Ascites is the fluid accumulation in the peritoneal cavity, and it is the consequence of a wide variety of entities, being liver cirrhosis the most common one. In this kind of patients, the development of ascites results from splanchnic vasodilation; decreased effective circulating volume; the activation of the sympathetic nervous system and the renin–angiotensin–aldosterone system; and a systemic inflammatory process. Its management is diverse and depends on the severity of the hemodynamic disturbance and other clinical manifestations. In recent years, therapeutic strategies have been developed, but they tend to result unconventional, so new evidence demonstrates the advantages of non-selective beta-blockers for the survival rate of patients with end-stage cirrhosis and ascites.
La ascitis es la acumulación de líquido en la cavidad peritoneal, y es consecuencia de una amplia variedad de entidades, siendo la cirrosis la más frecuente. En este tipo de pacientes, el desarrollo de ascitis resulta de la vasodilatación esplácnica, el volumen circulante efectivo disminuido, la activación del sistema nervioso simpático y el sistema renina-angiotensina-aldosterona, así como un proceso inflamatorio sistémico. Su tratamiento es diverso, y depende de la gravedad de la alteración hemodinámica y otras manifestaciones clínicas. En años recientes se han desarrollado estrategias terapéuticas poco convencionales, así como nueva evidencia demuestra los beneficios de los betabloqueadores no selectivos en la tasa de supervivencia de los pacientes con cirrosis en etapa terminal y ascitis.
Ascites is the fluid accumulation in the peritoneal cavity, and it is the consequence of a wide variety of entities, being liver cirrhosis the most common one with approximately 75% of the cases.1 The mortality rate of patients with cirrhosis and ascites is 21.9% at 1 year compared to 3.4% rate among people with cirrhosis and no ascites.2 There is an incidence of 5–7% per year; that is to say, in the 10 years after their diagnosis, about 60% of patients will have developed ascites, and 5–10% of those will be refractory ascites.3 The management of uncomplicated ascites depends on the stage of hemodynamic disturbance, mainly due to the intensity of portal hypertension and retention of sodium and water. This is represented through the severity of clinical manifestations in three grades: patients with cirrhosis and grade 1: mild ascites only detectable by ultrasound, with volumes as small as 100mL,4 do not need diuretics nor a low sodium diet. For patients with grade 2 ascites: who present a moderate symmetrical abdominal distension, therapy aims to counteract sodium retention with the use of diuretics. In patients with grade 3 marked abdominal distension, the method of choice is Large-volume paracentesis (LVP) in order to remove of more than 5–6L of ascitic fluid. Since refractory ascites develops due to a severe hemodynamic impairment, an approved effective therapy is repeated LVP and intrahepatic transjugular insertion portosystemic shunt (TIPS).5
