The human schistosomes or blood flukes are digenetic trematodes belonging to the Schistosomatoidea superfamily. The main species responsible for the clinical forms are Schistosoma mansoni , S. hematobium and S. japoni cum . Other species that affect humans ( S. mekongi and S. intercalatum ) have limited geographic distribution in Asia and Africa, respectively 1 . In the present review, we will discuss the S. mansoni form, which is the most widely distributed schistosome in humans and is found both in the Old and New Worlds 2 . Mansonian schistosomiasis is endemic in northeast Brazil, where it affects approximately 2.5 million people 3 . The decrease in the number of infected individuals in the last few decades and the geographic frontiers of schistosomiasis remain to be established in Brazil. The main reasons for this lack of epidemiological data are the presence of the snail Biomphalaria sp . , which is susceptible to S. mansoni infection in nonendemic areas, and migration 4 . Nowadays, in some areas such as the Caribbean Islands, Venezuela and Suriname, the prevalence of S. mansoni is very low (under 1%) 5 . Some authors believe that this might be due, at least partly, to the predatory action of some snails (e.g. Thiara granifera ) or the Biomphalaria glabrata6,7 . Such measures may be feasible in small areas, but not in our country where the Biomphalaria snails live in vast territories. In Brazil, an extensive S. mansoni control program based on treatment with oxamniquine and snail control resulted in a sharp decrease in hepatosplenic and other advanced forms of schistosomiasis 5 . The basic life cycle of the parasite alternates between generations, with a sexual stage of adult schistosomes in the definitive vertebrate host (man) and an asexual stage in a molluscan host ( Biomphalaria ). A relatively short-lived and free-swimming stage, the miracidium, hatches from the egg found in human stool and infects the snail intermediate host, where it gives rise to sporocysts that produce numerous free-swimming cercariae. These are infective both to man and to other vertebrate hosts. After skin penetration the cercariae are known as schistosomula, which migrate to the lungs and then to the liver. After maturation, both the male and female worms migrate to the terminal branches of the mesenteric veins where each female produces 100-300 eggs per day. A considerable number of eggs pass into the lumen of the bowel and are discharged into the feces. Some of these eggs become trapped in the intestinal wall and the remaining eggs are carried by the portal blood to the liver. Schistosomiasis is considered to be a «man-made disease» since humans are both the victims and the source of infection 8 . Insanitary habits among humans such as defecation in canal water and at the same time exposing themselves to this polluted water by bathing, swimming for recreation, washing utensils and clothes and other forms of contact characterize endemic areas 8 . Exposure to infection starts as early as at 6 months of age and maximal infection and severity occur in early childhood (10-14 years) followed by a progressive decrease. The lower prevalence in adults may be explained by lower exposure to contaminated water and to the development of acquired concomitant immunity 8 . Environmental changes that result from the development of water resources and population growth and migration can facilitate the spread of schistosomiasis. For example, the construction of the Diama Dam led to the introduction of S. mansoni into Mauritania and Senegal 9 .
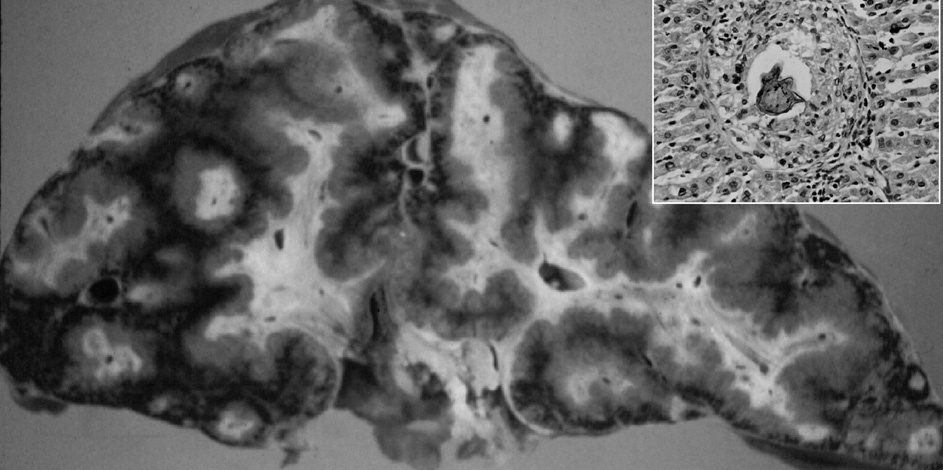
CLINICAL FORMS AND PATHOGENESISLiver disease in schistosomiasis results from the entrapment of eggs that are not excreted but instead lodge in portal venules that match the egg’s smallest diameter, about 50 µm 1 . Eggs in the liver remain viable for about 3 weeks and secrete products that elicit a characteristic initial response, the schistosome egg granuloma. In some persons with heavy infections, the final result of hepatic schistosomiasis is severe portal fibrosis (figs. 1 and 2). Advanced schistosomal hepatic fibrosis gives a gross appearance of greatly enlarged fibrotic portal tracts, described by Symmers in 1904 as resembling clay pipestems thrust through the liver, and now termed Symmers’ pipestem fibrosis 1 . Schistosome infection can be divided into distinct stages. The prepatent stage begins with cercarial invasion and ends with initiation of egg laying. The patent stage coincides with schistosome egg production and can be further divided into acute and chronic schistosomiasis 10 .
