The set of measures proposed by SEPD, AEEH, GETECCU and AEG are aimed to help departments in their resumption of usual activity. We have prepared a number of practical recommendations regarding patient management and the stepwise resumption of healthcare activity. These recommendations are based on the sparse, changing evidence available, and will be updated in the future according to daily needs and the availability of expendable materials to suit them; in each department they will be implemented depending upon the cumulative incidence of SARS-CoV-2 infection in each region, and the burden the pandemic has represented for each hospital. The general objectives of these recommendations include:
• To protect our patients against the risks of infection with SARS-CoV-2 and to provide them with high-quality care.
• To protect all healthcare professionals against the risks of infection with SARS-CoV-2.
• To resume normal functioning of our departments in a setting of ongoing risk for infection with SARS-CoV-2.
El artículo recoge el conjunto de medidas propuestas por la SEPD, la AEEH, GETECCU y la AEG que pretenden servir de ayuda a los servicios en su reincorporación a la actividad habitual. Hemos confeccionado una serie de recomendaciones prácticas respecto al manejo y a la reintroducción progresiva de la actividad asistencial. Estas recomendaciones están guiadas por la escasa y cambiante evidencia disponible y serán objeto de futuras actualizaciones, en base a las necesidades diarias y la disponibilidad del material fungible para adecuarse a las mismas; y se podrán implementar en cada servicio en función de la incidencia acumulada de SARS-CoV-2 en cada región y de la carga que la epidemia ha ocasionado en cada uno de los hospitales. Los objetivos generales de estas recomendaciones son:
• Proteger a nuestros pacientes de los riesgos de la infección por SARS-CoV-2 y prestarles una atención de calidad.
• Proteger a todos los profesionales sanitarios de los riesgos de la infección por SARS-CoV-2.
• Recuperar el normal funcionamiento de nuestros servicios en un entorno de riesgo continuado de infección por SARS-CoV-2.
Infection with the SARS-CoV-2 coronavirus and its potentially resulting disease, designated COVID-19, is causing significant concern among the general population, and — needless to say — healthcare professionals and patients.1,2 In this regard, it has had a highly significant impact on our gastroenterology and hepatology departments, which have reduced both their hospitalization activity (by more than 50%) and the number of diagnostic/therapeutic endoscopic procedures (by more than 50%, unpublished data). Besides affecting our activity, it also affected our work, with high numbers of gastroenterologists being moved to COVID areas. Finally, some — in fact many — of our colleagues have fallen ill as a consequence of caring for patients infected with SARS-CoV-2. Let us not forget that some of the procedures we carry out on a daily basis are associated with a high risk for COVID-19 transmission.3–5 Even if its incidence diminishes considerably, it will stay with us over the coming months, which should prompt us to take extreme precautions in a micro-environment with a high risk for coronavirus transmission as is the case with hospitals.
Times of crisis are usually accompanied by opportunities or else appropriate to reformulate activities and the way they are performed. In this crisis we had to respond to the exigencies of COVID-19, but must also carry on providing essential care as defined within our specialty. Because of this, this document also reflects on the opportunity to incorporate telemedicine into our usual practice in order to enhance the care we provide to our chronic patients. Since the present situation lacks consistency (different Autonomous Communities, hospitals, SARS-CoV-2 incidences, public/private centers, etc.), the right time to implement these recommendations may vary. Be it as it may, we propose that the transition from the current state of alarm, which has brought activity in our departments to a virtually complete standstill, to a more normal situation be accomplished in three phases: activity resumption phase, stabilization phase, and normalization phase. The length of these phases is difficult to foretell in such dynamic, highly changing scenario, but will not foreseeably be shorter than 2–4 months. Furthermore, when will the human and space resources redeployed to caring for COVID-19 patients be recovered by our departments remains yet unknown.
The set of measures proposed by SEPD, AEEH, GETECCU and AEG are aimed to help departments in their resumption of usual activity. We have prepared a number of practical recommendations regarding patient management and the stepwise resumption of healthcare activity. These recommendations are based on the sparse, changing evidence available, and will be updated in the future according to daily needs and the availability of expendable materials to suit them; in each department they will be implemented depending upon the cumulative incidence of SARS-CoV-2 infection in each region, and the burden the pandemic has represented for each hospital. The general objectives of these recommendations include:
- •
To protect our patients against the risks of infection with SARS-CoV-2 and to provide them with high-quality care.
- •
To protect all healthcare professionals against the risks of infection with SARS-CoV-2.
- •
To resume normal functioning of our departments in a setting of ongoing risk for infection with SARS-CoV-2.
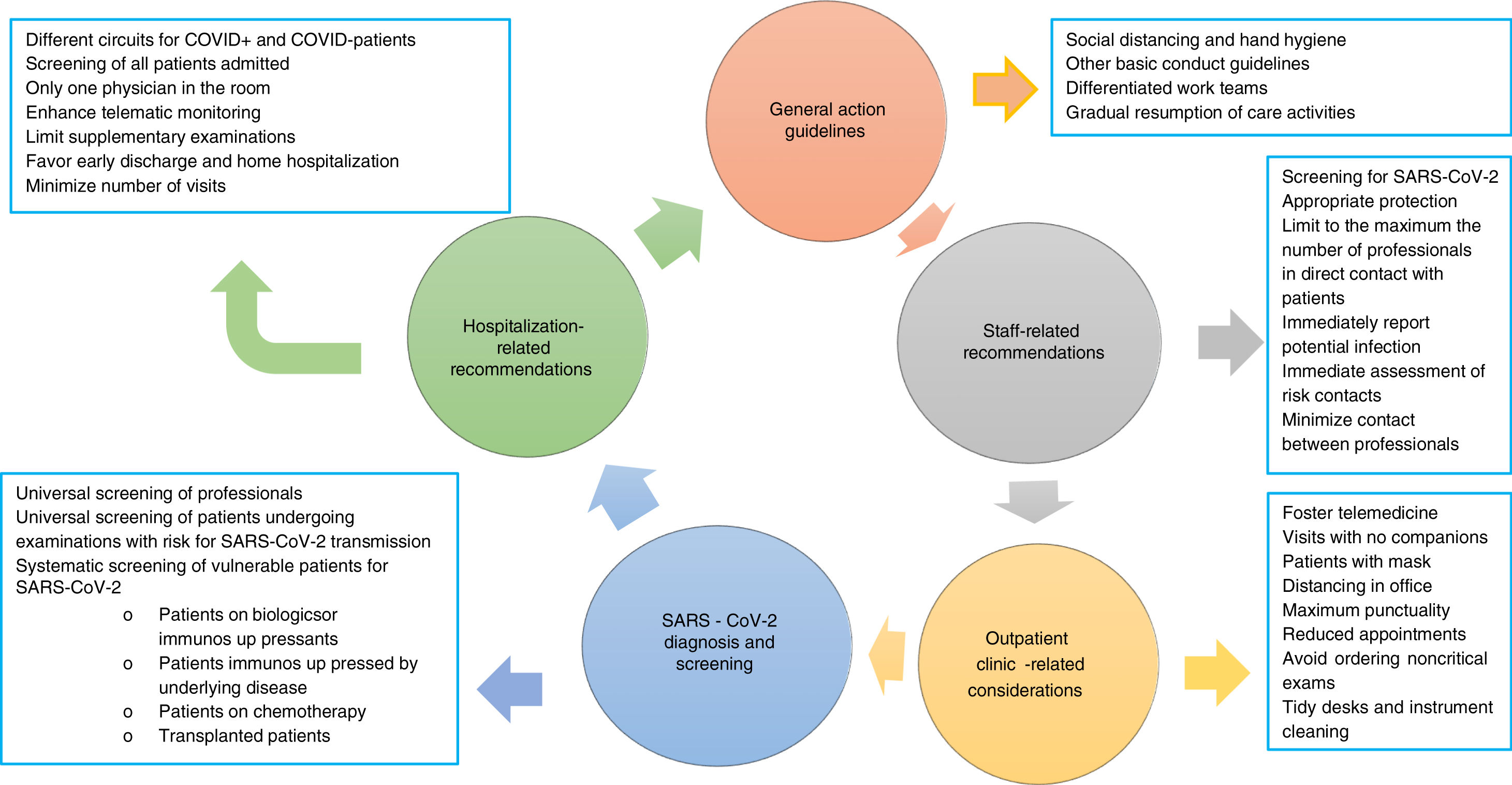
The risk for infection with SARS-CoV-2 has decreased as a result of the adoption of non-pharmacological measures primarily including isolation of confirmed or suspect cases, social distancing, and confinement of the population in their homes.6,7 The risk, however, has not disappeared, hence we must recommend:
- a.