Nevertheless, in recent years, therapeutic strategies have been developed, but they tend to result unconventional. This review will address the main management strategies for refractory ascites, focusing especially on non-selective beta-blockers (NSBBs).
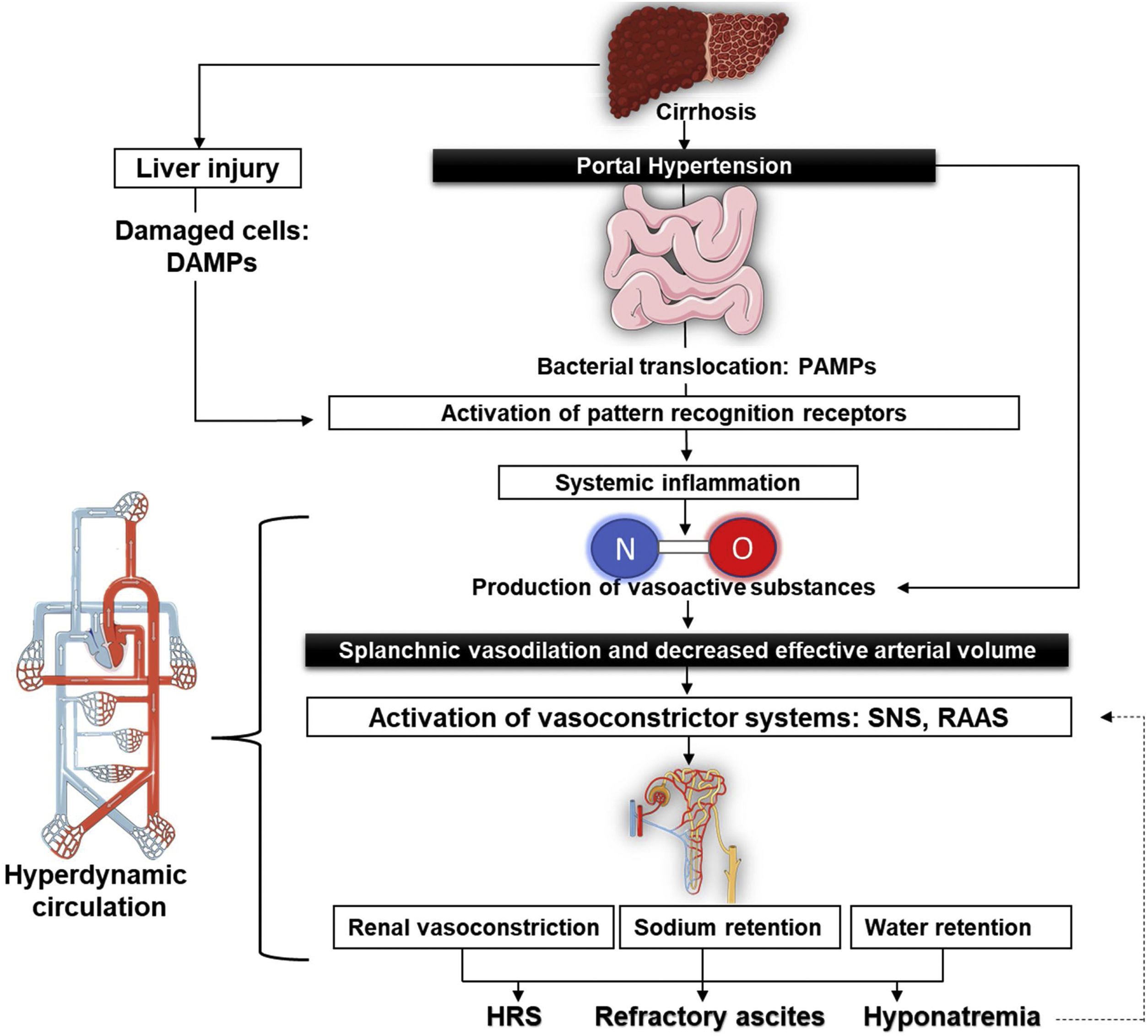
Pathophysiology of refractory ascitesThe structural changes associated with cirrhosis lead to an elevation in intrahepatic vascular resistance and, thus, causing the production of endogenous vasoactive substances such as nitric oxide (NO). The lessening in the degradation of these factors due to liver dysfunction generates splanchnic arterial vasodilation and so reduces the effective circulating volume.6 Some of these vasodilators are transferred to the systemic circulation through portosystemic shunts, produced by the reperfusion and dilatation of pre-existing vessels,7 but also through new vessels generated by angiogenic factors, like the vascular endothelial growth factor.8,9
Systemic vasodilation leads to a lower mean arterial pressure on account of a reduction in the systemic vascular resistance that, while in the early stages of cirrhosis, gets compensated with an increase in the cardiac output in order to keep mean arterial pressure within normal ranges.10 However, as cirrhosis progresses, the systemic vascular resistance lowers markedly, and the rise of cardiac output is no longer able to compensate for it. This conducts to the worsening of the effective circulating volume, relative hypovolemia, and systemic hypotension, thus explaining the hyperdynamic circulation in these patients.11
The increased cardiac output present in the hyperdynamic circulatory state elevates flow-mediated endothelial production and NO release in the systemic circulation and, therefore, the surge in shear stress is a feedback mechanism that improves the production of NO.12 In addition to these mechanical stimuli, pro-inflammatory cytokines contribute to NO-mediated vasodilation.13 Currently it is argued that systemic inflammation also plays an important role in the pathophysiology of ascites. Altered liver clearance produces an accumulation of damage-associated molecular patterns (DAMP) and pathogen-associated molecular patterns (PAMP), which are microbial molecules of translocated bacteria from intestinal lumen due to bacterial overgrowth, intestinal dysbiosis, and increased intestinal permeability interacting with their corresponding pattern recognition receptors of the innate immune system. This is associated with the rise of various plasma cytokines, such as interleukin-6 and tumor necrosis factor α (TNF-α), that contribute to the aforementioned splanchnic arterial vasodilation.14
Every event mentioned above triggers the activation of vasoconstrictor systems15: the sympathetic nervous system produces higher concentrations of norepinephrine to maintain renal perfusion16 while the renin–angiotensin–aldosterone system (RAAS) stimulates renal sodium retention, associated with dilute water clearance caused by nonosmotic hypersecretion of antidiuretic hormone to expand the intravascular volume. All of this may lead to hyponatremia, aggravating the apparition of ascites and, in some cases, even supports the development of Hepatorenal syndrome (HRS)17,18 (Fig. 1).
