Oral budesonide is a glucocorticoid of primarily local action. In the field of digestive diseases, it is used mainly in inflammatory bowel disease, but also in other indications. This review addresses the pharmacology, pharmacodynamics and therapeutic use of budesonide. Its approved indications are reviewed, as well as other clinical scenarios in which it could play a role, in order to facilitate its use and improve the accuracy of its prescription.
La budesonida oral es un glucocorticoide de acción fundamentalmente local. En la especialidad de Aparato Digestivo, se emplea sobre todo en la enfermedad inflamatoria intestinal, aunque también en otras indicaciones. Esta revisión aborda aspectos acerca de la farmacología, la farmacodinámica y el empleo terapéutico de la budesonida. Se contemplan sus indicaciones reconocidas y se especula acerca de otras situaciones en las que podría desempeñar un papel de interés, con el objeto de facilitar su uso y mejorar la exactitud de su prescripción.
Budesonide (BUD) is the only recognised pharmacological alternative for the treatment of mild, active ileal or ileocolic Crohn's disease (CD). Despite this, in the 2 decades since the publication of the controlled trials that led to its approval for this indication, relatively few publications or conferences have focused on updating its use in CD. Its idiosyncrasies (indication according to location and inflammatory activity of the disease, steroid with an optimised safety profile) make it a rara avis in the therapeutic arsenal of CD, particularly in the era of selective immunosuppressants (IS). In view of this situation, in 2016 a group of experts in CD met to reassess the role of BUD in the management of CD. In a second meeting held in 2017, we decided to prepare a document addressing issues surrounding both the indications (approved, suggested and potential) and mode of use (dosage, regimens, use of mineral and vitamin supplements) of BUD in inflammatory bowel disease (IBD) in order to bring gastroenterologists up to date with the latest evidence.
The aim of this article is to review the pharmacological characteristics of BUD and its accepted indications for the management of CD, and to evaluate treatment regimens and clinical situations for potential use based on the available evidence and expert opinion.
Description and pharmacological properties of budesonideBUD is considered the prototype “second generation” topical glucocorticoid. It is characterised by greater potency and lower systemic bioavailability, and is the most widely studied therapeutic option in IBD.1 Its affinity for glucocorticoid receptors is 195 times greater than hydrocortisone and 15 times greater than prednisolone.2,3 This means that 5mg BUD is therapeutically equivalent to 12mg prednisolone.4 Its rapid elimination, thanks to a 90% first-pass hepatic metabolism, results in a low systemic bioavailability which reduces its adverse effects (AE),5 something that does not occur in patients with portosystemic shunt, such as those with portal hypertension. Its metabolites are mainly excreted in the urine, and, to a lesser extent, in the faeces.6–8
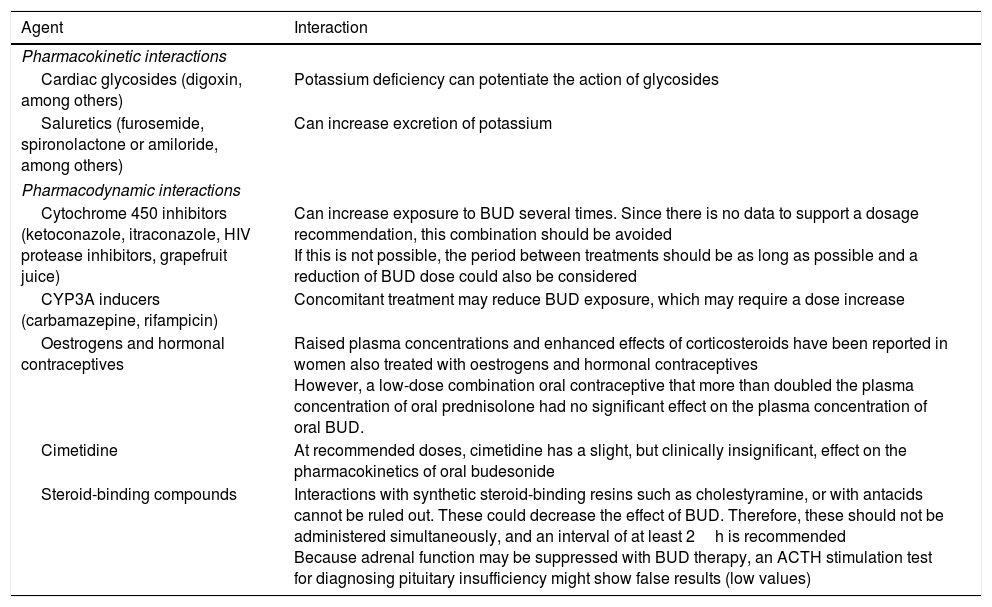
The metabolism of BUD, which is mainly CYP3A4-mediated, can be affected by several factors that interfere with its clearance and systemic bioavailability (Table 1).
Known pharmacological interactions with oral budesonide.
| Agent | Interaction |
|---|---|
| Pharmacokinetic interactions | |
| Cardiac glycosides (digoxin, among others) | Potassium deficiency can potentiate the action of glycosides |
| Saluretics (furosemide, spironolactone or amiloride, among others) | Can increase excretion of potassium |
| Pharmacodynamic interactions | |
| Cytochrome 450 inhibitors (ketoconazole, itraconazole, HIV protease inhibitors, grapefruit juice) | Can increase exposure to BUD several times. Since there is no data to support a dosage recommendation, this combination should be avoided If this is not possible, the period between treatments should be as long as possible and a reduction of BUD dose could also be considered |
| CYP3A inducers (carbamazepine, rifampicin) | Concomitant treatment may reduce BUD exposure, which may require a dose increase |
| Oestrogens and hormonal contraceptives | Raised plasma concentrations and enhanced effects of corticosteroids have been reported in women also treated with oestrogens and hormonal contraceptives However, a low-dose combination oral contraceptive that more than doubled the plasma concentration of oral prednisolone had no significant effect on the plasma concentration of oral BUD. |
| Cimetidine | At recommended doses, cimetidine has a slight, but clinically insignificant, effect on the pharmacokinetics of oral budesonide |
| Steroid-binding compounds | Interactions with synthetic steroid-binding resins such as cholestyramine, or with antacids cannot be ruled out. These could decrease the effect of BUD. Therefore, these should not be administered simultaneously, and an interval of at least 2h is recommended Because adrenal function may be suppressed with BUD therapy, an ACTH stimulation test for diagnosing pituitary insufficiency might show false results (low values) |
BUD is marketed in different formulations: as a nasal spray, oral tablets, or suppositories.9,10 In gastrointestinal disease, delayed-release tablets and suppositories are used to treat various intestinal diseases, including IBD and microscopic colitis, as well as autoimmune hepatitis. Orodispersible tablets are indicated to treat eosinophilic oesophagitis,9,10 although they are not currently available in Spain.