To strictly comply, both in the hospital at large and in our departments in particular, with the protective measures recommended for all citizenry: social distancing and hand hygiene.
- b.
Use of a mask must be mandatory, at least in all hospital premises, for patients and their companions.
- c.
Work areas should be kept well ventilated.
- d.
As far as possible for each hospital, we recommend setting up differentiated work teams for endoscopy areas,1 hospitalization wards, outpatient clinics, and on-duty services. We believe that such work dynamics could be loosened up during the second phase, and discontinued during the third phase.
- e.
The return of departments to care-as-usual activities should be slow.
SARS-CoV-2 has a high spread rate within hospitals, hence healthcare professionals are at high risk for infection, as demonstrated by the data reported in China (3300 cases) and Italy,8 and those estimated is Spain (12–15% of all recorded cases, data provided by the Health Ministry). To minimize infection risk we recommend:
- a.
Regular screening of all professionals (see below).
- b.
It is essential that professionals be equipped with appropriate protective means ccording to their work setting. At any rate, they must always wear personal protective equipment including surgical mask, scrubs, and closed footwear (if possible, specific for hospital use; otherwise, with shoe covers). In addition to providing protection, these measures will prevent care providers from serving as vectors for transmission in and out of hospitals.
- c.
All healthcare personnel with respiratory symptoms and/or fever and/or suspicion of recent contact with someone infected with SARS-CoV-2 must report it at the earliest possible time to the head of their department. Under no circumstances whatsoever must they go to their workplace in case of suspicion.
- d.
Social distancing and the other recommended measures must be maintained during the present pandemic.
- e.
The number of care providers in direct contact with patients must be kept to a minimum, both during hospitalization and when performing diagnostic or therapeutic procedures.
- f.
Professionals with suspected or confirmed COVID-19, even with a negative PCR result, must not work until 14 days after symptom onset. Whether a diagnostic test is repeated will be indicated by the departments of infectious diseases, occupational medicine or preventive medicine.
- g.
Professionals who have been in close contact with an individual infected with SARS-CoV-2, defined as the contact between a provider and a person (patient, companion, professional) with confirmed infection, must not work without the appropriate protective equipment.
- h.
Minimizing contact between healthcare providers. Only essential personnel (physician, nurse, nursing assistant, and anesthetist where appropriate) shall be present in endoscopy (or other procedures) rooms.
- a.
Patients must attend appointments alone, with no companions, unless they are disabled. In no case shall a patient visit with more than one companion. In case a companion is present, he or she should ideally be younger than 50 years.
- b.
Patients must always wear a mask and wash their hands with hydroalcoholic solution before entering the office.
- c.
Increase distancing in the office by separating the chairs from the desk as much as possible.
- d.
Examine patients only when imperative, and do so with appropriate protection (use gloves, wash hand and stethoscope with hydroalcoholic solution). Use a disinfectant to clean the examination table.
- e.
Desks must be kept as tidy as possible to facilitate appropriate cleaning of exposed surfaces on a regular basis.
- f.
Until after the third phase the number of on-site appointments must be reduced by lengthening their intervals in order to avoid waiting room overcrowding.
- g.
Efforts should be made to prescribe supplementary testing only when strictly necessary, and/or to lengthen their intervals.
- h.
Telemedicine must be favored, as well as other care modalities with value for patients that may also minimize transmission risk, both regarding patients and other health centers.
- i.
Wherever possible, pharmacy departments shall facilitate drug dispensation for longer periods, even home delivery, as is now the case with some hospitals.
Resuming activity should lead us to pender over the structure of our traditional consultation schedules. The use of telematic tools (consultations over the phone, video calls, other) should be promoted both for patient care and work meetings, as it significantly decreases exposure for both patients and care providers.9Objectives will be dependent on the current phase: in the first phase the primary goal is to reduce the risk for SARS-CoV-2 contagion among patients and professionals. In the second phase, and most particularly in the normalization phase, objectives will include: (a) reducing non-value-added, on-site care (reporting normal results, further prescribing the same therapy, ordering supplementary examinations, etc.); (b) facilitating care for patients unable to attend for work reasons; and (c) reducing usual overcrowding in our clinics. Some of the requirements telemedicine must meet are as follows10,11:
- •
Telemedicine should be considered for all intents and purposes a medical act. This type of visit must be included in electronic records and appear in the agenda as an “off-site” visit. The electronic medical record may include screen captures of the prescriptions. Having contact information available is important so that instructions and prescriptions may be mailed in writing and a follow-up strategy may be established.
- •
Ideally, telematic visits should be interspersed among in-person appointments in order to prolong intervals between the latter, thus reducing the number of people in waiting rooms.
- •
Appropriate coordination should be sought with primary care centers. We suggest appointing a department coordinator for each primary care center, who should currently favor telematic visits rather than referrals.
- a.
Differentiated circuits must be maintained for patients with and without COVID-19.
- b.
All admissions other than those strictly necessary should be avoided.
- c.
In all patients admitted to hospital infection with SARS-CoV-2 must be ruled out regardless of symptoms. PCR is currently the most suitable technique but each hospital should follow their own previously approved protocol.
- d.
Since the risk of community transmission still lingers on, further rapid testing for SARS-CoV-2 at 10–14 days after admission is advisable to minimize the risk for in-hospital outbreaks. Similarly, patients discharged after more than 10–14 days in hospital must be tested to prevent community outbreaks.
- e.
For patients who remain hospitalized:
- -
Only one adequately equipped physician shall enter the room. Stethoscopes and any other non-expendable materials coming in contact with patients will be subsequently cleaned with hydroalcoholic solution (or a disinfectant).
- -
Attempts should be made to monitor patients using telematic or telephony devices.
- -
Limit to a minimum all testing involving patient transportation within hospitals.
- -
Invasive procedures such as placement of nasogastric or bladder tubes should be avoided whenever possible, as well as ordering excessive lab tests.
- -
Foster early discharge and home hospitalization.
- -
- f.
The number of visits to inpatients must be minimized; this is particularly relevant for immunosuppressed patients. In no case should patients be accompanied by more than one person at a time.
- g.
It is advisable that patients visiting day hospitals to receive intravenous medications be tested for temperature before entering the facility, and that infusion chairs be at least 2 meters away from each other. Turns should be established if this is unfeasible. Chairs and rooms should be adequately cleaned after infusion completion.12 It is advisable that patients attend alone whenever possible.
- h.
In case of day-hospital overcrowding efforts will be made to prescribe drugs with subcutaneous formulation.
Once the confinement phase is over, massive screening will likely be a most effective measure to gain insight into the population's immune status regarding SARS-CoV-2 infection. Knowledge of this immune status will be particularly relevant in areas with high infection rates and, above all, among those who provide care for the rest of citizens. Because of this we recommend:
- a.
Regular, universal screening of professionals. Such screening will reveal immunization level, and will likely help establish the risk run by care providers, a key aspect for the management of a potential recurrence of the pandemic.
- b.
Universal screening of all patients who must undergo examinations involving SARS-CoV-2 infection transmission risks.
- c.
Systematic screening for SARS-CoV-2 among particularly vulnerable patients. Ideally, such screening should be primarily offered to:
- i.
Patients on biologics or immunosuppressants:
- -
Inflammatory bowel disease.
- -
Autoimmune liver disease.
- -
Other.
- -
- ii.
Patients with immunosuppression secondary to their underlying disease:
- -
Compensated and, most particularly, decompensated liver cirrhosis.