Treatment of uncomplicated ascitesPatients with cirrhosis and grade 1 ascites do not need diuretics or a low sodium diet; however, patients with grade 2 ascites with positive sodium balance would need a dietary sodium intake of 80–120mmol/day (that is to say, 4.6–6.9g of salt per day) and also diuretics as means to excrete more renal sodium.19 If the patient suffers from their first episode of grade 2 ascites, they may start a plan known as sequential treatment20 where they receive only spironolactone, starting with a dose of 100mg/day; and, in the case they are unresponsive, add up 100mg every 72h up to a maximum of 400mg/day. For patients with hyperkalemia or weight reduction of less than 2kg/week, it is possible to include furosemide (from 40mg/day to a maximum of 160mg/day, in increments of 40mg). Another strategy, called combination treatment, suggests an initial daily regimen of 100mg spironolactone and 40mg furosemide; and, if there is no appropriate response, the doses have to increase gradually while maintaining the constant ratio of both drugs.21
Finally, when it comes to patients with grade 3 ascites, paracentesis is imperative in most cases,22 so the recommendation is to provide 6–8g of albumin for each liter of fluid removed with LVP above 5l.23
Treatment for refractory ascitesThe focus of the newest treatments for patients with ascites is mostly on refractory ascites, defined as unresponsive to dual therapy with maximum doses of diuretics and dietary salt restriction for at least one week, having a mean weight loss of <0.8kg over four days, early recurrence of ascites (grade 2 or 3 ascites within 4 weeks of fluid drainage) and induced complications caused by diuretics.24 LVP is an effective treatment for refractory ascites, yet it should be applied with the co-administration of albumin as a means to prevent post-paracentesis circulatory dysfunction (PPCD).25