BUD is indicated to induce remission in patients with mild or moderate CD that affects the ileum, the ascending colon, or both. For this purpose, it is presented as enteric-coated (ethylcellulose matrix) pH-dependent modified release capsules that dissolves at a pH>5.5.7,8,11 This enables most of the drug (59–68%) to be absorbed in the ileum and caecum.7,8,11 The recommended daily dose to induce remission in adults is 9mg, administered in a single morning dose for up to 8 weeks, reaching its peak effect within 2–4 weeks.7,8 In Spain, BUD for this indication (Entocort® and Budenofalk®) is available in 2 formulations, which differ only in 2 aspects: start of release, which in the case of Entocort® is more proximal (although peak release occurs in both simultaneously); and Entocort® does not contain lactose, while Budenofalk® does.7,8 BUD is not yet marketed in a multi-matrix (MMX) formulation in Spain.
Table 2 summarises the existing recommendations for the use of BUD in IBD in the principle guidelines.12–18
Guideline recommendations for the use of budesonide as induction therapy in inflammatory bowel disease (ileal-ileocaecal Crohn's disease, ulcerative colitis, ulcerative proctitis).
| Guideline | Recommendation/comment |
|---|---|
| Adult guidelines | |
| British Society of Gastroenterology IBD guidelines Carter et al., 200412 | BUD has therapeutic benefit with reduced systemic toxicity in ileocaecal CD, or UC BUD is slightly less effective than prednisolone, but is an appropriate alternative for active ileo-ascending colonic disease BUD 9mg daily is appropriate for patients with isolated ileo-caecal disease with moderate disease activity, but marginally less effective than prednisolone (grade A) |
| CD guidelines American College of Gastroenterology. Lichtenstein et al., 200913 | Mild to moderate CD: BUD is more effective than mesalazine or conventional steroids (grade A). BUD (9mg/day) is effective when active disease is confined to the ileum and/or right colon (grade A) A clinical trial assessing the combination of ciprofloxacin (1g/day) and metronidazole (1g/day) in addition to budesonide (9mg/day) failed to demonstrate an additional benefit for patients receiving concomitant antibiotics despite a “trend” in post hoc analysis in favour of the supplemental antibiotics for patients with colonic disease Mild to moderate CD affecting the ileum or right colon: BUD 9mg/day has proven to be the best combination of short-term efficacy and safety in a series of well-controlled clinical trials. BUD is recommended for use as the preferred primary therapy in these patients |
| 3rd ECCO Consensus on CD Gomollón et al., 201614 | Mild ileocaecal CD: Oral BUD is the recommended treatment BUD 9mg daily is the favoured therapy to induce remission in mildly active, localised ileocaecal CD, because it is superior to placebo (RR 1.93, 95% CI 1.37–2.73). Although it is inferior to conventional steroids (RR 0.85, 95% CI 0.75–0.97), especially if severe disease (CDAI>300) is present (RR 0.52, 95% CI 0.28–0.95), it has fewer AEs (RR 0.64, 95% CI 0.54–0.76). Fifty to sixty percent of patients with mild ileocaecal CD attain remission at 8 weeks on BUD. A Bayesian meta-analysis confirmed that BUD should be the preferred option in mild/moderate ileocaecal CD. Moderate CD: neither BUD nor prednisolone are appropriate as initial induction therapy. Prednisolone is very effective, although with more AEs than BUD. BUD 9mg has consistently shown benefits for active ileal or ileocolic CD, but is less effective than prednisolone, especially in severe cases. Ileal release BUD has no role in treating colonic disease, unless it primarily affects the proximal colon. BUD is advocated in preference to prednisolone in ileal or ileocaecal Crohn's disease BUD is still associated with AEs typical of steroids at a lower or similar frequency, although they are less severe than those caused by prednisolone. BUD causes less reduction in bone mineral density than prednisolone (mean −1.04% vs. −3.84% over 2 years in a randomised study of 272 patients, p=0.0084) |