- -
- iii.
Patients with liver cancer.
- iv.
Transplant recipients.
- i.
Two important considerations apply regarding the above screening:
- •
Obviously, a systematic screening of all these populations cannot be carried out simultaneously, hence we recommend setting up local screening plans.
- •
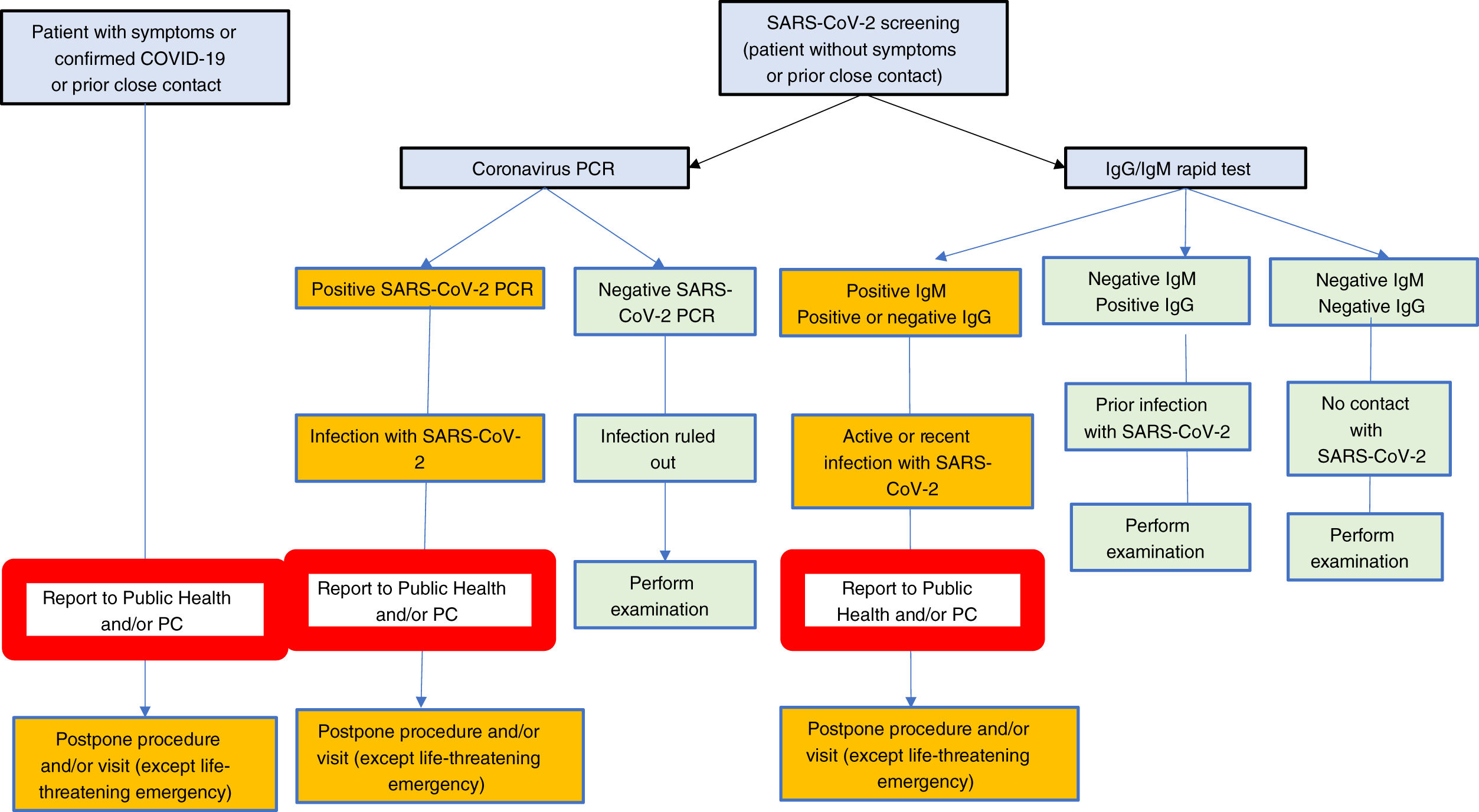
Screening is for the asymptomatic population; should a patient present with symptoms suggestive of infection with SARS-CoV-2, he or she should be diagnosed (PCR and/or serologic tests and/or chest X-rays and/or chest CT scan); in no case should the patient undergo an elective dianostic test.
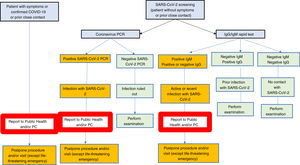
In our view, the best screening strategy should be established at any given time depending on the availability of rapid antibody tests and/or PCR and/or serological techniques such as ELISA, as well as on endemic evolution.
- •
Interpretation of results obtained in asymptomatic patients without previous close contacts.
- -
IgG negative, IgM negative. Individual with no prior exposure to SARS-CoV-2.
- -
IgM positive, IgG negative or positive. Recent, potentially active infection. A PCR shall be made to rule out active infection.
- -
IgM negative, IgG positive. Prior infection with SARS-CoV-2. Exceptionally, patients with no history of symptoms and with no close contact with an IgG+, IgM- infected patient may have a positive PCR.
- -
Rarely may the result be indeterminate.
- -
- (1)
Ultrasound:
- •
Both the patient and physician must wear surgical face masks during examinations.
- •
Activity shall be immediately resumed (first phase) for patients with urgent or preferential indications, and/or for undelayable therapeutic procedures.
- •
The rate of activity resumption shall depend on local characteristics, both regarding pandemic incidence and healthcare personnel/ancillary staff availability.
- •
- (2)
Hepatic elastography with Fibroscan:
- •
Transient elastography is only exceptionally an urgent procedure.
- •
Both the patient and physician must wear surgical masks during the examination.
- •
Activity shall be gradually resumed, preferentially starting in the second phase.
- •
- (3)
Manometry and pH-metry:
- •
High-risk examinations beause of aerosol formation.
- •
Only exceptionally urgent.
- •
Resumption shall be held off until the third phase.
- •
Should an urgent indication arise in the first phase (highly unlikely), the course of action shall be the same as for gastroscopy, including screening for SARS-CoV-2 and using appropriate protective equipment.
- •
- (4)
Breath tests:
- •
High-risk examinations beause of aerosol formation.
- •
Only exceptionally urgent.
- •
They may be substituted for by other testing modalities (fecal antigens, commercial tests).
- •
These tests shall not be resumed until the third phase.
- •
- (5)
Portal hemodynamics:
- •
Portal hemodynamics is a moderate-risk study regarding SARS-CoV-2 transmission (position and duration).
- •
During the first phase of activity resumption in our departments a conservative attitude is advisable, prescribing this examination only in two situations:
- •
Liver biopsy in cases of severe acute liver failure where the test may play a key role.
- •
Urgent placement of TIPS for intractable bleeding secondary to portal hypertension.
- •
In the second phase it seems reasonable to perform any necessary procedures to assess portal hypertension in patients with hepatocellular carcinoma potentially amenable to surgical resection.
- •
- •
Finally, during the third phase (normalization) all indicated procedures will be carried out the same as before the crisis.
- •
The COVID-19 pandemic represents an unprecedented challenge to our health system, and as regards specifically patients with inflammatory bowel disease (IBD) multiple concerns arise in connection with their management, as many are on treatment with immunity-impairing therapies. Furthermore, IBD is a condition that evolves in flares alternating with remission periods, and may have potential complications that often require urgent, or at least preferential, care.
A variable proportion of patients have digestive complaints such as nausea, vomiting, bowel habit changes, or abdominal pain.13 These symptoms are common in patients with IBD, hence the importance of excluding COVID in our patients. Furthermore, the virus has been reported to be present in the stools of COVID-19 patients, regardless of the presence of diarrhea, and to persist there even after respiratory symptoms are over or detection in the oropharynx is no longer feasible, its significance being uncertain concerning infectivity during endoscopic procedures or potential fecal-oral transmission.14
Reciprocal influence between IBD and COVID-19The first question we posed ourselves from the start of the pandemic was whether patients with IBD are at increased risk of infection. Patients with IBD do not seem to have a greater risk for infection with SARS-CoV-2 or for development of COVID-19. According to data from Bergamo in Lombardy, a region especially affected of Italy, no patient among their 522 cases of IBD was diagnosed with, or admitted to hospital for COVID-19.15 A possible reason explaining this lower number of cases of COVID-19 in IBD patients may be this population's adherence to protective measures.
