchanged. Among the patients who ever used steroids (n=11) data of steroid withdrawal was available for seven patients. With this information, the corticosteroid-free remission rate was 28.6% (2/7).
Adverse eventsDuring the study period, five patients presented one or more adverse events (AE). Most of the AE were classified as mild and included: infections (two patients), headache (one patient), dizziness (one patient), increased transaminases (one patient). Overall, VDZ infusions were well tolerated. However, one patient with history of allergic reaction to anti-TNFs, developed urticaria as an infusion reaction, which led to the discontinuation of VDZ.
DiscussionIn this real-world data study conducted in Colombia, we report the effectiveness and safety of VDZ in patients with IBD. IBD is a chronic and progressive entity that requires goal-oriented treatment to improve prognosis, reduce disease-related complications, and enhance patient quality of life.30,31 The treatment for IBD has evolved over time, with early initiation of biologic agents becoming a mainstay of therapy, especially for patients with moderate and severe manifestations and for CD patients at higher risk of complications and surgical interventions.32,33 As different therapeutical agents are available for IBD treatment, understanding their real-life use is essential for patient-centred clinical decisions.
In this cohort, we found a clinical response in 87.1% of patients at week 14 (induction), with 65.5% achieving remission. These results are similar to findings from other observational studies.34,35 A Brazilian multicenter observational multicenter study of VDZ, reported a remission rate of 46% in CD and 42% in UC at week 52, with slightly higher response rate in CD compared to UC.20 Similarly, our results showed clinical and endoscopic improvements in patients, although the effect was less pronounced in CD patients, likely due to the small number of CD patients in this subgroup (only six patients in follow-up).
As found in other publications, prior exposure to anti-TNF agents was associated with a reduced probability of achieving clinical remission.36 In our cohort, 61.3% of the patients had previous exposure to anti-TNFs, and 25.8% had failed to two prior anti-TNFs. Despite high clinical response and remission in all patients, anti-TNF naïve patients exhibited higher clinical response during induction and maintenance therapy, as well as higher rates of deep remission.
The loss of response in our study was lower than previously reported.37 However, it is important to note that 50% of the subjects in our study did not reach 42 weeks of follow-up treatment, which may suggest a possible under-identification of the event. Loss of response was observed in a patient with penetrating and perianal CD, suggesting a potential relationship between more severe disease activity and treatment response.
Endoscopic healing is considered an important goal associated with improved clinical outcomes. However, in real-life scenarios, endoscopic response, an indirect reference to endoscopic healing, is more commonly evaluated.38 Clinical trials of VDZ, such as the VERSIFY38 and LOVE CD studies39 reported endoscopic response rates of 24.8% and 40% at week 26, respectively. A real-world study involving 117 Italian patients with UC and CD reported endoscopic response rates of 47% and 38,2% for UC and CD, respectively, one year after VDZ initiation.40 In our study, endoscopic response rates were 61.9% for UC and 66.7% for CD at the end of induction therapy (week 14). However, it is important to note that the baseline endoscopic status of 36.8% of the patients in our cohort was unknown, and definitions of endoscopic response vary between studies, which limits comparability.
Adverse events were rare in our study, with only seven patients reporting adverse events, primarily related to mild infections. Discontinuation of VDZ was necessary in one case due to urticaria as an infusion reaction. The difference in our safety data, with an adverse event proportion of 18% and only one serious case, compared to other reports where the proportion of patients with adverse events is around 30–40%,4,20 could potentially be attributed to the small number of patients in our cohort and the possibility of under-reporting.
Despite the limitations of our study, including its small sample size, retrospective design, variable follow-up duration, and missing data, it contributes to the published experience with VDZ in Latin America.
To our knowledge, it is the second real-world study on this subject in our region.20 Although the study sample size is small, these results provide valuable evidence regarding the effectiveness and safety of VDZ in treating IBD patients in Latin American, where data is scarce.
In conclusion, the EXVEDOCOL study showed that VDZ provides clinically relevant benefits in terms of clinical, endoscopic, and biochemical outcomes for IBD patients. Notably, our results suggest that anti-TNF naïve patients may derive substantial benefits from VDZ therapy, indicating its potential as a first-line biologic agent in the management of IBD. The observed improvements in disease activity and endoscopic response further support the notion that VDZ plays a crucial role in achieving disease control and promoting mucosal healing. Furthermore, the low incidence of adverse events and the overall tolerability of VDZ in our study population highlight its favourable safety profile. Our results are consistent with those reported by other authors. While our study provides valuable insights, it is important to note the need for larger-scale, prospective studies to validate these findings and assess the long-term benefits and safety of VDZ in the Latin American context.
Data availabilityThe data underlying this article will be shared on reasonable request to the corresponding author.
Ethical considerationsThis study was conducted following the ethical guidelines of the Declaration of Helsinki, and the local ethics committee of each participating centre approved it. This research was deemed to carry minimal risk due to its utilization of retrospective data, thus obviating the need for patient informed consent in accordance with national legislation.
Authors’ contributionsAll authors participated in the acquisition, analysis, and interpretation of the data, drafted the initial manuscript and revised the article critically for relevant intellectual content.
FundingThe study was funded by Baxalta Colombia SAS (Takeda). Data analysis and manuscript preparation was performed by Epithink, Health Consulting and funded by Baxalta Colombia SAS (Takeda).
Conflict of interestsFP reports sponsorship by Takeda to attend scientific meetings. EP reports honoraria for lectures, presentations, speakers, manuscript writing, and educational events sponsored by Takeda and Pfizer, and participated at advisory boards from Takeda. GR reports honoraria for lectures, presentations, speakers, manuscript writing, and educational events sponsored by Takeda. FG is an employee of Baxalta Colombia SAS.
The authors would like to thank all the site research teams for their participation and contribution in the EXVEDOCOL study.