Acute form (Katayama fever)Acute schistosomiasis is due to a fresh infection. Clinical manifestations may be absent, particularly in individuals living in an endemic area 11 . Occasionally some individuals may develop the syndrome after an initial contact with water contaminated by S. mansoni cercariae . The most common manifestations are fever, chills, weakness, headache, anorexia, nausea, vomiting, and general malaise. Severe watery diarrhea, sometimes bloody stools, nonproductive cough, and rapid weight loss may develop. Occasionally, patients with acute schistosomiasis manso-ni have been diagnosed as having typhoid fever, hepatitis, pancreatitis, miliary tuberculosis, myelitis and appendicitis 12 , only to be cured when the schistosomiasis has been discovered after a long search 13 . On the other hand, many patients have been erroneously diagnosed with schistosomiasis in endemic areas where this disease is common 14 . Hepatomegaly is virtually a constant finding and is frequently accompanied by slight right upper quadrant tenderness. Transitory generalized lymphadenopathy and splenomegaly frequently appear but there is no clinical evidence of jaundice. Laboratory examinations show leucocytosis with eosinophilia, which can reach 70% and slight changes in serum aminotransferase 11,15 . Ultrasonographic studies are still scarce in patients with acute schistosomiasis 12 . Cesmeli et al reported the case of a patient with acute schistosomiasis who presented nodular liver lesions on abdominal ultrasound and computed tomography scan 16 . Such lesions, however, were not found by others 17 , suggesting that these sonographic features are uncommon 12 . Acute schistosomiasis usually progresses to an asymptomatic period and eventually to the chronic forms 11 . In patients with acute schistosomiasis admitted to hospital be-cause of the intensity of their symptoms, treatment should be started with corticosteroids, followed by schistosomicides 18 . However, these situations are fairly uncommon.
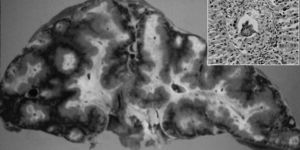
Fig. 1. Symmers’ periportal fibrosis of the liver with Schistosoma mansoni showing an egg at the center of a granuloma (detail).
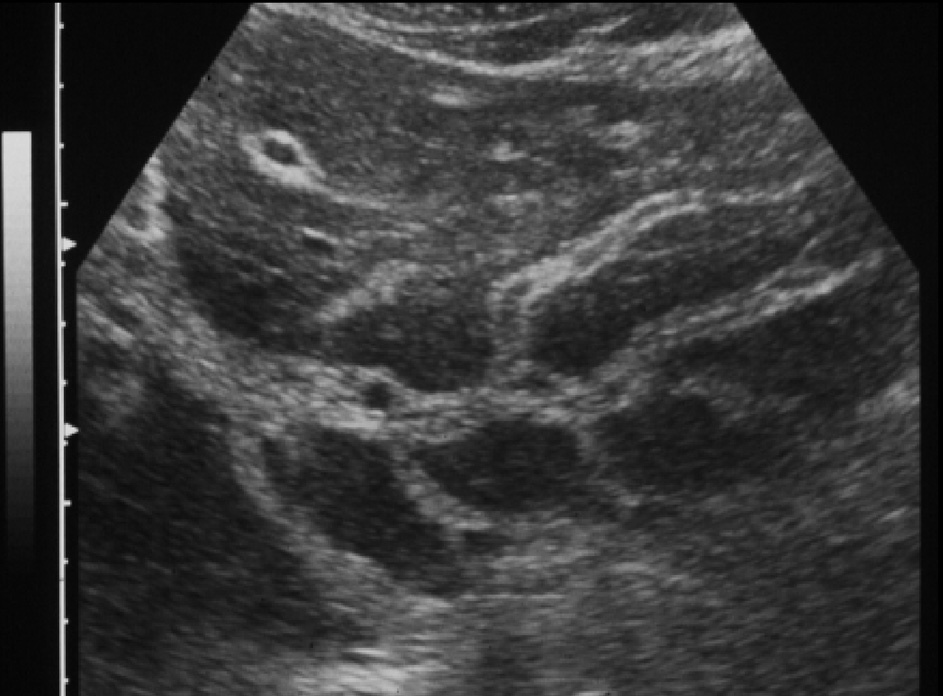
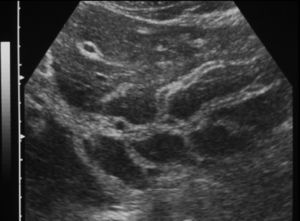
Fig. 2. Sonographic features of periportal fibrosis in schistosomiasis mansoni.
Chronic forms
The life cycle of the parasite in the host, which begins with penetration of cercariae into the skin and ends with the adult worms lodging in the terminal branches of the portal vein, explains why S. mansoni can damage several organs other than the intestines and liver 19 . However these localizations are by far the most frequent.
Intestinal and hepatointestinal forms
Intestinal manifestations such as diarrhea and colicky pain are occasionally observed, but asymptomatic forms are more common. A modulation of the egg granuloma is observed during the transition of the acute form to the chronic form: the exudative-necrotic granuloma of the acute phase becomes smaller and with fewer inflammatory cells and, consequently, it becomes less pathogenic to the liver cells 5 . Some authors believe that in patients with poor modulation of the granuloma, the disease tends to evolve to the hepatosplenic form 20 . Because the intestinal form is frequently asymptomatic, the diagnosis is based on the finding of S. mansoni eggs in stools. Schistosomotic intestinal polyposis is uncommon in Brazil 5 is but frequently observed in Egypt 21 . The subdivision into an intestinal form and a hepatointestinal form is not uniformly accepted 5,22 , but enlargement of the liver, mainly of the left lobe, may be seen in schistosomotic individuals living in highly endemic areas, and characterizes the hepatointestinal form 2 . Recently, our group has reported portal and splenic overflow in both the hepatointestinal and hepatosplenic forms of schistosomiasis 23 .