| IBD Consensus of the Pan American Crohn's and Colitis Organisation Yamamoto-Furusho et al., 201715 | BUD 9mg/day is recommended as a first-choice treatment of mild localised ileocaecal CD. Level of evidence: 1a. Level of agreement: 82% Remission rate with BUD: 51–60% in 8–10 weeks BUD has been shown to be superior to placebo (RR 1.96, 95% CI: 1.19–3.23) and mesalazine (RR 1.63, 95% CI: 1.23–2.16). BUD is preferred over prednisolone because it is associated with minor AEs (RR 0.64, 95% CI: 0.28–0.95) BUD is an alternative for patients with chronic pouchitis (>4 weeks) who do not respond to antibiotic treatment. Level of evidence: 2. Level of agreement: 91% In UC, BUD multimatrix (MMX), is indicated for inducing remission in patients with mild-to-moderately active UC of any extension that is resistant to aminosalicylates. This can be tried before the use of systemic steroids. Level of evidence: 1. Level of agreement: 91% Randomised controlled studies with oral budesonide MMX have shown it to be more effective than placebo, and to be as effective as oral 5-ASAs for inducing remission. However, this has not been demonstrated with other formulations of ileal-release budesonide, such as Entocort® and Budenofalk®, whose effectiveness was lower than placebo and 5-ASAs Compared with conventional steroids, BUD has fewer systemic adverse events (33% vs. 55%) and it has not been associated with a significant decrease in bone mineral density Rectal steroids are suggested as second-line therapy for inducing complete remission in patients with mild-to-moderate ulcerative proctitis who do not respond to topical 5-ASAs. Level of evidence: 1b. Level of agreement: 91% |
| French national CD consensus. Peyrin-Biroulet et al., 201716 | Indications in first line of treatment with BUD: a) CD with mild activity; b) CD with moderate uncomplicated activity without poor prognosis factors. Definition of poor prognosis factors: elevated gastrointestinal damage, small bowel damage, severe ileal involvement, severe rectal involvement, perianal disease, severe endoscopic lesions (large or deep ulcers) and a young age at diagnosis |
| Paediatric guidelines | |
| Systematic review of paediatric IBD. British Society of Paediatric Gastroenterology, Hepatology and Nutrition. Wilson et al., 201017 | Studies: Levine 2003 (mild/moderate, 58% ileocaecal, 27% ileocolic, 15% colitis, BUD 19, prednisolone 14): 9mg/day BUD 8 weeks, followed by tapering over 10 weeks. Results: remission at 12 weeks: BUD 47%, prednisolone 50%. AE: BUD 32%, prednisolone 71% (NS, p<0.05) Escher 2004 (ileal or ascending colon CD; BUD 22–9mg 8 weeks, followed by 6mg, prednisolone 1mg/kg 4 weeks, followed by dose reduction): terminated prematurely, with fewer AEs and higher morning values of cortisol with BUD. Results: remission BUD 55%, prednisolone 71% Kundhal 2001 (CD: BUD 32, ileal 23, ileocaecal 9): BUD 9mg/day 8 weeks, followed by 6mg/day. No significant remission rates Levine 2001 (mild/moderate CD without excluding distal colonic involvement, BUD 62, prednisolone 58): BUD 0.45mg/kg/day, maximum 9mg/day. Results: remission BUD 48%, prednisolone 77%. One case of cranial hypertension with BUD |
| BSPGHAN IBD Working Group. Kammermeier et al., 201618 | For mild/moderate ileocaecal Crohn's disease, BUD is an alternative option with fewer steroid-related AEs (maximum: 12mg/day, tapering over 2–4 weeks) |
5-ASA: 5-aminosalicylic acid; ACG: American College of Gastroenterology; BUD: budesonide; CD: Crohn's disease; IBD: inflammatory bowel disease; RR: relative risk.
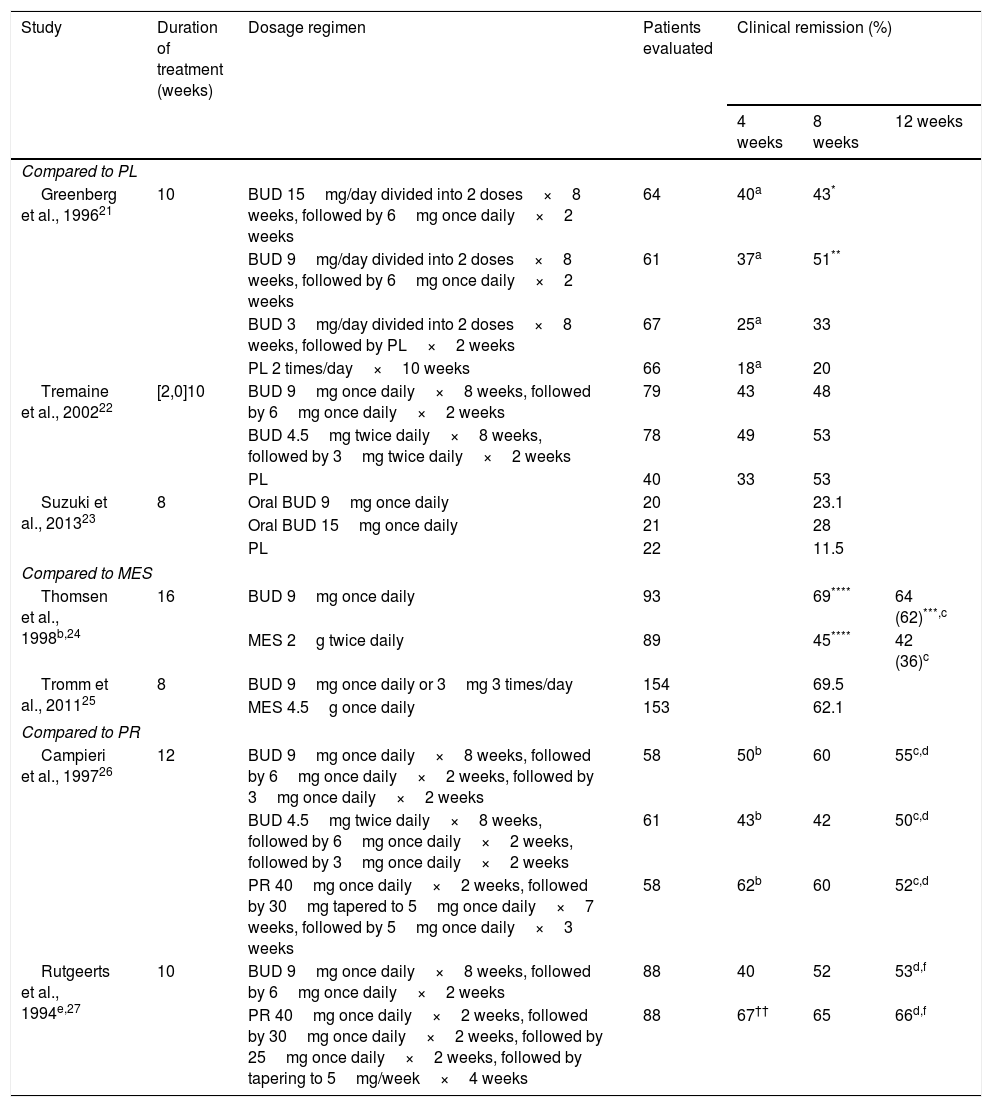
Several controlled clinical trials have shown that BUD is superior to placebo in inducing remission of CD, and equivalent to prednisolone for the control of CD of right-sided ileal or ileocolic involvement, and mild or moderate activity.6 Although BUD is less effective in the short-term than conventional steroids, particularly in patients with severe disease or more extensive colonic involvement, there is less likelihood of AEs and adrenal suppression with BUD.19 The most important controlled trials in induction of clinical remission in CD with BUD are shown in Table 3.