Another common question is whether COVID-19 may cause an IBD flare-up; current evidence does not seem to support that, albeit available data are scarce and caution is here advisable.16
Finally, the next question we posed ourselves was whether suffering from IBD may condition the course of COVID-19. Answering this is difficult since multiple factors may play a role: age, comorbidities, inflammatory activity, and trestments received, the available information being limited about these. There is an international registry called SECURE-IBD17 that aims to collect the data of patients diagnosed with IBD where COVID-19 has been confirmed (positive testing).1 At the time of writing these recommendations a total of 457 patients have been recorded, 78 of them from Spain. The overall rate of hospitalization has been 30%, and those of ICU admissions, ventilation requirement, and mortality have been 4%, 4%, y 3%, respectively. Therefore, it seems that the course of COVID-19 in patients with IBD is not worse than in the general population, but we should bear in mind that our IBD patients are younger than the general population. In this respect the fact should be highlighted that patients with moderate-high activity required ICU care/ventilation or died (pooled variable) in 17% of cases, versus 5% for patients with remission or low activity, with 27% of subjects with an untoward outcome being on steroids.
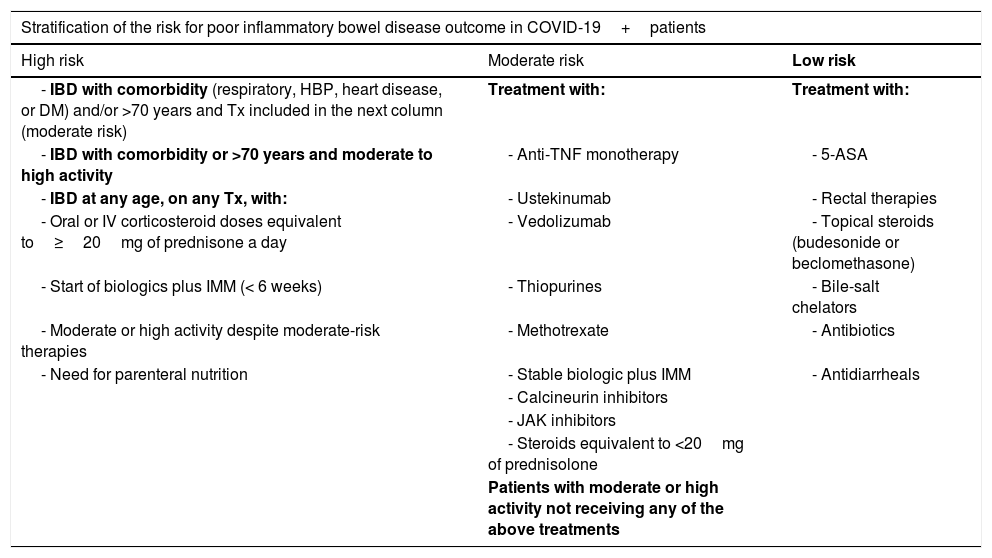
Below we include a table with a theoretical stratification of the risk for poor outcomes based on recommendations by the British Society of Gastroenterology (BSG),18 although, again, the dearth of data available about the therapies used for IBD should be borne in mind (Table 1). Drug half-life must be taken into account, and so patients who discontinued immunosuppressants or biologics within the last 3 months remained exposed to their effects when it comes to risk categorization.
Theoretical stratification of the risk for poor outcomes based on the recommendations issued by the British Society of Gastroenterologt (BSG).18
| Stratification of the risk for poor inflammatory bowel disease outcome in COVID-19+patients | ||
|---|---|---|
| High risk | Moderate risk | Low risk |
| - IBD with comorbidity (respiratory, HBP, heart disease, or DM) and/or >70 years and Tx included in the next column (moderate risk) | Treatment with: | Treatment with: |
| - IBD with comorbidity or >70 years and moderate to high activity | - Anti-TNF monotherapy | - 5-ASA |
| - IBD at any age, on any Tx, with: | - Ustekinumab | - Rectal therapies |
| - Oral or IV corticosteroid doses equivalent to≥20mg of prednisone a day | - Vedolizumab | - Topical steroids (budesonide or beclomethasone) |
| - Start of biologics plus IMM (< 6 weeks) | - Thiopurines | - Bile-salt chelators |
| - Moderate or high activity despite moderate-risk therapies | - Methotrexate | - Antibiotics |
| - Need for parenteral nutrition | - Stable biologic plus IMM | - Antidiarrheals |
| - Calcineurin inhibitors | ||
| - JAK inhibitors | ||
| - Steroids equivalent to <20mg of prednisolone | ||
| Patients with moderate or high activity not receiving any of the above treatments | ||
Recommendations concerning the treatment of IBD are based on those issued by the International Organization for the study of IBD (IOIBD), BSG, and American Gastroenterology Association (AGA),19 differentiating between uninfected, SARS-CoV-2 infected, and COVID-19 patients.
- (1)
General recommendations regarding the treatment of patients with IBD, which must remain in force during the gradual resumption of activities:
- a.
Patients must not discontinue medication or visits to the infusion center, or start self-medication, without consulting with their doctor first.
- b.
Medication must be available at home in case an isolation period is required.
- c.
Smoking should be stopped as it increases the risk and severity of COVID-19. Smoking augments gene expression of angiotensin converting enzyme 2 (ACE2), the receptor for viral entry.20
- a.
- (2)
Patients with IBD not infected with SARS-CoV-2:
- a.
In case of suspect symptoms and a negative SARS-CoV-2 PCR, the potential for false negative results should be considered, as well as a repeat test.
- b.
Treatment must be maintained to prevent non-adherence-related relapse, which may represent a higher risk of infection because of steroid or hospitalization needs.
- c.
When possible, thiopurines, methotrexate, and tofacitinib should be avoided if other options are available, given their potential to increase susceptibility to viral infection.
- d.
When biologics are required, monotherapy without an immunomodulator should be the preferred option.
- e.
When planning to initiate therapy with biologics or immunosuppressants, it is advisable that SARS-CoV-2 testing be included in the previous assessment routine.21
- f.
Steroids use should be minimized. If required, rapid tapering by 10mg/week is advisable.
- a.
- (3)
Patients with IBD infected with SARS-CoV-2 without COVID-19 manifestations:
- a.
If the patient is on steroids, reduce dose to below 20mg or switch to budesonide should the clinical scenario allow.
- b.
Consider temporarily discontinuing thiopurines, methotrexate, and tofacitinib, monitoring the course of COVID-19.
- c.
Consider delaying for 2 weeks anti-TNF, ustekinumab, and vedolizumab doses, monitoring the course of COVID-19. Serological tests to measure IgM M and IgG will be helpful to guide the reintroduction of biologic therapy.
- d.
Treatments with oral/topical mesalazine, local steroids (budesonide, beclometasone), antibiotics for bacterial overgrowth or perianal disease, antidiarrheals (loperamide), and bile salt chelators (cholestyramine resin) may be maintained.
- a.
- (4)
Patients with IBD and COVID-19
- a.
Consider intestinal inflammatory activity and COVID-19 severity levels when making therapeutic decisions.
- b.
Treatments with oral/topical mesalazine, locally released corticosteroids (budesonide, beclomethasone), antibiotics for bacterial overgrowth or perianal disease, antidiarrheals (loperamide), and bile salt chelators (cholestyramine resin) may be maintained if necessary to manage IBD.
- c.
Steroid dosage should be reduced or the drug switched to budesonide whenever possible.22
- d.
Discontinue thiopurines, methotrexate and tofacitinib until 14 days after discharge.
- e.