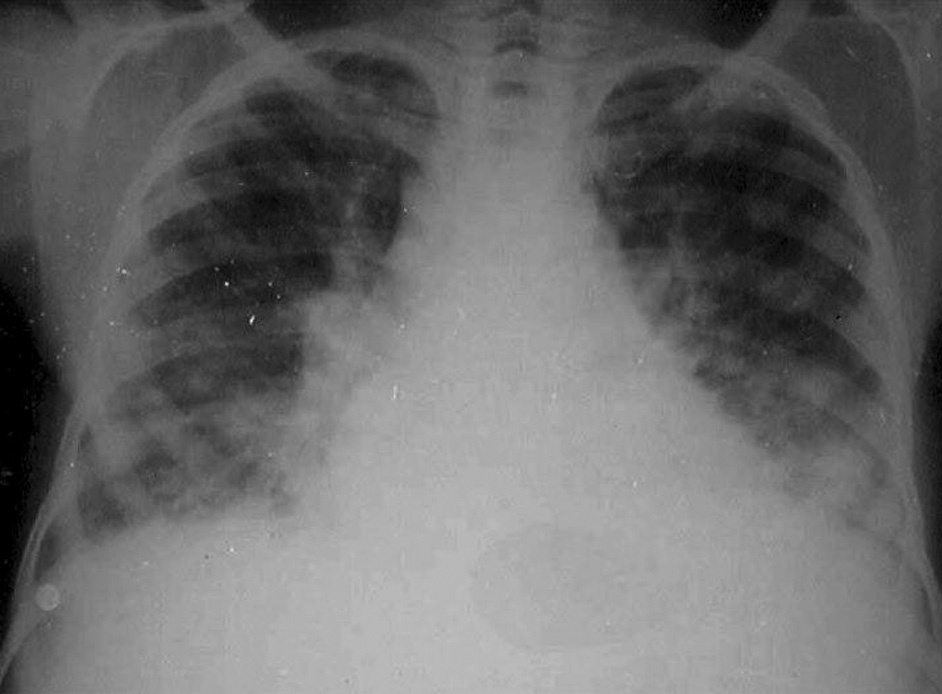
Fig. 3. «Dead worm pneumonitis» after chemotherapy in a patient with heavy infection.
Hepatosplenic form and portal hypertension
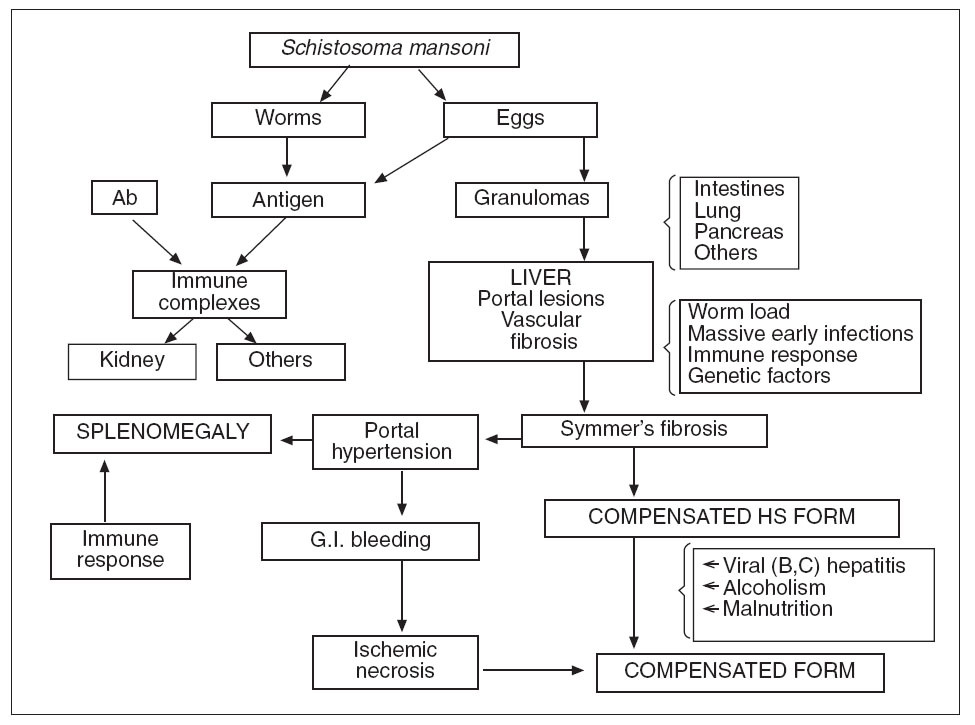
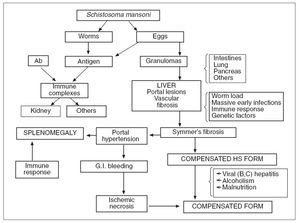
Pathophysiology. The pathogenesis of the distinct stages of schistosomiasis and of its clinical manifestations is not well understood. Many authors agree that the egg granuloma is the main process causing tissue damage in organs such as liver, intestines, lung and pancreas. However, immunocomplexes derived from parasite antigens are responsible for special lesions such as glomerulopathy 19 . Hepatic granulomas are initiated endovascularly and are presinusoidal, mostly at inlet venules or terminal portal venules. Following initial endothelial proliferation, the blood vessels rapidly become occluded with eventual obliteration of the lumen and displacement of hepatocytes by the developing granuloma 24 . Symmers’ fibrosis of the liver, which may result in portal hypertension and congestive splenomegaly, occurs in individuals aged 6– to 20-years or 5-15 years after the onset of infection 15 . It is the most severe form and the most common cause of morbidity and death 2 . However, in heavily infected young patients there is a «noncongestive» phase of the disease with splenomegaly but without evident signs of portal hypertension. Data from Brazil show that in such cases, the spleen may become impalpable after adequate chemothe 2,19,25 . A strong correlation between the hepatosplenic form and worm load in young patients has repeatedly been shown 26 . Furthermore, experimental studies suggest that massive infections during early periods of exposure to cercariae may be important. Failure of immunologic modulation of inflammatory reactions seems to play a role 27 . These and other factors (see below) explain the presence of hepatosplenic schistosomiasis in only a small percentage of patients with heavy worm burden 19 . Hepatic periportal fibrosis is caused by the T cell-depen-dent granuloma that develops around schistosome eggs. Experimental models of infection have shown that granuloma and fibrosis are highly regulated by cytokines. However, why advanced periportal fibrosis occurs only in certain individuals is unknown. A study performed in 75 patients with no or mild fibrosis and in 24 patients with advanced fibrosis suggested that interferon (IFN)-gamma plays a key role in the protection of S. mansoni -infected patients against periportal fibrosis, whereas TNF-alpha may aggravate the disease 28 . In some patients with heavy infection chemotherapy may produce a peculiar type of pulmonary lesion, «dead worm pneumonitis» (fig. 3), which has been attributed to a hyperergic pulmonary reaction to the products of dead worms. The pathogenesis and pathophysiology of the hepatosplenic and other forms of S. mansoni infection are presented in figure 4. It is now well established that some experimental models can reproduce a «pipe-stem» fibrosis. According to Andrade 29 , a peculiar pattern of vascular intrahepatic lesions, described later, seems to depend on 2 mechanisms: egg embolization, with a partial blocking of the portal vasculature, and the appearance of small portal collaterals along the intrahepatic portal system. The continuous lodging of newly arrived eggs into these thin and anastomosing blood vessels leads to vascular obstruction and