Principle controlled studies performed with budesonide in adults with Crohn's disease.
| Study | Duration of treatment (weeks) | Dosage regimen | Patients evaluated | Clinical remission (%) | ||
|---|---|---|---|---|---|---|
| 4 weeks | 8 weeks | 12 weeks | ||||
| Compared to PL | ||||||
| Greenberg et al., 199621 | 10 | BUD 15mg/day divided into 2 doses×8 weeks, followed by 6mg once daily×2 weeks | 64 | 40a | 43* | |
| BUD 9mg/day divided into 2 doses×8 weeks, followed by 6mg once daily×2 weeks | 61 | 37a | 51** | |||
| BUD 3mg/day divided into 2 doses×8 weeks, followed by PL×2 weeks | 67 | 25a | 33 | |||
| PL 2 times/day×10 weeks | 66 | 18a | 20 | |||
| Tremaine et al., 200222 | [2,0]10 | BUD 9mg once daily×8 weeks, followed by 6mg once daily×2 weeks | 79 | 43 | 48 | |
| BUD 4.5mg twice daily×8 weeks, followed by 3mg twice daily×2 weeks | 78 | 49 | 53 | |||
| PL | 40 | 33 | 53 | |||
| Suzuki et al., 201323 | 8 | Oral BUD 9mg once daily | 20 | 23.1 | ||
| Oral BUD 15mg once daily | 21 | 28 | ||||
| PL | 22 | 11.5 | ||||
| Compared to MES | ||||||
| Thomsen et al., 1998b,24 | 16 | BUD 9mg once daily | 93 | 69**** | 64 (62)***,c | |
| MES 2g twice daily | 89 | 45**** | 42 (36)c | |||
| Tromm et al., 201125 | 8 | BUD 9mg once daily or 3mg 3 times/day | 154 | 69.5 | ||
| MES 4.5g once daily | 153 | 62.1 | ||||
| Compared to PR | ||||||
| Campieri et al., 199726 | 12 | BUD 9mg once daily×8 weeks, followed by 6mg once daily×2 weeks, followed by 3mg once daily×2 weeks | 58 | 50b | 60 | 55c,d |
| BUD 4.5mg twice daily×8 weeks, followed by 6mg once daily×2 weeks, followed by 3mg once daily×2 weeks | 61 | 43b | 42 | 50c,d | ||
| PR 40mg once daily×2 weeks, followed by 30mg tapered to 5mg once daily×7 weeks, followed by 5mg once daily×3 weeks | 58 | 62b | 60 | 52c,d | ||
| Rutgeerts et al., 1994e,27 | 10 | BUD 9mg once daily×8 weeks, followed by 6mg once daily×2 weeks | 88 | 40 | 52 | 53d,f |
| PR 40mg once daily×2 weeks, followed by 30mg once daily×2 weeks, followed by 25mg once daily×2 weeks, followed by tapering to 5mg/week×4 weeks | 88 | 67†† | 65 | 66d,f | ||
Location in ileum or descending colon, analysis by intention to treat and remission rates at the end of treatment unless otherwise indicated. Clinical remission defined as reduction of CDAI to ≤150.
BUD: budesonide; MES: mesalazine; PL: placebo; PR: prednisone.
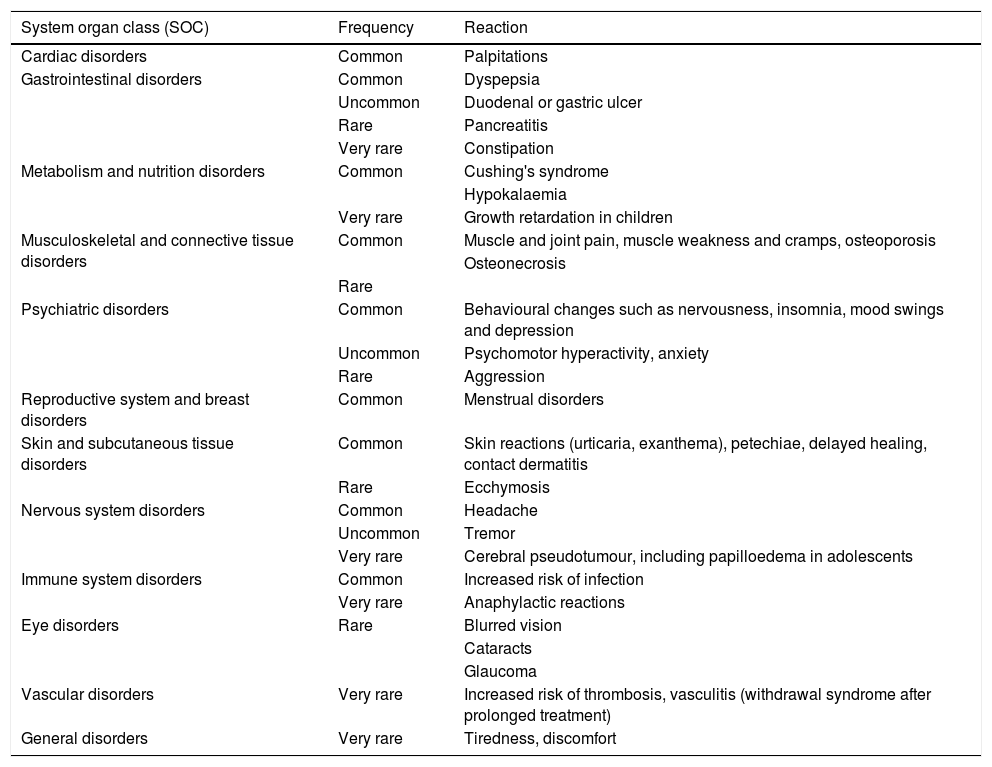
Systemic glucocorticoids are associated with various AEs, ranging from aesthetic changes, such as moon facies, hirsutism and acne, to psychic disturbances (nervousness, insomnia) and even more permanent and serious conditions, such as reduced growth rate, infections, hypertension, diabetes, osteoporosis and glaucoma.28
BUD can present the typical side effects of systemic glucocorticoids (Table 4). However, most of the AEs observed in clinical trials were mild to moderate and non-severe. The availability of BUD in modified-release capsules has reduced the incidence of glucocorticoid-related AEs by approximately half compared to prednisolone at therapeutically equivalent doses.7 In addition, BUD appears to cause less reduction in bone mineral density (BMD) than prednisolone.14,15
Known adverse reactions for oral budesonide indicated for Crohn's disease.