In case of mild COVID-19 (outpatients, inpatients without evidence of pneumonia and O2 saturation >94%) consider delaying biologics for 2 weeks.
- f.
In case of moderate or severe COVID-19 discontinue biologics until COVID resolution. The use of serologic testing, when available, may guide drug reintroduction following the clinical resolution of the infection.
- g.
Rule out other causes of digestive complaints such as infection with Clostridium difficile, which requires specific treatment.
- a.
IBD patients already underwent specific follow-up prior to the COVID-19 crisis. Most IBD units have clinics or free-access mechanisms in case of flare-ups to avoid visits to the emergency room (ER).23 Two further characteristics should be considered: nursing consultation24 and telemedicine, long implemented in our IBD units pioneered in our country by GETECCU platforms such as TECCU.25
Scheduled consultations. In an initial phase it is recommended that all scheduled follow-up visits take place telematically. The duration of follow-up for patients in remission according to their medication should be met or at least deviated the least possible from the standards published and accepted by GETECCU.26 In the initial phase it is recommended that patients with severe disease requiring specific physical examinations attend. In the stabilization phase patient numbers will be adjusted according to the above indications.
Non-scheduled consultations. During the initial and stabilization phases, aiming to avoid physical visits to hospital, patients with urgent consultations should be advised to contact the IBD unit via the nursing clinic, telephone, or email. Should the issue be serious or unsolvable through telematic means a visit will be scheduled according to the above indications. Infection with SARS-CoV-2 must be ruled out if fever and diarrhea are present.
Day hospital and drug infusion unitsApproximately, one third of patients with IBD receive biologics and must visit hospital both to be administered intravenous infusions (infliximab, vedolizumab, first dose of ustekinumab) and to collect oral or subcutaneous medications (adalimumab, golimumab, maintenance ustekinumab, tofacitinib) from the pharmacy. The recommendation stands that they go to hospital as little as possible and with the greatest safety measures available.
- a.
It is advisable that, in the first phase, patients receiving oral and subcutaneous medications avoid visiting the hospital. It is recommended that medications be delivered by pharmacy departments to patient homes. Only patients initiating their treatment shall visit the hospital to be instructed in self-administration, as well as patients incapable of self administering their drugs. Nurses shall telematically monitor adherence and solve any emerging doubts.
- b.
Switching from an intravenous molecule to a different subcutaneous one in a patient in remission is not recommended since not only no studies support it but data are available suggesting that it may be detrimental for patient activity.27
- a.
It is recommended that patients with severe Crohn's disease or ulcerative colitis flare-ups refractory to outpatient management be admitted to hospital. Also patients with subocclusion and with septic complications.
- b.
In case of high suspicion of COVID-19 despite a negative test result on admission, it is advisable to place the patient in a pre-COVID-19 area and then repeat testing given the potential for false negative results.28
- a.
Take into account the presence of the virus in the feces, hence the risk of contagion during their manipulation. Consider delaying testing for fecal calprotectin in patients in deep remission.
- b.
Given the limitation of endoscopic procedures because of the COVID-19 pandemic CMV viral load may be used rather than identification in endoscopic biopsies for patients with ulcerative colitis.
- c.
Consider imaging tests as an alternative to endoscopic procedures, according to availability in each center.
Endoscopic examinations are common and necessary for IBD patients, but their resumption must be gradual.
- a.
For patients with highly suspected IBD, severe manifestations, and presence of high biomarker levels colonoscopy is recommended to confirm the diagnosis and start treatment.
- b.
We must prolong the deadlines for scheduled colonoscopies and for those indicated only to assess inflammatory activity, prioritizing the use of biomarkers. Colonoscopy shall be reintroduced first for patients requiring biopsy taking in case of suspected overinfection with CMV, and then for those requiring an urgent change of therapy when biomarkers are inadequate.
Almost half of patients with Crohn's disease and 20% of those with ulcerative colitis will require surgery during the course of disease.
- a.
It is currently recommended that all scheduled surgeries be delayed, both intestinal resections and perianal procedures.
- b.
Urgent surgery such as colectomy for a refractory flare-up, perianal sepsis, or subocclusion with unresolved strictures cannot be delayed.
- c.
It is advisable that the introduction of scheduled surgical procedures during the initial and stabilization phases be agreed upon in the interdisciplinary committees according to severity, absence of medical alternatives, and patient characteristics.
- d.
Treatments to prevent post-surgical recurrence shall comply with the same rules and indications discussed for disease activity.
- a.
Many clinical trials have been interrupted by their sponsors.
- b.
Consider the benefit of an otherwise inaccessible treatment for an individual patient versus the risk of undergoing surgery or receiving corticosteroids, bearing in mind the unknown effects of the newer therapies on the course of COVID-19 and the risk of on-site visits.
- c.
Try to implement telematic visits.
- d.
Bear in mind the potential need to unblind treatment arms in blind studies in case of COVID-19.
- e.
Consider reducing and facilitating bureaucratic aspects using sponsor amendments given the care burden and activity reassignments of participating physicians.
Probably, from a healthcare perspective, managing endoscopy units is the most challenging activity of any gastroenterology department. Patient presence is mandatory, work there involves risk for both patients and care providers (and society at large), and no published or accessible protocols deal with activity resumption.
On the way back to normalization, we must bear in mind that the latter does not result from overcoming the pandemic but rather a reduction in infection rate allowing to decrease hospital overload, which may in turn permit a recovery of regulated activity. Therefore, one must at all times recall that the risk of infection may persist both for patients and care workers. In general, social distancing and the use of adequate personal protective equipment must be maintained. It is crucial that consideration be given to the amount of hospital resources devoted to caring for patients with COVID-19, and how their recovery for the care of non-COVID patients is anticipated. When opening agenda windows the hospital's contingency plan to reclaim non-COVID areas as COVID areas should a new peak occur has to be taken into consideration. Furthermore, the number of professionals available at the unit itself and their risk of infection must also be weighed up. A key point is the need for endoscopy units to have available all the materials necessary for a potential increase in activity (at least 3 PPEs, for physician, nurse, and assistant, per procedure (4 if additional staff is required: anesthetist, nurse, etc.). Without this minimum of materials (defined below in the present document) no endoscopic procedure should be performed.
Care activity in endoscopyIn preparation for activity resumption we should bear in mind a number of variables:
- (1)
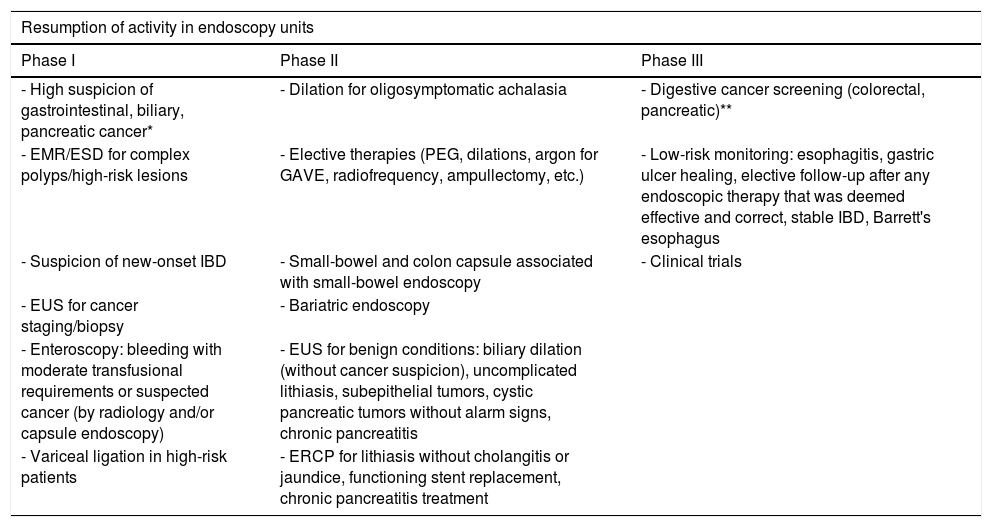
Type of procedure. It is key that the use of the various types of endoscopic procedure be defined according to the different scenarios brought about by the SARS-CoV-2 pandemic. Considering that procedures deemed to be urgent have been maintained during the most problematic phase (Table 2),29 we have established three well-differentiated phases for procedure performance according to each endoscopy unit reactivation phase, and the circumstances surrounding infection with SARS-CoV-2 (Table 3).29 Transitions between phases will be directly dependent on the situation in each hospital regarding the SARS-CoV-2 pandemic.