periportal fibrosis 30 . In more advanced cases, a thin septal fibrosis, which dissects the parenchyma in several directions, is observed. Its pathogenesis is not well established. The distribution of schistosomal antigen immunoglobulins and complement C3 was studied by our group using biopsy specimens of human liver from 21 patients with hepatosplenic schistosomiasis. The kinetics of the granuloma formation and IgG deposits along the sinusoidal wall were described. The irreversibility of schistosomotic fibrosis has been seriously disputed. Experimentally, chemotherapy markedly decreases mortality in infected mice soon after treatment. Later, periportal fibrosis can undergo degradation in a way similar to that of periovular granulomas. In a study based on family heredograms, Tavares-Neto and Prata 31 found a highly significant concentration of hepatosplenic forms among children from the same nuclear family, particularly when the mother was affected by the same clinical form of the disease. Furthermore, this clinical form is more frequent in whites than in blacks, even though Brazilian whites are more socially and economically advantaged. All these findings would point to a genetic component, which is not yet well characterized.
Fig. 4. Pathogenesis and pathophysiology of human hepatosplenic (HS) schistosomiasis (G.I.: gastrointestinal; Ab: antibodies).
–Association of schistosomiasis with viral hepatitis Band hepatitis C. In the last few decades, several authors have pointed out the role played by hepatitis B virus infection in increasing morbidity due to schistosomiasis 32 . It is generally accepted that S. mansoni infection alone does not lead to cirrhosis 12 . Liver biopsy studies have shown a significant increase in chronic hepatitis and hepatic decompensation in schistosomotic patients with hepatitis B virus infection. According to Strauss 33 , the higher frequency of hepatitis B virus markers in the hepatosplenic form than in the hepatointestinal form is due to therapeutic measures in patients who seek medical care. This point of view is advocated by some authors 34 , but not by others 32 . Seventeen cases of acute hepatitis C in previously infected patients with mansonian schistosomiasis were compared with 15 non-schistosomotic control patients, also with acute hepatitis C. Spontaneous viral elimination was observed in 5 controls, but in none of the patients with schistosomiasis 34 . Furthermore, structural liver changes were more severe in patients with double infection and predominant Th 2 immune response. The relationship between hepatitis C virus infection and different clinical forms of mansonian schistosomiasis was investigated by Pereira et al 35 , who demonstrated that hepatitis C viral infection is an important pathogenic factor in decompensated forms of schistosomiasis.–Hemodynamic features . Some intrahepatic angiographic changes are highly suggestive of hepatosplenic schistosomiasis 19 .
1. Hepatic veins. Hepatic veins are enlarged and there is a marked reduction of branching, along with a somewhat pronounced arching of large branches 36 . In 50 patients studied by Mies et al 36,37 the gradient between hepatic vein wedged and free pressures was at the upper limit of normality (7 ± 4.4 cm H 2 O). In about 40% of patients, however, it was slightly raised: the gradient ranged from 8 to 17.5 cm H 2 O. These findings were in agreement with those reported by Coutinho 38 .