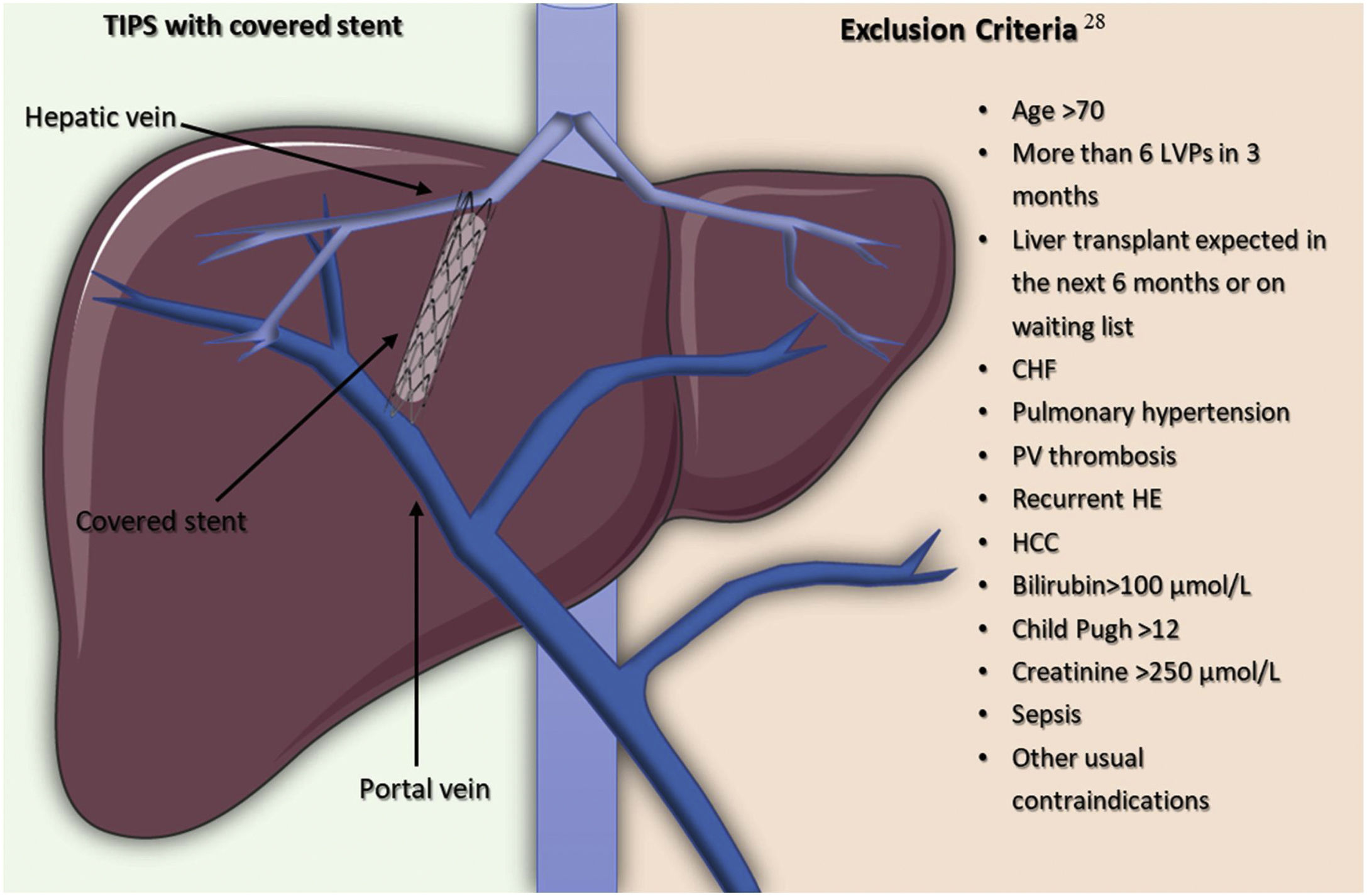
In order to improve the survival rate, an alternative strategy to LVP is to get an interventional radiologist to create and place a TIPS so as to improve the survival rate of selected patients by reducing portal hypertension through a portocaval shunt inserted between an intrahepatic high-pressure portal branch to a low-pressure hepatic vein26 (Fig. 2).
The introduction of stents covered in polytetrafluoroethylene (PTFE) help to avoid pseudo intimal hyperplasia growing inside the stent and, so, they have been a major breakthrough due to improved clinical outcomes and the decrease in the rate of shunt dysfunction, like stent stenosis or thrombosis.27 It is crucial to select accurately patients with refractory ascites for TIPS placement to optimize the benefits and diminish adverse effects. Serum bilirubin and platelet count are able to predict 1-year of the survival rate of patients with a platelet count above 75×109/L and a bilirubin level lower than 3mg/dl, compared to those with a platelet count below 75×109/L or a bilirubin level higher than 3mg/dL (73.1% vs 31.2%, respectively).28 Also, taking into consideration the exclusion criteria for TIPS insertion shown in Fig. 2, coming from a randomized controlled trial involving 62 patients with cirrhosis and recurrent ascites, 93% of the patients in the TIPS with polytetrafluoroethylene-coated stents group, had a 1-year liver transplant-free survival rate