- a.
Phase I: initiating the recovery of regulated activity. Part of this activity will be conditioned by bed availability for urgent (complications) or elective (ERCP, stent placement, etc.) hospitalization, as well as activities related to other undelayable services (surgery, oncology).
- b.
Phase II: normalization of regulated activity. Here elective admission for delayable activities would be possible.
- c.
Phase III: normality is fully recovered. Low-risk screening and follow-up protocols are reinstated.
Table 2.Urgent, undelayable indications during the SARS-CoV-2 pandemic.
Undelayable endoscopy indications - Unstable GI bleeding and/or high transfusional requirements amenable to endoscopic therapy - Acute esophageal obstruction (foreign bodies, punctiform stricture, cancer where a stent is required) - Endoscopic therapy for perforations/leaks - ERCP (± EUS) for acute cholangitis/jaundice secondary to malignant/benign biliary obstruction - ERCP (± EUS) for acute biliary pancreatitis and/or cholangitis with stones and jaundice - Infected pancreatic collections/WON - Nutritional support that is deemed urgent for an inpatient (PEG/NJT) - Gastrointestinal obstruction, for decompression and stent placement Table 3.Indications during the phases of resumption of activities in endoscopy units.
Resumption of activity in endoscopy units Phase I Phase II Phase III - High suspicion of gastrointestinal, biliary, pancreatic cancer* - Dilation for oligosymptomatic achalasia - Digestive cancer screening (colorectal, pancreatic)** - EMR/ESD for complex polyps/high-risk lesions - Elective therapies (PEG, dilations, argon for GAVE, radiofrequency, ampullectomy, etc.) - Low-risk monitoring: esophagitis, gastric ulcer healing, elective follow-up after any endoscopic therapy that was deemed effective and correct, stable IBD, Barrett's esophagus - Suspicion of new-onset IBD - Small-bowel and colon capsule associated with small-bowel endoscopy - Clinical trials - EUS for cancer staging/biopsy - Bariatric endoscopy - Enteroscopy: bleeding with moderate transfusional requirements or suspected cancer (by radiology and/or capsule endoscopy) - EUS for benign conditions: biliary dilation (without cancer suspicion), uncomplicated lithiasis, subepithelial tumors, cystic pancreatic tumors without alarm signs, chronic pancreatitis - Variceal ligation in high-risk patients - ERCP for lithiasis without cholangitis or jaundice, functioning stent replacement, chronic pancreatitis treatment - a.
- (2)
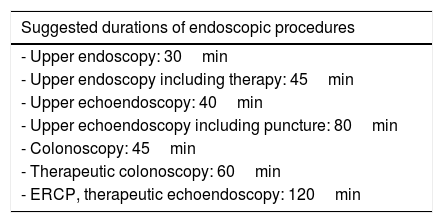
Schedule readjustment strategy. A key point is the adjustment of the appointment schedule, which depends on the duration of endoscopic procedures (Table 4). At present the times necessary to change clothes, clean instruments and room, disinfect, avoid waiting room overcrowding, etc., should be estimated. Our proposal, considering transmisison risks and the need for cleaning and/or protective measures in case of examining a high-risk patient, is as follows29:
- a.
Phase I: attempt to reach 50% of usual activity at the endoscopy unit.
- b.
Phase II and III: attempt to reach 75% of usual activity at the endoscopy unit.
Table 4.Intervals between endoscopic procedures according to EFICAD.
Suggested durations of endoscopic procedures - Upper endoscopy: 30min - Upper endoscopy including therapy: 45min - Upper echoendoscopy: 40min - Upper echoendoscopy including puncture: 80min - Colonoscopy: 45min - Therapeutic colonoscopy: 60min - ERCP, therapeutic echoendoscopy: 120min In order to define intervals between procedures when examining high-risk patients at least 45min should be added to the established duration according to the EFICAD study (Table 4).
- a.
- (3)
Patient management:
- a.
Appointments: patients will be called up on the day before endoscopy to fill out a risk checklist, which will be done again on the day of the procedure. The ideal scenario we must doubtless pursue is the running of a SARS-CoV-2 test in all patients before the treatment (see above).30,31
- b.
Access to the endoscopy unit: a surgical mask and gloves (or hydroalcoholic solution for hand washing) will be placed on the patient, and temperature will be measured.
- c.
The patient shall attend at most with one companion, who will not enter the unit unless the patient requires specific help. Social distancing is key.
- a.
- (1)
Risk. The risk of infection spread is dependent upon the potential risk of patient infection and the type of endoscopic procedure.
- •
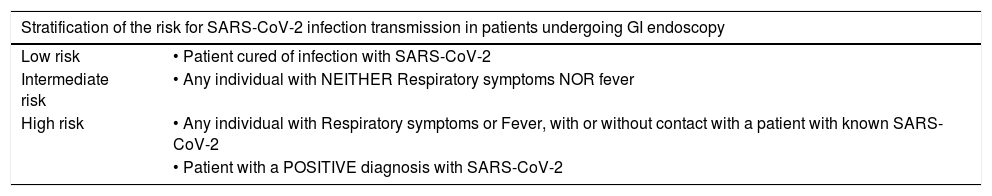
Risk by patient type. The risk of transmission by patient type may be seen in Table 5.
Table 5.Stratification of the risk for SARS-CoV-2 transmission according to type of patient scheduled for GI endoscopy.
Stratification of the risk for SARS-CoV-2 infection transmission in patients undergoing GI endoscopy Low risk • Patient cured of infection with SARS-CoV-2 Intermediate risk • Any individual with NEITHER Respiratory symptoms NOR fever High risk • Any individual with Respiratory symptoms or Fever, with or without contact with a patient with known SARS-CoV-2 • Patient with a POSITIVE diagnosis with SARS-CoV-2 - •
Risk by procedure type. Two kinds of procedure must be differentiated according to their potential to generate aerosols32:
- i.
Aerosol-generating procedures, namely those involving upper endoscopy (ERCP, gastroscopy, upper echoendoscopy, upper enteroscopy); these are deemed to be high-risk. When possible, sedation must be used for all upper examinations in order to reduce the risk for aerosol formation.
- ii.
Non-aerosol generating procedures, namely those involving lower endoscopy (colonoscopy, lower enteroscopy, lower echoendoscopy) or ostomy; these are deemed to be low-risk.
- i.
- •
- (2)
Protective level
- a.
Some level of protection must be used during access to the endoscopy unit, including common areas (administrative area, corridor, living area, washing area, recovery room, etc.). A mask, body protection with scrubs and overcoat, and hospital-specific footwear. Continuous use of gloves would not be required but regular hand washing would.
- b.
Once in the room the protective level will vary according to the risk allotted to each patient and procedure32–34:
- •
Low risk: surgical mask, face protection (goggles or screen), disposable cap, body protection (scrubs+overcoat±waterproof lab coat), gloves, and hospital-specific closed shoes would be required.
- •
Intermediate risk: N95 (FFP2/FFP3) mask (preferably), face protection (goggles or screen), disposable cap, body protection (scrubs+overcoat±waterproof lab coat), double gloves, and hospital-specific closed shoes with covers would be required.
- •
High risk: N95 (FFP2/FFP3) mask, face protection (goggles or screen), disposable cap, body protection (scrubs+overcoat+waterproof lab coat), double gloves, and hospital-specific closed shoes with covers would be required.
- •
- a.
- (3)
Place where endoscopic procedures are undertaken.
- a.
Initially, endoscopic procedures should be carried out in the usual manner, within the GI endoscopy area, with the necessary precautions.
- b.
Should a procedure be performed in a high-risk patient (SARS-CoV-2, confirmed or suspected), two options are currently available according to the infrastructure and their availability in each center:
- –
Perform the endoscopic study in a hospital-designated room (usually in the surgical area).