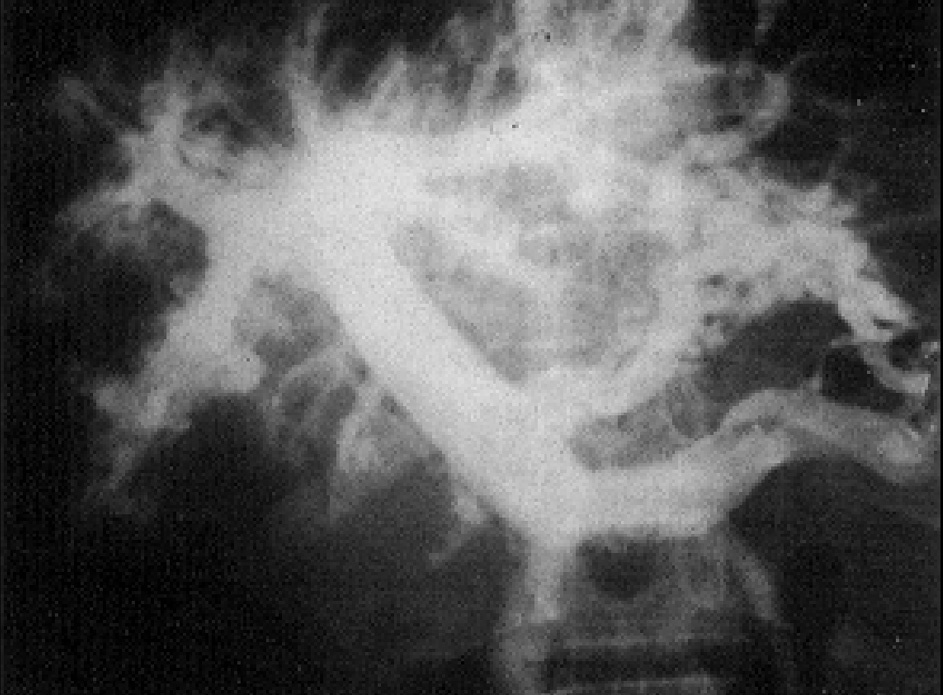
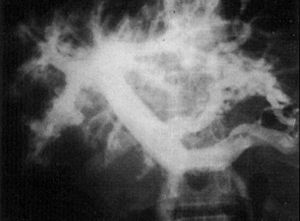
2. Intrahepatic pattern of the portal system . Contrast material injected into the portal system shows an intrahepatic pattern characterized by gross amputations of large branches and by the appearance of an intense network of small-caliber vessels around portal branches. These features were described by Bogliolo in 1957 39 and are known as «Bogliolo’s sign» (fig. 5).
3. Extrahepatic portal system and portal pressure . Enlargement of splenic and portal veins is usually present. Portal pressure is raised to levels similar to those seen in liver cirrhosis. In an analysis of 20 scientific papers with 432 patients in whom portal pressure was measured by various methods, usually by intrasplenic puncture, Carneiro 40 found an average pressure of 33 cm H 2 O.
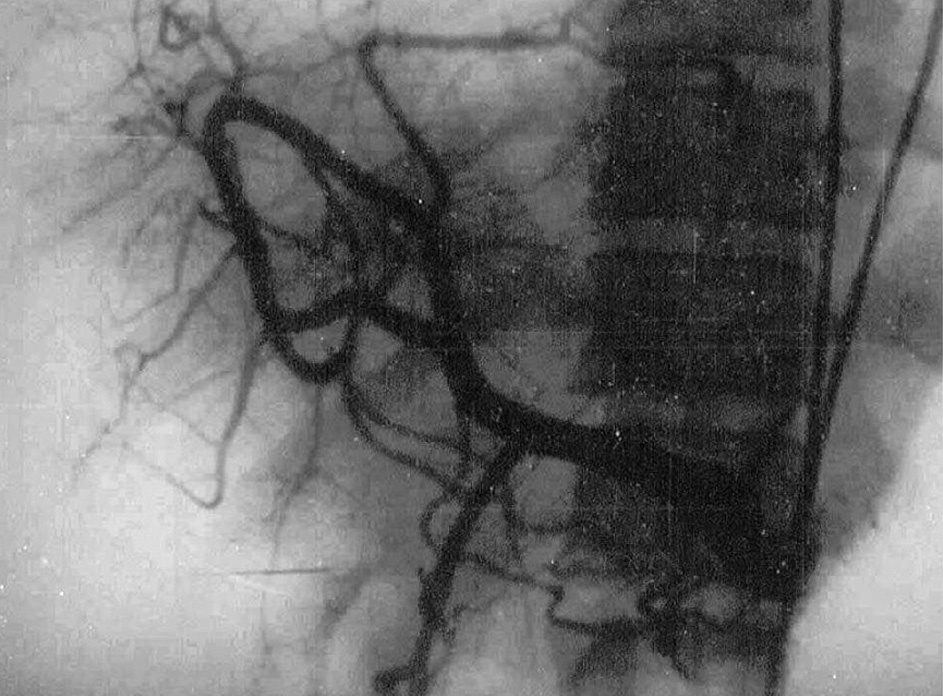
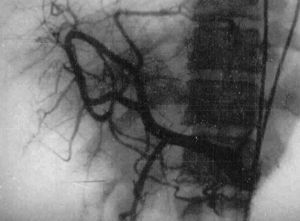
4. Hepatic artery. Angiographic studies have shown a reduced diameter of the hepatic artery, with thin, arched branches outlining vascular gaps (fig. 6). The tortuous aspect characteristic of liver cirrhosis is not observed 41 . Interestingly, increased hepatic arterial flow was demonstrated in infected hamster 42 . These findings suggest that the poor intrahepatic arterial vascularization demonstrated by selective arteriography in human hepatosplenic schistosomiasis is due to «functional deviation» of arterial blood to the splenic territory 19 . In advanced schistosomiasis, hypertrophy of the hepatic artery may be found 43 .