and the incidence of hepatic encephalopathy did not increase in comparison to patients in the LVP plus albumin group.29 A recent meta-analysis highlights the importance of patient selection for TIPS placement since they reported a 1-year mortality of 33%, a number that becomes even lower when taking into account more recent studies on treatment with TIPS. The benefit of this can be compared to the 1-year mortality rate of 44% (95% CI 32–58%) and 45% (95% CI 38–53%) in patients treated with peritoneovenous shunts and LVP, respectively.30
Vasopressors such as oral midodrine, which forms an active metabolite, desglyMidodrine, being an alpha1-agonist, rises peripheral resistance by arteriolar constriction, with some venoconstriction in capacitance vessels.31 Accordingly, there are investigations regarding the beneficial effect in hypotensive cirrhotic patients with refractory ascites due to the reports of non-azotemic patients having an increment in mean arterial pressure and a hemodynamic improvement.32,33
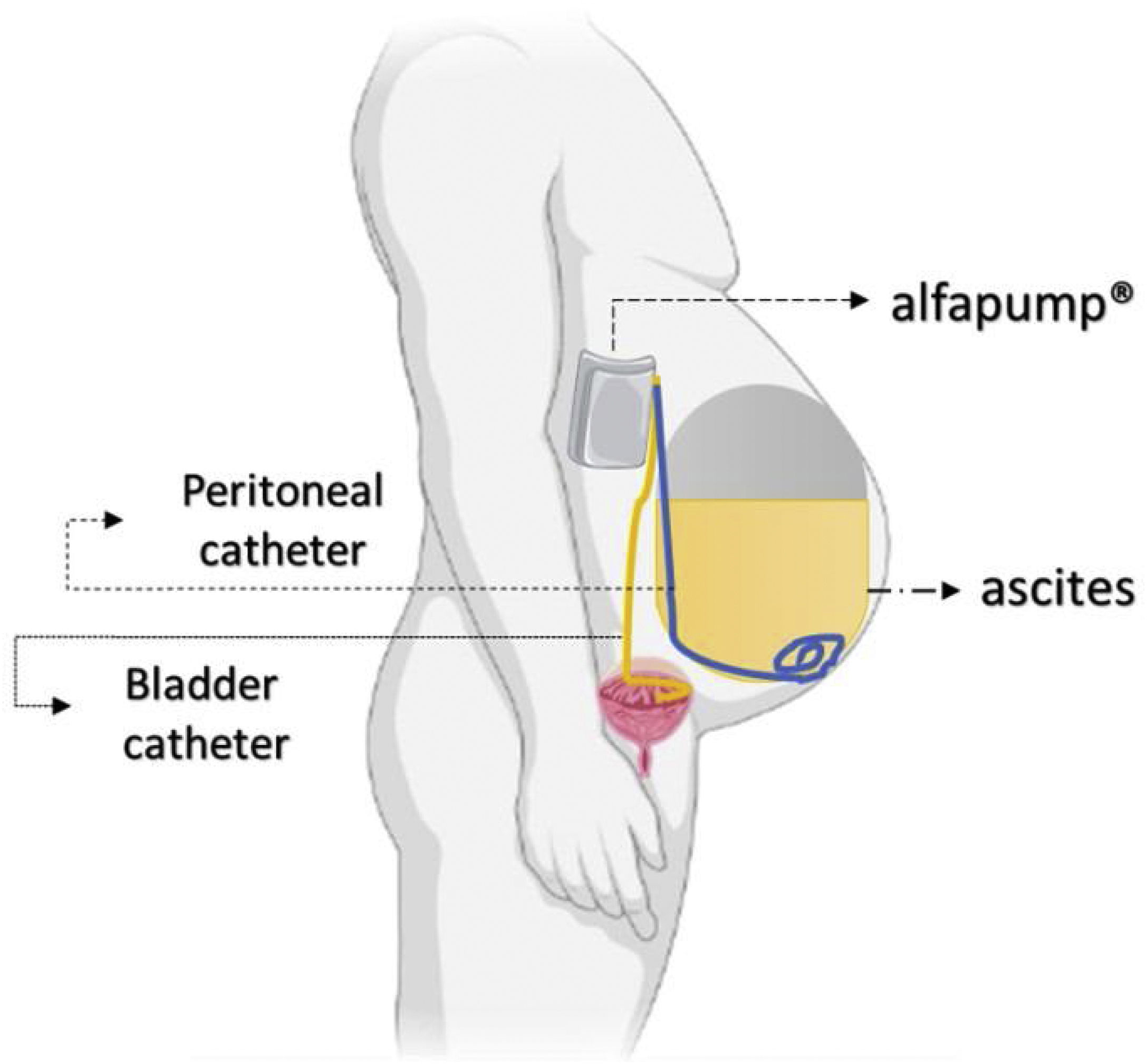
The automated low-flow ascites pump system (ALFApump) consists of a subcutaneously implanted battery-powered programmable pump (Fig. 3) connected to catheters in order to transfer ascites from the peritoneal cavity to the bladder for the purpose of eliminate it through urine. It moves ascites in small portions (usually 5–10mL) every 5–10min, from 0.5 to 2.5L of fluid per day, without needing to administer albumin.34 The pump has internal sensors monitoring the pressure in the abdominal cavity and the bladder to allow it to stop when there is no ascites or when the bladder is full, making the device fully automated.