- –
Perform the endoscopic study within the endoscopy unit. In this case, it would be advisable to designate one specific room for these patients, with the procedures being undertaken at the end of the agenda in order to allow time and resources to clean the specific room.
- –
- a.
- a.
The recommendation by scientific societies is that endoscopes and reusable expendable materials undergo the standard reprocessing and disinfection procedure with bactericidal, mycobactericidal, fungicidal, and virucidal properties, which will minimize transmission risk for any type of virus.
- b.
Channel cleaning brushes must be single-use, and plastic connections to aspiration must be disposed of.
- c.
Endoscopes must travel to cleaning areas in a closed container (e.g., plastic bag); upon entering the disinfection room they must undergo immediate manual washing before entering the automated washing system.
- d.
Surfaces having been in contact with patients or their secretions and with the staff must be cleaned and disinfected with bleach or sodium hypochlorite solution containing 1000ppm of active chlorine.
- e.
Residues shall be disposed of and managed according to the relevant regulations in force, and a differentiated circuit should be available for this purpose.34–36
With telematic and phone-based care, during the present pandemic we have learned that patient acceptance is very high and their response to disease has changed. Patients are now more reluctant to undergo procedures unless they are absolutely necessary. Hence, the possibility and/or need emerges to clear the scheduled wait list. In this context patients could be contacted before their assigned appointment by a unit physician to assess their need for the scheduled procedure according to indication and clinical status. The possibility that the procedure could be delayed because of the pandemic should be laid out for their consideration.
HepatologyRecommendations in case of patients with stable chronic liver disease (Table 6)There is no evidence that patients with stable chronic liver disease of any origin will be more susceptible to infection with SARS-COV-2, even though many of them have comorbidities such as hypertension and diabetes mellitus, which are associated with greater severity, particularly in patients with advanced fat deposition disease.37
- a.
Once started, the treatment of patients with B or C must be maintained, with maximum intervals between visits. Delaying treatment for newly diagnosed patients with hepatitis C seems reasonable except for decompensated cases. In patients where antiviral therapy was started efforts should be made to deliver medications to their usual pharmacy or their homes, with doses for at least 8–12 weeks.
- b.
In case of established autoimmune disease reducing immunosuppressive therapy is not advisable except for special considerations. In newly diagnosed autoimmune hepatitis cases treatment may be started with steroids, particularly in patients with moderate to severe disease. In mild cases delaying treatment onset for a few weeks should be considered, with azathioprine being held back for as long as possible.
- c.
Since infection with SARS-CoV-2 may alter LFTs, it is sensible to rule out this infection in the presence of a compatible clinical picture.
- d.
Cirrhotic patients must be screened for HCC, but minimally increasing monitoring intervals seems a reasonable thing to do.
- e.
In patients with chronic liver disease on treatment for infection with SARS-CoV-2 liver function must be monitored as there is a risk of hepatotoxicity, usually mild, associated with drugs such as remdesivir and tocilizumab. In these patients, their use should be warned off outside of clinical trials.
- f.
In our opinion, portal hemodynamics measurements should only be carried out during the first phase for patients with hepatocellular carcinoma where knowledge of the portal pressure gradient may result in therapy changes; transjugular biopsy may be advisable in case of acute liver failure. TIPS placement may only be recommended in the setting of a life-threatening emergency.
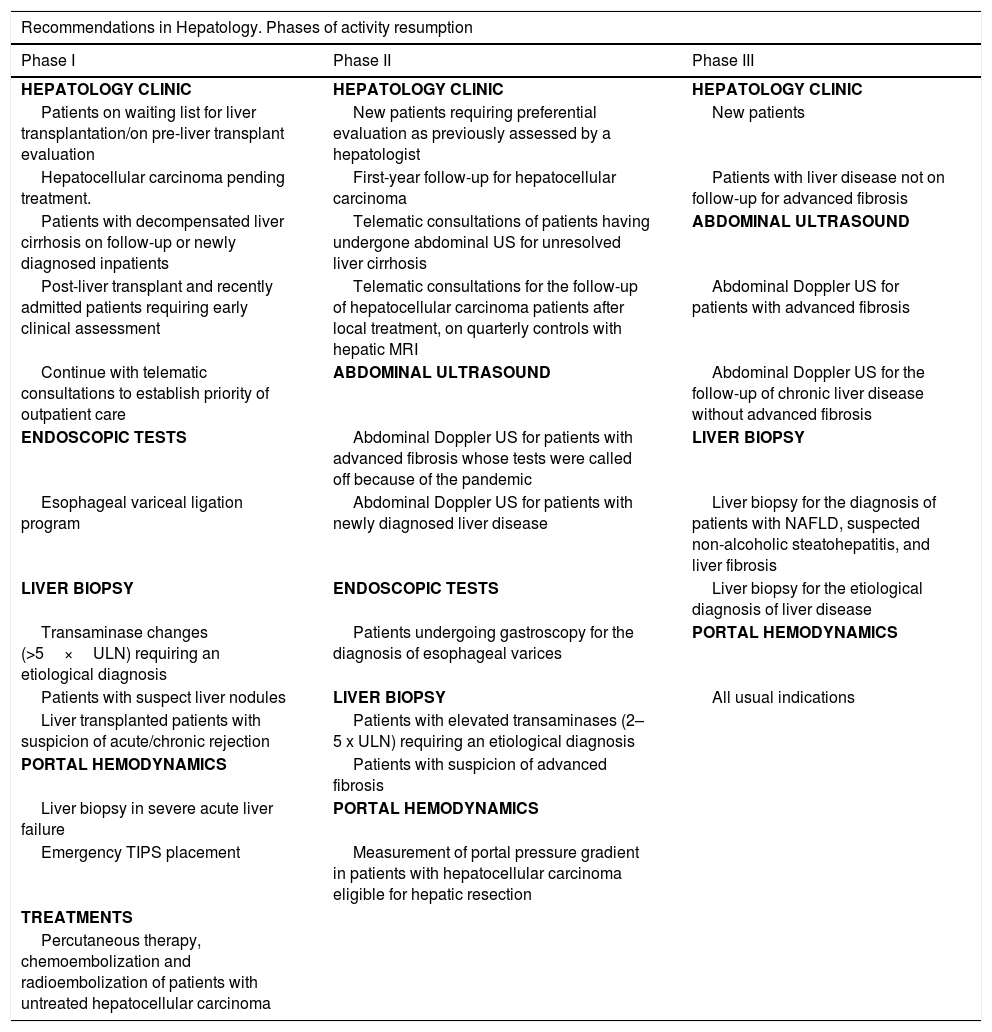
Recommendations for resumption of activities in hepatology units.