| System organ class (SOC) | Frequency | Reaction |
|---|---|---|
| Cardiac disorders | Common | Palpitations |
| Gastrointestinal disorders | Common | Dyspepsia |
| Uncommon | Duodenal or gastric ulcer | |
| Rare | Pancreatitis | |
| Very rare | Constipation | |
| Metabolism and nutrition disorders | Common | Cushing's syndrome |
| Hypokalaemia | ||
| Very rare | Growth retardation in children | |
| Musculoskeletal and connective tissue disorders | Common | Muscle and joint pain, muscle weakness and cramps, osteoporosis |
| Osteonecrosis | ||
| Rare | ||
| Psychiatric disorders | Common | Behavioural changes such as nervousness, insomnia, mood swings and depression |
| Uncommon | Psychomotor hyperactivity, anxiety | |
| Rare | Aggression | |
| Reproductive system and breast disorders | Common | Menstrual disorders |
| Skin and subcutaneous tissue disorders | Common | Skin reactions (urticaria, exanthema), petechiae, delayed healing, contact dermatitis |
| Rare | Ecchymosis | |
| Nervous system disorders | Common | Headache |
| Uncommon | Tremor | |
| Very rare | Cerebral pseudotumour, including papilloedema in adolescents | |
| Immune system disorders | Common | Increased risk of infection |
| Very rare | Anaphylactic reactions | |
| Eye disorders | Rare | Blurred vision |
| Cataracts | ||
| Glaucoma | ||
| Vascular disorders | Very rare | Increased risk of thrombosis, vasculitis (withdrawal syndrome after prolonged treatment) |
| General disorders | Very rare | Tiredness, discomfort |
The efficacy and safety of BUD in conventional indications has prompted investigators to explore other possible uses of the drug. The following is a list of clinical situations in which administration of BUD could be considered.
Alternative or first choice in particular circumstancesCD can coincide with clinical situations that call for changes in the usual management of the disease, and BUD could be a useful tool in this context. One such situation is previous or current malignant disease. IS are usually avoided in cancer patients because they are believed to have a negative effect on tumour immune surveillance and can therefore increase the risk of cancer recurrence or extension. This is why the use of IS in patients with IBD and cancer, current or in the last 5 years, is highly controversial. In the opinion of the experts, IS should be avoided in these patients during the first 2–5 years after cancer diagnosis. If the disease is active, it should be managed with corticosteroids, enteral nutrition or aminosalicylates.29 In this situation, BUD has the advantage of being more effective than aminosalicylates and can be administered safely for longer than standard corticosteroids.30 In this context, therefore, it should be the first-line treatment in mild to moderate ileal or ileocaecal CD.
Aside from patients with cancer, there are other particular circumstances in which BUD is a very attractive alternative. Traditional IS and corticosteroids are known to increase the risk of infection, particularly in the elderly,31 and should be avoided in children if they are not clearly indicated, such as in mildly active CD. Again, BUD can be a good alternative in these circumstances, since it can be administered for longer periods than conventional corticosteroids and in a much safer manner.
Finally, although there is little information available on the administration of oral BUD during pregnancy, both the data available on inhaled BUD and the European Crohn's and Colitis Organisation (ECCO) guidelines have shown it to be a valid alternative in the treatment of active ileal CD during pregnancy.32–34
PouchitisAcute pouchitis is the most frequent complication in patients with ulcerative colitis that requires total proctocolectomy with ileal pouch-anal anastomosis, and affects up to half of all patients 10 years after surgery.35 Initial treatment is the use of antibiotics, but, despite this, up to 15% of patients antibiotics, but, despite this, up to 15% of patients will develop chronic pouchitis. Few randomised studies have explored this situation, and evidence usually comes from case series (including experience with anti-TNF). As a result, there are few pharmacological options available for this complication. Practically no drugs have shown efficacy in clinical trials, apart from the probiotic VSL#3®, which can prevent new episodes, but this drug is not funded by the Spanish national health system.
Initial evidence for the use of oral BUD in pouchitis comes from a small case series in which 60% of patients responded well to the treatment.36 Two subsequent studies confirmed its effectiveness; the use of 9mg for 8 weeks in pouchitis refractory to antibiotics achieved a clinical and endoscopic remission rate of 75–80%.37,38 An endoscopic remission rate of 72% was also observed in a group of patients with pouchitis associated with primary sclerosing cholangitis, although BUD had no effect on liver function tests.39 It is interesting to note that few BUD-related AEs were reported in all the foregoing series.
It is still to be determined whether topical application of the drug would be more appropriate and effective in this context, or even if combined oral and rectal treatment could be considered.
Bridging therapy for slow-onset drugsCertain drugs, such as thiopurinic IS, have a slow mechanism of action,40 and effectiveness can only be measured after 2–3 months of use. This is also true of new drugs such as vedolizumab, particularly in CD where the possibility of additional doses is considered in week 10 if partial improvement is observed following administration of the standard 0, 2 and 6-week regimen. Starting a course of BUD combination with these drugs could be a safe option for improving symptoms while awaiting for them to take effect, provided the flare-up is not serious and the disease location is indicated for BUD.
Alternative to escalating biological agentsBUD could be an alternative in patients with ileal or ileocaecal CD who present secondary loss of response to anti-TNF drugs. A recent meta-analysis concluded that one third of anti-TNF responders lose response over time.41 There is no consensus regarding the most effective alternative in this situation, and most clinicians shorten the dosage interval or double the dose of the anti-TNF agent. It has also been suggested that the association of an IS can recapture response by reducing the formation of antibodies against anti-TNF drugs, and this synergy between thiopurines and infliximab has recently been explored.42 Finally, some authors have suggested anti-TNF re-induction and maintenance of the previous regimen.43 However, an unexplored option would be to try to recapture response with the temporary administration of BUD, depending on the location of the disease. Finally, the temporary addition of BUD could provide rapid symptom relief while the clinician decides whether to intensify treatment or change the biological agent, particularly if the patient presents mild or mild to moderately active CD.