The pump system removed 90% of ascitic fluid and significantly reduced the median number of LVP sessions,35 although still one-third to half of the cases presented directly adverse effects. In a multicenter RCT with patients with refractory ascites, ALFApump had no effect on survival rate and had a significantly higher incidence of serious adverse events (85.2 vs. 45.2%), mostly acute kidney injury.36
As an alternative to albumin infusions, Japanese authors have proposed a cell-free and concentrated ascites reinfusion therapy (CART) aimed to maintain serum albumin levels by filtrating and concentrating the removed ascitic fluid, followed by intravenous reinfusion of the collected proteins. In a retrospective observational study, Kozaki et al. performed 24 procedures in 11 patients with decompensated cirrhosis and showed the effectiveness and safety of CART.37 There is a clear emphasis on the benefit of albumin reduction, although the instruments’ cost for CART is a drawback since it is higher than that of the albumin solution. Table 1 shows a summary of these and other management options for recurrent and refractory ascites.19
Treatment of patients with recurrent and refractory ascites.
| Diagnosis | Treatment |
|---|---|
| Recurrent ascites | Oral clonidine, 0.1mg 2 times a day, ±20g albumin/week, plus sodium restriction and diuretics |
| Oral midodrine, 7.5mg 3 times a day±20g albumin/week, plus sodium restriction and diuretics | |
| Intravenous terlipressin, 2mg daily±20g albumin/week, plus sodium restriction and diuretics | |
| Large volume paracentesis in non-responders to clonidine or midodrine | |
| Transjugular intrahepatic portosystemic shunt (TIPS) | |
| Refractory ascites | Large volume paracentesis+6–8g of albumin/L of liquid removed above 5L |
| TIPS in non-responders to high volume paracentesis | |
| Automatic low flow pump for ascites evacuation (ALFApump System) | |
| Concentrated and Cell-Free Ascites Reinfusion Therapy (CART) | |
| Extracorporeal ascitic fluid ultrafiltration | |
| Liver transplant | |
| Vasopressors (midodrine, terlipressin, clonidine) | |
New interventional radiology techniques have shown great benefit in the management of patients with refractory ascites. Partial Splenic Embolization (PSE), originally described as a procedure to avoid splenectomy in patients with splenic trauma,38 consists of embolization of splenic artery branches using mainly 300–700μm of polyvinyl alcohol particles, gelatin sponge compresses and coils, inducing splenic infarction and, therefore, the reduction of splenic blood flow contributes to decreased portal pressure with increased hepatic and superior mesenteric blood flow,39 with clinical benefits on hepatic encephalopathy, variceal hemorrhage and hypersplenism in patients with portal hypertension.40 However, serious adverse effects such as splenic abscess and/or left subphrenic abscess, left refractory pleural effusion, pneumonia, acute liver failure and portal vein thrombosis have been reported.41 The development of Proximal Splenic Artery Embolization (PSAE) allowed avoiding the large spleen infarction and therefore the adverse effects of PSE; new evidence suggests clinical improvement by reducing refractory ascites in patients with cirrhosis who are not candidates for TIPS or liver transplantation.42 Used as a complementary therapy in patients with refractory ascites treated with TIPS, showed a decreased incidence of encephalopathy.43 Also as a first-line treatment, case series reports, demonstrated a partial or total improvement of refractory ascites in liver transplant patients.44,45
Moreover, with a combination of interventional radiology and NSBBs, a patient with hepatitis C virus-related decompensated cirrhosis with portal systemic liver failure, ascites and refractory encephalopathy had a successful treatment. Their HVPG, blood ammonia level, ascites, hepatic encephalopathy and portal hypertensive gastropathy improved after undergoing a balloon-occluded retrograde transvenous obliteration (BRTO) followed by a partial splenic embolization (PSE) and NSBBs administration.46 Long-term efficacy of interventional radiology techniques as a therapeutic option in patients refractory ascites should be studied in larger case series.