| Recommendations in Hepatology. Phases of activity resumption | ||
|---|---|---|
| Phase I | Phase II | Phase III |
| HEPATOLOGY CLINIC | HEPATOLOGY CLINIC | HEPATOLOGY CLINIC |
| Patients on waiting list for liver transplantation/on pre-liver transplant evaluation | New patients requiring preferential evaluation as previously assessed by a hepatologist | New patients |
| Hepatocellular carcinoma pending treatment. | First-year follow-up for hepatocellular carcinoma | Patients with liver disease not on follow-up for advanced fibrosis |
| Patients with decompensated liver cirrhosis on follow-up or newly diagnosed inpatients | Telematic consultations of patients having undergone abdominal US for unresolved liver cirrhosis | ABDOMINAL ULTRASOUND |
| Post-liver transplant and recently admitted patients requiring early clinical assessment | Telematic consultations for the follow-up of hepatocellular carcinoma patients after local treatment, on quarterly controls with hepatic MRI | Abdominal Doppler US for patients with advanced fibrosis |
| Continue with telematic consultations to establish priority of outpatient care | ABDOMINAL ULTRASOUND | Abdominal Doppler US for the follow-up of chronic liver disease without advanced fibrosis |
| ENDOSCOPIC TESTS | Abdominal Doppler US for patients with advanced fibrosis whose tests were called off because of the pandemic | LIVER BIOPSY |
| Esophageal variceal ligation program | Abdominal Doppler US for patients with newly diagnosed liver disease | Liver biopsy for the diagnosis of patients with NAFLD, suspected non-alcoholic steatohepatitis, and liver fibrosis |
| LIVER BIOPSY | ENDOSCOPIC TESTS | Liver biopsy for the etiological diagnosis of liver disease |
| Transaminase changes (>5×ULN) requiring an etiological diagnosis | Patients undergoing gastroscopy for the diagnosis of esophageal varices | PORTAL HEMODYNAMICS |
| Patients with suspect liver nodules | LIVER BIOPSY | All usual indications |
| Liver transplanted patients with suspicion of acute/chronic rejection | Patients with elevated transaminases (2–5 x ULN) requiring an etiological diagnosis | |
| PORTAL HEMODYNAMICS | Patients with suspicion of advanced fibrosis | |
| Liver biopsy in severe acute liver failure | PORTAL HEMODYNAMICS | |
| Emergency TIPS placement | Measurement of portal pressure gradient in patients with hepatocellular carcinoma eligible for hepatic resection | |
| TREATMENTS | ||
| Percutaneous therapy, chemoembolization and radioembolization of patients with untreated hepatocellular carcinoma | ||
In this scenario, where a high number of patients require admission and intensive care, medical resources are diverted from the care for other patients, including patients with cancer. It is unclear whether liver cancer patients are more susceptible to COVID-19 or have more severe SARS-CoV-2 manifestations, but both situations have been described for patients with other solid tumors, particularly when receiving chemotherapy.38 We specifically recommend:
- a.
Consider surgery and transplantation for these patients according to resource availability in each center. In centers where these procedures are to be delayed bridge therapies such as ablation or intra-arterial treatment (TACE, radioembolization) may be used.
- b.
Ablation or intra-arterial procedures, while potentially limited by the availability of anesthesiologists or hospitalization beds, usually require short stays and no intensive care, the latter likely being the most critically scarce resource during this pandemic. Nevertheless, candidate selection shall be rigorous to eliminate patients with higher risk for complications.
- c.
Patients should only be included in clinical trials when no other therapeutic option is available. Hence, among advanced cases candidate to systemic therapy, only those on second-line treatment should be enrolled. Sorafenib or lenvatinib should be used as first line.
- d.
Virtual interdisciplinary committees should be maintained to ensure decision making, often removed from usual practice during the present crisis.
Information about the impact of SARS-CoV-2 infection on patients with decompensated cirrhosis or on the transplant waiting list is very limited. Many units found themselves forced to perform no transplantations during this period of time because of lacking resources. Efforts should be made to normalize this situation as soon as resources in each hospital return to normal. In this setting transplant units must maintain an ongoing assessment of the patients on their wait list, particularly in cases with high MELD for tumor progression, considering their risk-benefit ratio. It is acceptable that in selected stable cases transplantation be postponed in spite of available donors.
Recommendations in case of patients with liver transplantImmunocompromised patients might be more susceptible to SARS-CoV-2 infection, even though solid evidence is lacking in this respect. However, some data suggest that immune response is a key factor in pulmonary involvement, and so immunosuppression may even be protective.39–41 In fact, post-transplant immunosuppression has not represented a risk factor for mortality during the SARS or MERS C coronavirus pandemic.40 Our recommendations include:
- a.
Reducing immunosuppression in patients without SARS-CoV-2 infection is not indicated, but they should be instructed to minimize infection risk by prolonging confinement beyond official recommendations, and above all maximizing hand hygiene and social distancing.
- b.
In patients with mild infection of the upper airways with SARS-CoV-2 (fever, cough, no infiltrates on X-rays) a reduction or discontinuation of mycophenolate mofetil or everolimus should be considered, and calcineurin inhibitors (tacrolimus or cyclosporine) should be reduced to the lowest reasonable level.
- c.
In patients with SARS-CoV-2 pneumonia (with or without severity criteria) it is advisable to reduce or discontinue mycophenolate or everolimus doses, and to discontinue or reduce calcineurin inhibitors to the lowest reasonable level.
- d.
The use of azithromycin and LPV/r involves a high risk of drug-drug interactions and requires close monitoring. Particularly significant is the interaction between LPV/r and calcineurin and/or mTOR inhibitors (everolimus, silorimus). Cyclosporine, tacrolimus, sirolimus, or everolimus levels may increase significantly in case of coadministration with LPV/r, hence transient discontinuation is advisable with trough-level monitoring after 48–72h followed by dose adjustments. LPV/r also increases systemic corticosteroid levels, and may modufy mycophenolate mofetil levels since both compounds may inhibit glucuronidation. For appropriately dosing these drugs we recommend visiting COVID-19 (www.covid19-druginteractions.org), HIV (www.hiv-druginteractions.org) or general (https://reference.medscape.com/drug-interactionchecker) online resources.42
- e.
In case of acute rejection usual treatment may be started including high-dose steroids.42
- f.
Whenever possible early discharge should be favored, as well as monitoring through home hospitalization.
- g.
Population screening for COVID-19 in asymptomatic liver transplant patients. It may start with measuring antibodies (IgM and IgG) in capillary blood (rapid test, see above); however, we have no information regarding their behavior in immunosuppressed patients such as transplant recipients.
In case the LT program was interrupted and resumption is under consideration, its restart will be subjected to the availability of an adequate number of ICU/CCRU beds and COVID-19-free hospitalization areas. The course of the pandemic shall always be taken into account.
- •
Regarding donation, we shall preferentially use excellent donors with no risk factors whatsoever for COVID-19.
- •
In case of an offer, a coordinator will interview the potential recipient over the phone to assess the presence of COVID-19.
- •
Performing an RT-PCR test on nasopharyngeal exudate samples from both donor and recipient is key to rule out COVID-19; also a pulmonary assessment of the recipient should be undertaken (this will be according to each transplant group). In all cases a second recipient should be ready for the procedure.
- •
When possible, acording to each center, the candidate recipient should undergo testing for measuring antibodies (IgM and IgG) in capillary blood, which will supplement the information obtained with the RT-PCR. This obviously extends and complicates logistics, but is indispensable.
- •
Confirmed COVID-19 cases must be excluded as donors until at least 21 days after symptom disappearance and therapy completion. Cases deemed cured are described in the ONT document of April 13, 2020,42 as well as other technical issues whose review we deem advisable.
- •
Interpreting RT-PCR and serological test results:
- ∘
Trasplantation contraindicated:
- •
RT-PCR positive. Active infection confirmed.
- •
RT-PCR positive, IgM positive, IgG negative or positive. Active infection.
- •
RT-PCR negative and IgM serology positive: likely a false negative result of RT-PCR because of no longer detectable viral load in upper airway samples when collecting lower airwy samples is unsafe.
- •
When a chest CT scan is performed in a recipient candidate, and it is suggestive of pneumonia by COVID-19.
- •
Any combination of the above.
- •
- ∘
Liver transplantation possible
- •
RT-PCR negative, IgG negative, IgM negative. Patients with no previous exposure to SARS-CoV-2. Potential false negative result (uncommon, consider other diagnostic tests).
- •
RT-PCR negative, IgM negative, IgG positive. Prior infection with SARS-CoV-2.
- •
- ∘
The recommendations expressed in this document are aimed at helping departments in the resumption of their usual healthcare activity, which has been almost completely postponed in some of them. We are facing a changing reality that demands considerable plasticity of all of us; a relevant part of our way of working will change, and we must play a leading role in this process. For the above recommendations we have relied on pragmatism, although the scarce, changing evidence available will require future updates. The start of this journey toward a changing normality in every department will depend on the cumulative incidence of SARS-CoV-2 infection in each region, as well as on the burden the pandemic has inflicted on each hospital.
This article is published simultaneously in [The Spanish Journal of Gastroenterology, DOI: 10.17235/reed.2020.7141/2020] and [Gastroenterology and Hepatology, DOI: 10.1016/j.gastre.2020.04.001], with the consent of the authors and editors.