Prophylaxis against post-surgical recurrenceAlthough BUD is not usually included as an option in clinical guidelines, and a recent systematic review44 concluded that it does not reduce the risk of post-surgical endoscopic recurrence of CD (RR 0.86, 95% CI, 0.61–1.22), a recent Cochrane review concluded that the evidence from trials performed to date is insufficient to reach solid conclusions about its usefulness for this indication. This calls for a careful analysis of existing clinical trials. In a preliminary, high-quality, double-blind, randomised controlled study,45 129 patients with ileocolic resection due to inflammatory activity or obstruction, but not due to perforation, were given 6mg of controlled ileal release BUD or placebo. The primary outcome variable (endoscopic recurrence defined by a Rutgeerts’ index score of ≥i1) 1 year after surgery was similar in both groups (52% BUD and 58% placebo). In a sub-analysis of patients operated for persistent inflammatory activity, endoscopic recurrence at 1 year was lower in the group receiving BUD (32% vs. 65%, p<0.047). In a subsequent, less robust study,46 83 patients were treated with 3mg daily of BUD or placebo. The rate of endoscopic or clinical recurrence at 1 year was 57% in the treated group vs. 70% in the control group (no significant difference). These studies, therefore, included a population at moderate-to-low risk of recurrence, the sample size was insufficient, and the dose was probably inadequate. Nevertheless, some data suggest a certain degree of efficacy in some subgroups of patients. In any event, BUD is not a suitable candidate for the long-term treatment required for prophylaxis of post-operative recurrence, and should only be used as an additional treatment (as with imidazole drugs).
High-output ileostomy and protein-losing enteropathyStudies have reported that BUD can be effective in the treatment of patients with high-output ileostomies for CD.47,48 The mechanisms involved were not linked to an anti-inflammatory effect, but rather to enhancing the net absorption of water.47,48 Since it is unlikely that randomised studies will be performed for this indication, which is fortunately rare, it is worth remembering that BUD can be an alternative in such difficult to manage patients.
BUD has also been used successfully in the treatment of protein-losing enteropathy associated with the correction of congenital heart disease using the Fontan procedure.49
Non-ileal CDThe effectiveness of BUD in locations outside the ileum is not known. Given its safety profile, it could be considered in jejunal sites before intensifying therapy to control a flare-up of proximal CD, provided that the lesions are uncomplicated and limited in extent.
Controversial aspects regarding the use of budesonideCan budesonide dependence occur in Crohn's disease?Steroid dependence, a common clinical situation in IBD, is characterised by a relapse of symptoms following a reduction in the dose of steroids or shortly after stopping them in patients who initially respond to these drugs. This makes it necessary to increase the dose or re-start the treatment to maintain remission. Steroid-free remission is the foremost objective in the treatment of IBD because, given their AEs, these drugs cannot be used as long-term maintenance therapy.14
Although BUD has been proposed in this context50,51 there is very solid evidence to recommend thiopurines as a steroid-free approach to maintaining long-term remission. Furthermore, steroid dependence in CD has been associated with cases of colonic involvement and smoking.52 A possible association between variants in the IL10 and ABCB1 genes (which encode for a transporter involved in steroid metabolism) and steroid dependence has also been described in CD.53
It is still to be determined whether BUD dependence shares epidemiological and clinical characteristics with conventional steroid dependence. Although it appears to be a common occurrence, so far no studies have specifically evaluated this aspect of BUD. Trials evaluating the efficacy of BUD to induce remission of CD have a follow-up of less than 12 weeks,22,26,27,54 and the primary maintenance trials and real-life cohorts analysed the effect of fixed doses of BUD for 1 year,54–56 or compared BUD dosing regimens without tapering.21,57–61 None of these trials describe BUD dependence.
The magnitude of the problem could be estimated from the number of patients in maintenance trials who received the lowest doses of BUD and relapsed at 3 or 6 months. However, the heterogeneity of these trials (different induction doses, determination at different time points) makes it difficult to determine the frequency of BUD dependence. An indirect estimate of BUD dependence can be inferred from the higher relapse rates in patients treated with 3mg/day compared to those treated with 6mg/day. Similarly, although BUD dependency figures in patients with autoimmune hepatitis and microscopic colitis have not been published, prevalence could be as high as 60% in patients with microscopic colitis treated with this drug.62,63
None of the published studies have addressed the treatment of BUD-dependent CD patients, and this is why no specific recommendations are made in clinical practice guidelines. In clinical practice, patients with BUD dependence are treated with conventional steroids or IS. However, it is important to bear in mind that direct comparative trials have shown conventional steroids (prednisone, methylprednisolone) to be only slightly more effective than BUD, and equally as effective in patients with low disease activity.64,65 Therefore, it is tempting to hypothesise that conventional steroids have no significant added value in BUD dependence. Moreover, the pathophysiological mechanisms of BUD resistance and dependence could well be similar to those of conventional steroids.
The ECCO guidelines, meanwhile, recommend that patients with early relapse (which could include those with BUD dependence) should start immunomodulatory therapy14 to control the activity of the disease in the long term. Although a subgroup of patients with mild CD classified as L1-B1 (ileal and inflammatory phenotype) in the Montreal system will not need maintenance treatment in the short/medium term after a preliminary cycle of BUD to induce remission, BUD dependence could raise the need for long-term maintenance treatment. In any event, although this strategy has not been assessed, given its optimal safety profile, using BUD in patients with dependence on conventional steroids could allow the initial dose to be tapered until thiopurines achieve their effect.
Can a dose of more than 9mg of budesonide be useful?The latest data from the aforementioned Cochrane meta-analysis19 and the recommendations of clinical practice guidelines and consensus documents1,16,17,66 confirm the standard clinical practice of administering 9mg/day BUD to induce remission in mild or mild-to-moderate ileal or ileocolic CD. This dose has greater benefit and no major AEs or clinical complications compared to placebo, and has fewer AEs and complications than prednisolone.