Non-selective β-blockersClinically significant portal hypertension (CSPH), defined by a hepatic venous pressure gradient (HVPG) of ≥10mmHg, is correlated with esophageal variceal formation related to upper gastrointestinal bleeding, ascites, infection and hepatic encephalopathy.47 The PREDESCI trial observed the prevention of decompensation in liver cirrhosis with the use of NSBBs, in which the assessed the hemodynamic response to beta-blockers by measuring HVPG in 201 patients who were provided randomized to NSBBs or placebos. In a median follow-up of 37 months, the primary outcome, defined as the incidence of a decompensation event such as ascites, portal hypertension-related gastrointestinal bleeding, overt hepatic encephalopathy or even death, decreased substantially in patients that received NSSBs: 27% vs. 16%, (hazard ratio [HR] 0–51, 95% CI 0.26–0.97, p=0.041).48
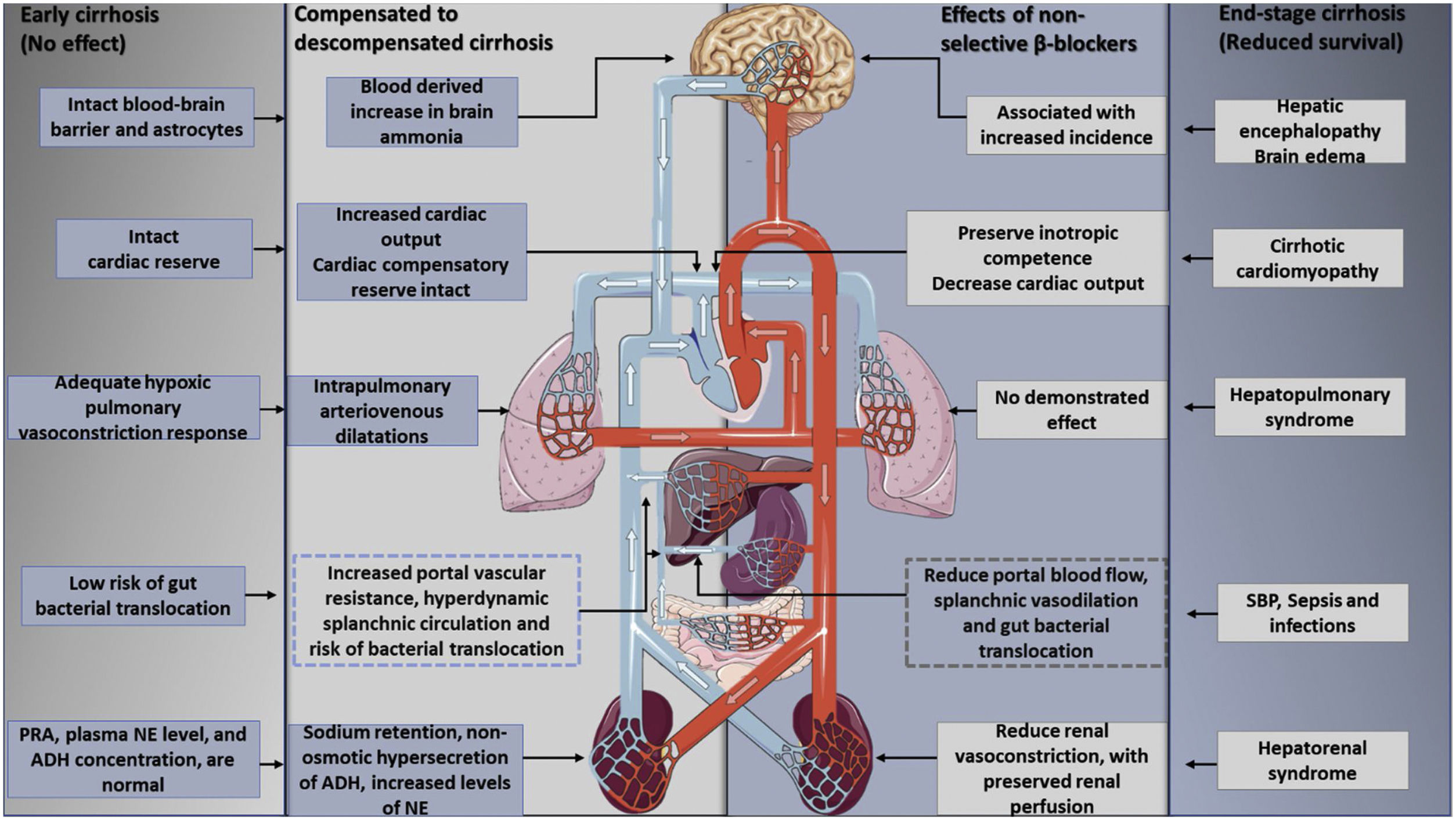
Furthermore, the utilization of non-selective beta-blockers is able to reduce the mortality rate in decompensated cirrhosis: Beta 1 adrenergic blockade lowers cardiac output, while Beta 2 adrenergic blockade decreases portal blood flow with splenic vasoconstriction. This is explained by the so-called window hypothesis49,50 that stipulates that treatment with beta-blocking should start at the beginning of cirrhosis decompensation, a stage characterized by elevated SNS and RAAS activity, and also prone to gut bacterial translocation with the presence of medium and large esophageal varices. In cases like this, treatment has shown a lower risk of variceal bleeding and bacterial translocation, thus diminishing cases of SBP.51–54 This effect still needs a demonstration in relation to early cirrhosis, since, at this point, the portal pressure is high, but has still not reached the CSPH threshold, with no beneficial effects of NSBBS on complications of portal hypertension.55 The window hypothesis closes with the development of end-stage cirrhosis with refractory ascites for there is a progressive reduction in cardiac reserve, enhanced by the betablocker, that results in a lower renal perfusion pressure and an increased risk of developing hepatorenal syndrome56 (Fig. 4).