5. Liver blood flow. Total liver blood flow, measured by various methods, is normal 44,45 , suggesting that increased
portal blood flow is accompanied by a reduction of arterial blood flow 44 and that there is an interaction between portal and arterial blood flow 46 . As already shown, the diameter of the common hepatic artery increases after occlusion of the splenic artery by a balloon catheter 47 or after splenectomy 48 or selective portal decompression. Some dynamic studies have demonstrated that high splenic blood flow may be responsible for the increased portal blood flow. The splenic component and the abundant network around portal branches («Bogliolo’s sign») may predominate (hyperflow component) over the obstruction of the intrahepatic portal branches produced by parasitosis (presinusoidal block), at least in cases of severe splenomegaly 2 . However, ultrasound studies show that intense periportal thickening of the liver can be found in some patients without a palpable spleen, despite the presence of portal hypertension, probably as a consequence of spontaneous shunts 49 . Furthermore, schistosomic portal hypertension is not restricted to splenic hyperdynamic circulation. Mies et al 50 showed that despite small changes in sinusoidal pressure and liver function, schistosomotic patients present with high cardiac index and low systemic vascular resistance compatible with a systemic hyperdynamic circulatory status similar to that of cirrhosis.Fig. 5. Intrahepatic pattern of the portal vein characterized by gross amputations of large branches and by the appearance of an intense network of small-caliber vessels around portal branches (Bogliolo’s sign).
Clinical and laboratory changes. Hepatosplenic schistosomiasis can be clinically divided into compensated and decompensated forms. The latter is characterized mainly by muscular wasting, low serum albumin, and occasionally, chronic ascites. However, other causes, such as alcoholism, and hepatitis B and C virus infection, are frequently responsible for the decompensated form (fig. 4).
S.mansoni shows a wide range of clinical manifestations, varying from asymptomatic to highly symptomatic forms. Sometimes the disease is accidentally discovered, either by a routine stool examination of by findings of an enlar ged liver or spleen on physical examination 22 . The main symptoms of the hepatosplenic form are related to splenomegaly and hemorrhage. Spontaneous ascites is uncommon and usually appears after an episode of bleeding or infection or in association with other liver lesions. Spontaneously appearing neuropsychiatric symptoms are very rare in hepatosplenic schistosomiasis, even with abundant collateral circulation. This syndrome is only seen after precipitating factors, mainly hemorrhage or infection. In patients with compensated hepatosplenic schistosomiasis, arterial blood ammonia determination shows normal or slightly increased levels after hemorrhage or after an oral ammonium chloride load. This contrasts with the significant elevation of ammonia in compensated forms after portocaval anastomosis and in decompensated forms 51 . Anemia with delayed somatic and sexual development may be observed in young people. Spider nevi, gynecomastia, and palmar erythema are rare in uncomplicated schistosomiasis. A hard, enlarged liver with a predominant increase of the left lobe is frequently palpable. The spleen is enlarged, and Symmers’ fibrosis is one of the main causes of huge spleens in Brazil. Leukopenia and thrombocytopenia are found in most cases of hepatosplenic schistosomiasis. Anemia is common but is also due to other mechanisms, such as malnutrition, ankylostomiasis, chronic bleeding, and hemolysis 19 . In hepatosplenic schistosomiasis, the clotting defects are due to an impaired rate of protein synthesis by the liver or localized consumption coagulopathy, or both 52 . The relative role of these 2 factors is not well established. Some changes in plasma coagulation systems may be partially corrected by subcutaneous heparinization and mainly by splenectomy 53 .
Fig. 6. Angiographic study showing reduced diameter of the hepatic artery, with thin, arched branches outlining vascular gaps.
–Diagnosis. Direct diagnostic methods are based on the demonstration of eggs or of miracidia. Eggs may be found in the stools by the Kato-Katz quantitative method 54 . The hatching test is a simple, cheap, and useful method, without the need for microscopy. Rectal biopsy is used in suspected cases with negative parasitological tests.
Immunologic methods, indirect immunofluorescence, indirect hemagglutination, and enzyme-linked immuno absorbent assay (ELISA) give a high degree of positi- 55,56 . Serum precipitins have been demonstrated by immunodiffusion 57 ; its low sensitivity was greatly increased by counter immunoelectrophoresis and enzyme-lin-ked-immunoelectrodiffusion assay (ELIEDA) 58 . Antibody titers are usually higher in the hepatosplenic form than in the hepatointestinal form. It is well known that antibody titers present an early and significant increase after chemotherapy as a consequence of worm death 56 . One major diagnostic problem concerned the demonstration of periportal fibrosis, even with wedged liver biopsies. Fortunately, ultrasonography has become a reliable and sensitive method of detecting these lesions 59 .