Few randomised studies have evaluated the clinical efficacy of high-dose BUD to induce remission, and it is not mentioned in guidelines for adults. Greenberg's 1994 study54 is the only one included in the Cochrane meta-analysis that analyses this regimen. The study was conducted on 258 patients with ileal or ileocolic disease involving no more than half of the transverse colon, and compared 3 different doses (9, 6 and 3mg/day) of controlled release BUD vs. placebo. After 8 weeks, the best remission rate was achieved in the group taking 9mg (51%), although there were no statistical differences compared to the 15mg (43%) group. The author observed a dose-related reduction of basal cortisol and adrenocorticotropin-stimulated cortisol, but no relevant clinical toxicity at any dose. Similarly, in a 2013 study comparing 77 patients, Suzuki et al.23 found no difference in 8-week remission rates between the 9mg and 15mg regimen (23% and 25%, respectively), although they observed higher rates of adrenal axis suppression and AEs with the 15mg dose. In 2000, Irvine et al.67 found no differences in a study evaluating quality of life in patients with ileal and ileocaecal CD. In a recently published network meta-analysis, doses of 15 and 18mg/day (OR 3.28, CI: 1.4–7.5) vs. 9mg/day (2.93; CI 1.5–5.4) showed similar response compared with placebo.30
Herfarth et al., however, in a study conducted in 2004 using 6, 9 and 18mg doses of pH59-dependent release BUD in patients with ileocolic CD irrespective of disease activity or colonic extension, with evaluation at 6 weeks, found dose-dependent clinical remission rates of 36%, 55% and 66%, respectively. Furthermore, although they considered the 9mg dose to be sufficient in most patients, a sub-analysis revealed better response with the 18mg dose vs. the standard 9mg regimen in patients with greater activity (CDAI≥300) or with ileocolic disease manifestation distal to the transverse colon. These results are similar to those reported in a paediatric study68 that also included patients irrespective of colonic involvement. A 12mg induction dose during the first month, followed by a standard 9mg regimen achieved a decrease in C-reactive protein and a significantly higher remission rate.
Doses of more than 9mg are only mentioned in the recent (2016) British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN) guidelines,18 which recommend treating mild to moderately active ileal or ileocolic CD with BUD “at maximum doses of 12mg/day, tapering over 2–4 weeks”. The guideline does not mention the evidence on which the recommendation for doses greater than 9mg is based.
The 12-mg dose could have its therapeutic niche, and will perhaps be explored in greater depth in new studies. It would be relevant in patients with moderately active disease in whom oral corticosteroids should be avoided for whatever reason. It is important to bear in mind that BUD reduces, in a linear, dose-dependent manner, basal and stimulated-release levels of plasma cortisol, and that the incidence of toxic effects increases with doses greater than 9mg. It is unlikely that the 12-mg dose for 4–8 weeks will cause significant clinical harm compared to the 9-mg regimen, since none has been reported in doses of 15–18mg. In any event, in these cases it is advisable to monitor each patient closely, and administer supplemental calcium and vitamin D to prevent bone loss.
Tapering budesonide regimenThe standard regimen of 9mg BUD for 2 months rarely suppresses the adrenal-pituitary axis, clinical manifestations of hypercorticism are uncommon, and it does not appear to be associated with suppression effects following abrupt discontinuation of steroids.19,65 No studies published so far have shown that tapered withdrawal is preferable to prevent AEs.19,65 No significant damage compared with placebo has been observed in safety studies lasting up to 1 year. Cases of sudden corticoid withdrawal syndrome have only been associated with prolonged treatment with BUD.69
After achieving remission with a standard regimen of 9mg/day for 8 weeks, guidelines and consensus documents differ with regard to the need for a tapering regimen to prevent the AEs associated with abrupt discontinuation, and to the specific regimen that should be used in such cases. This ambiguity has led to the different approaches adopted in clinical practice. The pharmacokinetics of BUD are dose-proportional between 3 and 15mg (ranges of clinical use), and the low systemic bioavailability of the drug minimises AEs compared to systemic corticosteroids.70 However, BUD has a relatively wide range of bioavailability (9–21%),70 which increases under certain conditions, such as intake of grapefruit juice and cytochrome CYPA3 inhibitor drugs, or in patients with cirrhosis. A BUD regimen of 9mg can induce significant plasma cortisol suppression (45%) at 5 days of treatment (area under the curve), although this is lower than with prednisone 20mg (78%).10 It is also clear that suppression of the adrenal axis occurs with low doses of 3 and 6mg, and a potentially clinically significant, linear, dose-dependent effect is observed in regimens of 9mg and over.
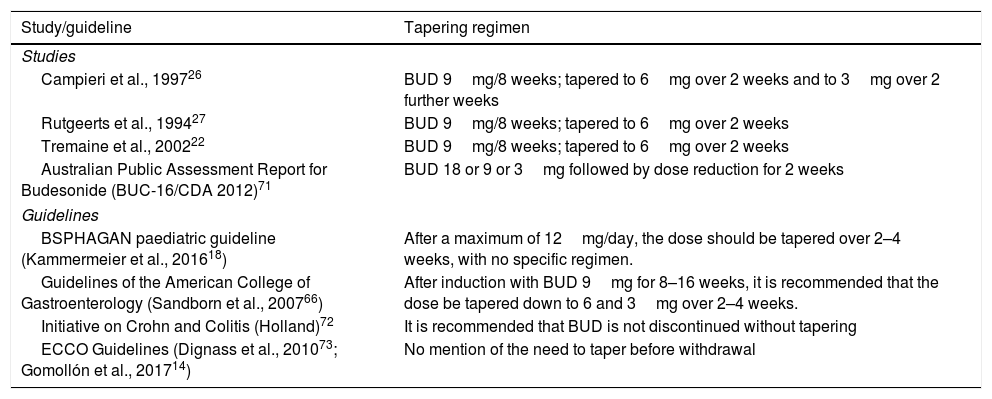
All of the above, coupled with the unforeseeable individual variations and idiosyncrasies encountered in daily practice, would justify the routine practice of tapering BUD systematically over 2–4 weeks in all cases, as is usually done with systemic corticosteroids. Because of this, various studies or consensus documents suggest using a tapering regimen (Table 5).
Budesonide tapering regimens used in clinical trials or recommended in guidelines.
| Study/guideline | Tapering regimen |
|---|---|
| Studies | |
| Campieri et al., 199726 | BUD 9mg/8 weeks; tapered to 6mg over 2 weeks and to 3mg over 2 further weeks |
| Rutgeerts et al., 199427 | BUD 9mg/8 weeks; tapered to 6mg over 2 weeks |
| Tremaine et al., 200222 | BUD 9mg/8 weeks; tapered to 6mg over 2 weeks |
| Australian Public Assessment Report for Budesonide (BUC-16/CDA 2012)71 | BUD 18 or 9 or 3mg followed by dose reduction for 2 weeks |
| Guidelines | |
| BSPHAGAN paediatric guideline (Kammermeier et al., 201618) | After a maximum of 12mg/day, the dose should be tapered over 2–4 weeks, with no specific regimen. |
| Guidelines of the American College of Gastroenterology (Sandborn et al., 200766) | After induction with BUD 9mg for 8–16 weeks, it is recommended that the dose be tapered down to 6 and 3mg over 2–4 weeks. |
| Initiative on Crohn and Colitis (Holland)72 | It is recommended that BUD is not discontinued without tapering |
| ECCO Guidelines (Dignass et al., 201073; Gomollón et al., 201714) | No mention of the need to taper before withdrawal |
BUD: budesonide.
This can be done in 3 ways. First, the definition of corticodependence includes the inability to reduce glucocorticoid levels (prednisolone 10mg or BUD 3mg over a 3-month period),14,73 so induction therapy should not last longer than 12 weeks. Given that 5mg of BUD and 12mg of prednisolone are clinically equivalent, after inducing remission with 9mg BUD for 8 weeks, it is reasonable to recommend dose tapering in all cases in order to avoid the effects of sudden discontinuation: 6mg for 15 days, followed by 3mg for a further 15 days. Another option would be to use this regimen only when it has been decided to prolong, switch or increase the standard dose and duration of treatment with BUD:
- 1.
Prolonged induction regimen in the “American Clinical Practice Guideline” of 9mg for more than 8 weeks (8–16 weeks).66
- 2.
Corticosteroid-dependent patients with inactive CD who the clinician has decided to switch from oral systemic corticosteroids to BUD as a bridge to other treatments and as a way of reducing corticosteroid-induced AEs.50
- 3.
If it has been decided to give maintenance therapy with 6mg for 3 months as a strategy to delay relapse.60
- 4.
When induction doses greater than 12mg are used.
Various scientific societies74–76 recommend giving calcium (1–1.5g/day) and vitamin D (800IU/day) supplements to prevent the development of BMD alterations (BMDA) in patients undergoing treatment with steroids. Osteoporosis, which increases the risk of fractures, especially in elderly patients, undermines quality of life and is a considerable burden on the health system. However, some aspects of the pathogenesis of BMDAs in patients with IBD are still controversial, such as the impact of inflammatory activity on bone metabolism, the type of steroid used, the dose given and the route of administration, the duration of exposure, and possible recovery of BMD after discontinuation (especially in patients with an exposure time of less than 3 months).
Given its low systemic bioavailability, BUD will probably have less or even no impact on BMD; however, the data is very heterogeneous. In an open, controlled study in patients with active ileal or ascending colon CD randomised to receive prednisolone (40mg/day) or BUD (9mg/day), in the BUD group, loss of bone mass at 24 months was only lower in patients who had never received steroids.77 Regarding fractures, only 1 asymptomatic vertebral fracture occurred in the prednisolone group, and 2 traumatic fractures in each group. In terms of baseline parameters, 44% of patients previously exposed to steroids and 58% of corticosteroid-dependent patients had BMDA, compared to 34% of those who had never received steroids (p=0.006). Interestingly, BMDA prevention therapy was not a study protocol criterion in either of the 2 treatment groups. Nevertheless, the calcium and vitamin D supplements that some patients received before being included in the study did not change, and was comparable in both groups (around 15% vitamin D and 50% calcium).77 These results contrast with those of another study in patients with inactive CD treated with low dose, long-term BUD or prednisone. No differences in BMD were observed between study groups; however, this was not a randomised study and there were major differences between groups in terms of disease location, previous exposure to steroids, and administration of calcium and vitamin D supplements.78
Few studies in the field of IBD have evaluated whether these supplements are effective or necessary to preserve or improve BMD,79 and none have evaluated them in the context of BUD treatment.
In conclusion, given the impossibility of stratifying the risk of BMDA in patients with IBD (apart from pre-established population factors), and specifically in patients receiving BUD, the administration of calcium and vitamin D is a safe and probably cost-effective measure. In the subpopulation of elderly patients, there are solid data to show that such supplements reduce the fracture rate,80–82 and a meta-analysis83 has shown that vitamin D supplements are effective in preventing falls by acting through skeletal muscle receptors to increase muscle strength.84
ConclusionsBUD is an alternative to conventional steroids in the treatment of CD. Its effectiveness depends on the severity and location of the disease. Although its position among the drugs used for this indication is clear, we believe that prescribers need to know the details of BUD therapy provided in this review. It is also interesting to note the therapeutic situations in which real-world use has filled in the gaps remaining after the preclinical development stage better than the summary of product characteristics. We hope to have contributed to promoting the appropriate use of a good drug in the patients that need it, and to have clarified its place in the therapeutic arsenal for CD and other digestive tract diseases.
Conflicts of interest- -
Antonio López-Sanromán: Consultancy, speaker's fees or research grants from AbbVie, MSD, Tillotts, Ferring, FAES Farma, Shire, Hospira, Kern-Celltrion, Takeda, Pfizer.
- -
Joan Clofent: no conflict of interest to declare.
- -
Esther Garcia-Planella: Consultancy, speaker's fees or research grants MSD, AbbVie, Kern, Gebro, Pfizer, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Tillotts Pharma.
- -
Luís Menchén: consultancy, speaker's fees or research grants from MSD, AbbVie, Takeda, Janssen, Pfizer, Tillotts Pharma, Dr Falk Pharma, Shire, Ferring.
- -
Pilar Nos: consultancy, speaker's fees or research grants from MSD, Otsuka, AbbVie, Takeda, Kern, Biogen and Ferring.
- -
Iago Rodríguez-Lago: Consultancy fees from Tillots Pharma.
- -
Eugeni Domènech: consultancy, speaker's fees or research grants from AbbVie, MSD, Takeda, Hospira, Kern, Shire Pharmaceuticals, Ferring, Tillots Pharma, Otsuka Pharmaceuticals, Pfizer, Celgene, Grifols.
Please cite this article as: López-Sanromán A, Clofent J, Garcia-Planella E, Menchén L, Nos P, Rodríguez-Lago I, et al. Revisando el papel terapéutico de la budesonida en la enfermedad de Crohn. Gastroenterol Hepatol. 2018;41:458–471.