Ultrasonography in schistosomiasis. A review of the literature on the use of ultrasonography in schistosomiasis showed that ultrasonography can provide direct information on lesions in internal organs and can thus provide information about patterns of morbidity and regression of pathological changes after treatment 60 . The method is non-invasive and can be used under field conditions. However, the methodology used for ultrasonography has varied considerably so that valid comparisons between results obtained in different places or at different times are difficult to make 60 . From the clinical point of view, periportal fibrosis, seen in ultrasonography as echodense areas along the portal vein (fig. 2), is an important diagnostic feature, and also indicates the possible existence of portal hypertension 59 . Until fibrosis reaches an advanced stage, thickened patches are typically scattered throughout the liver. Experience shows that the portal vein and the structures around it can best be visualized in the left liver lobule 61 . A combined clinical sonographic classification of hepatosplenic schistosomiasis mansoni was proposed by Lambertucci et al 49 for use in field-based studies. In 741 individuals out of 892 (83%) living in an endemic Brazilian area who underwent physical and parasitological examination, splenomegaly was diagnosed by abdominal palpation in 71 patients (9.6%). By using abdominal palpation and ultrasound examination, 4 groups of individuals have been identified: (i) palpable spleen and intense periportal thickening in 9 individuals (1.2%); (ii) spleen not palpable and intense periportal thickening in 15 (2%); (iii) palpable spleen with light-to-moderate periportal thickening in 32 (4.3%) and (iv) palpable spleen with a normal liver on ultrasonography in 30 (4%). Based on these results, the authors 49 concluded that the definition of hepatosplenic schistosomiasis in field-based studies as the finding ofS.mansoni eggs in the stools in an individual with splenomegaly is no longer acceptable. According to recent studies, the parameters associated with variceal bleeding in patients with hepatosplenic schistosomiasis are endoscopic (variceal size, red signs, congestive gastropathy, and fundic varices) and ultrasonographic (portal vein diameter and periportal thick-ness) 62 . The most important combined parameters are gastropathy and red signs, portal vein diameter and variceal size, and variceal size and gastropathy 62 . A series of recommendations on qualitative and quantitative data obtained by ultrasonography in studies performed in Africa and Brazil were presented at a recent symposium on ultrasound in Schistosomiasis held in Minas Gerais, Brazil (Niamey-Belo Horizonte protocol). Imaging patterns of liver and portal hypertension assessment were analyzed 63 . These patterns were more recently reviewed 64 and include: non-specific minor thickening of the walls of portal branches («starry sky»); fibrosis of the peripheral portal branches or of the main portal system and of the right and left branches can occur alone or simultaneously. Finally, in agreement with previous reports 59,65,66 , a Doppler ultrasonographic study by our group 23 showed that hepatic left lobe hypertrophy and right lobe atrophy are commonly observed in hepatosplenic schistosomiasis. Increased portal blood flow in patients with the hepatointestinal form of schistosomiasis, diagnosed by the absence of gastroesophageal varices and of splenomegaly and by the presence of hepatomegaly, as shown by our group 23 , would be unexpected. However, the possibility of lowgrade portal hypertension not detected by clinical and ultrasonography in these patients cannot be ruled out 49 . In summary, our group showed that portal overflow may be found in both forms of schistosomiasis 23 .
Extrahepatic schistosomiasis
Glomerulopathy. The incidence of nephrotic syndrome and chronic renal failure is much higher in tropical than in temperate climates, suggesting that parasitic diseases, principally schistosomiasis, might be involved in the pathogenesis of glomerular disease 67 . A recent study conducted in a series of patients with schistosomal glomerulopathy allowed localization of schistosomal antigens in glomerular structures 68,69 . The kidneys play an important role in the disposal of circulating antigens. In S. mansoni infection, circulating antigens derive from the adult worm (gut antigens) or from the schistosome eggs 67 . In hepatosplenic schistosomiasis the shunting of portal blood carrying the primary load of antigens into the systemic circulation is the main mechanism by which the liver may be excluded from processing the schistosomal antigens, which are then supplied to the kidneys in larger amounts 67,70 . Earlier studies have suggested that mesangioproliferative glomerulonephritis and membranoproliferative glomerulonephritis type I are the more common forms of schistosomal glomerulopathy 68,71,72 . Clinically, patients with this disease may either be asymptomatic, with only minor urinary changes, such as proteinuria or hematuria, or may manifest a nephrotic syndrome that may evolve to endstage renal failure 69 . The transformation of mesangioproliferative glomerulonephritis into membranoproliferative glomerulonephritis was demonstrated by De Brito et al 73 . Nephrotic syndrome seen in association with schistosomiasis presents with an increased plasma globulin concentration and normal cholesterol levels in one-third of affected patients 12 . Notably, schistosomal antigens were demonstrated for the first time in the glomeruli of humans with active schistosomiasis mansoni for the first time in the transplanted kidney of a patient with focal and segmental glomerulosclerosis 74 and later by our group in patients with mesangioproliferative glomerulonephritis 75 . The outcome of treatment of nephrotic syndrome associated with hepatosplenic schistosomiasis with antischistosomal drugs, corticosteroids and immunodepressors, has been disappointing 76 .
Pulmonary forms. Pulmonary manifestations may be observed in the acute phase, but are far more common in the chronic phase. Besides the rare «pneumonias» produced by dead worms after chemotherapy 77 , pulmonary hypertension can be found in 13% to 16% of patients with hepatosplenic schistosomiasis and, more rarely (0% to 2%), cor pulmonale 12 . Anti-schistosomal therapy usually has no effect, but is given to prevent further progression of the disease. Pulmonary embolism of dead worms may produce an abrupt in-crease in pulmonary pressure 12 .
Other forms. Other forms of schistosomiasis, including neuroschistosomiasis are clinically less important than those previously described. The course of Salmonella and Escherichia infections in patients with schistosomiasis is unusual; the infection becomes chronic with prolonged fever and blood cultures that remain positive for months 15 . These infections can only be cured by antischistosomal treatment.
Course and prognosis
The prognosis of hepatic schistosomiasis is good in the acute and hepatointestinal forms. The prognosis of the hepatosplenic forms depends on whether the liver disease is compensated or decompensated. Fortunately, most cases belong to the compensated form: the prognosis is better than that of patients with cirrhosis. Recovery after hematemesis is rapid and patients live longer 2 . Hepatic coma and ascites after hemorrhage are less frequent and easier to control. A few patients with severe liver failure have a poor prognosis, similar to those with cirrhosis 19 .
Treatment
Treatment of helminthiasis. Treatment of helminthiasis is indicated in all stages and clinical forms of mansonian schistosomiasis, but some patients with a very advanced form or with unrelated conditions or diseases may not tolerate chemotherapy. There are two highly effective chemotherapeutic drugs: oxamniquine, with different doses in Latin America and Africa, and praziquantel. Both drugs are administered through the oral route. A double-blind trial published by our group in 1986 78 showed that both drugs have the same efficacy for the Brazilian strains of Schistosoma ; however, praziquantel produces fewer neuropsychiatric symptoms. Furthermore, this drug is also effective against other species of Schisto soma . As reported by the World Health Organization, praziquantel has become the most widely used of the antischistosomal drugs 79 . Praziquantel can also be safely given to patients with the compensated hepatosplenic form of the disease 80 . Cessation of egg-induced immunopathology is reflected by a return to normal of serum procollagen-III-propeptide and neopterin concentrations 81 .
Treatment of portal hypertension.Nonsurgical treatment of bleeding esophageal varices.
The management of patients with esophageal and gastric varices remains challenging, particularly when prophylactic procedures are considered 82 . Although high portal pressure and large varices are associated with an increased risk of bleeding, the literature is still not clear and the methods of measuring size and pressure are still controversial 83 . Some endoscopic changes that are thought to indicate a high risk of bleeding include large varices, cherry-red spots, varices on varices, erosions on varices 84 and hypertensive gastropathy. These, and probably other endoscopic signs, including ultrasonographic changes, should be used to identify patients for prophylactic procedures, including drug therapy, sclerotherapy and surgery.
1. Propranolol.β -blockers such as propranolol have mainly been used for the management of portal hyperten sion in liver cirrhosis. Their utility in chronic schistoso miasis, however, is not well established. In a murine schistosomiasis model, the development of portal syste mic shunting was decreased or prevented by oral propra nolol through the reduction of flow and pressure in the portal system 85 . A prospective study of 42 patients with at least one episo de of gastrointestinal bleeding showed a significant re duction in episodes in treated patients when compared with a historical control 86 . Therefore, propranolol seems to protect patients with schistosomiasis against gastroin testinal rebleeding during the short period of time that precedes definitive surgical treatment. Indeed, oral admi nistration of a single «blockade dose» may significantly decrease azygos blood flow 86 . The same group showed that administration of proprano lol corrects hyperdynamic circulation, aggravates pulmo nary hypertension, does not alter portal pressure and re duces sectorial portal blood flows, especially of the azygos vein, with maintenance of total hepatic blood flow 87 . A controlled trial with non-cirrhotic portal fibrosis pa tients, most of whom had schistosomiasis, showed that continuous oral propranolol treatment is effective in pre venting recurrent upper gastrointestinal bleeding. Despite these favorable results withβ -blockers, we wondered whether we could obtain good compliance over a long pe riod of time 82 . Furthermore in some clinical trials, high doses of propranolol were necessary for a good pharmacological results, hampering the clinical utility of the 88,89.
2. Sclerotherapy of esophageal varices . In retrospective study of sclerotherapy of bleeding esophageal varices in schistosomiasis, varices were eradicated in nearly 70% of patients during a follow-up period lasting between 48 and 132 months. Sclerotherapy was effective in controlling rebleeding in 97% of the patients in the group who had previous portal hypertension surgery and in 73% of the group without previous portal hypertension surgery, with a smaller number of sclerotherapy sessions per patient in the former group; these differences were statistically significant 83 . Favorable results were also obtained by another group after emergency or elective endoscopic sclerotherapy 90 . A prospective non-randomized trial comparing elective sclerotherapy with a control group with a 5-year follow-up showed that the incidence of rebleeding was 28% and 45%, respectively. This difference was not statistically significant; however, mortality from rupture of esophageal varices was 3% in the sclerotherapy group and was 28% in the control group, which was a statistically significant difference 91 .
Surgical treatment of portal hypertension. The morphological, hemodynamic and clinical features previously described show that mansonic schistosomiasis is an excellent model for the study of portal hypertension and of surgery used to treat bleeding varices. Until 1986, three major types of operations were used in Brazil 41 : (i) esophagogastric devascularization plus splenectomy (EGDS); (ii) Splenectomy with splenorenal shunt (SRS), and (iii) selective portal decompression (SPD) or distal splenorenal shunt (DSS). In 1986 an article based on a prospective randomized trial was published by our group 92 comparing the results of the three surgical techniques. It became clear that SRS frequently produces portosystemic encephalopathy (39%); for this reason we interrupted this trial. Later, a long-term follow-up study (85.7 ± 33.1 months) was performed by the same group 93 in the same patients, excluding 9 patients (9.6%) who were lost to follow-up. This study showed that gastrointestinal tract bleeding occurred in 24.1% of the patients, without statistical differences among the three groups, but rebleeding due to varices was more frequent after EGDS. Hepatic encephalopathy was significantly greater after proximal SRS (39.3%) than after DSS (14.8%) or EGDS (0%). Lethality was also significantly higher after SRS (42.9%) than after DSS (14.8%) or EGDS (7.1%). Indirect hyperbilirubinemia was absent after EGDS and was more frequent after DSS (52%), although it was also present after SRS (29.6%). Because of the absence of encephalopathy and the low mortality rates, EGDS was demonstrated to be the best option 93 . As emphasized by Conn 94 many questions remain unanswered. For example, would endoscopic sclerotherapy with splenectomy be as effective? Would esophagogastric devascularization without splenectomy be as effective? Our study was initiated before endoscopy sclerotherapy, variceal ligation, β-adrenergic blockade and transjugular intrahepatic portal-systemic shunt (TIPS) became established as viable forms of therapy in portal hypertension. In our Department of Gastroenterology, patients with schistosomiasis and bleeding esophageal varices are treated with esophagogastric devascularization with splenectomy and, if rebleeding occurs, with sclerosis of the varices19,95.
Correspondencia: Dr. L.C. Da Silva. Rua Silva Correia, 153, ap. 41 ZC 04537-040 São Paulo. Brazil Correo electrónico: fjcarril@usp.br
Recibido el 20-4-2004; aceptado para su publicación el 29-9-2004.