The effects of nonselective beta-blockers on the therapeutic window in cirrhosis. Beta-blockers have no clear effects in early cirrhosis without ascites or in end-stage cirrhosis, aspects that mark the upper and lower limits of the therapeutic window. The onset of hyperdynamic circulation, with the development of sodium and water retention and ascites formation, reflects an adaptive response to peripheral vasodilatation and effective hypovolemia. However, as cirrhosis progresses, the cardiovascular response eventually loses its compensatory capacity. At this stage, previous evidence suggested that the hemodynamic effects of beta-blockers in reducing blood pressure and cardiac output may actually result in decreased survival in these patients. PRA: plasma renin activity, NE: norepinephrine, ADH: antidiuretic hormone.
Nevertheless, recent evidence suggests a betterment in the survival rate for patients with advanced liver disease. In a single-center cohort, patients with cirrhosis, referred and evaluated for liver transplantation, were given NSBBs and their short-term survival rate improved (lower 90-day mortality, 6% vs. 15%, with a risk hazard ratio of 0.27; 95% confidence interval [CI] 0.09–0.88, p=0.03).57 In contrast, in an analysis that included 3 randomized control trials and 8 observational studies of propranolol, carvedilol, nadolol and metoprolol in patients with cirrhosis and ascites, the use of NSBBs did not raise significantly the all-cause mortality rate at 6, 12, 18 and 24 months in patients with ascites (relative risk [RR] 0.95; 95% CI 0.67–1.35), nonrefractory ascites (RR 0.96, CI 0.5–1.82) or refractory ascites (RR 0.95, CI 0.57–1.61).58
Another recent meta-analysis of 15 clinical trials showed that patients with cirrhosis and ascites who respond to treatment with NSBBs have are less prone to refractory ascites, variceal hemorrhage, spontaneous bacterial peritonitis or hepatorenal syndrome when compared to nonresponders (odds ratio [OR] 0.27; 95% CI, 0.16–0.43); these results are based in reductions in HVPG. For patients with cirrhosis, with or without ascites, the odds of liver transplantation or death were lower among responders (OR, 0.47; 95% CI, 0.29–0.75).59
There are few methods to assess the efficacy of a treatment, the gold standard being the measurement of HVPG, defined as the pressure difference between wedged hepatic venous pressure (WHVP) and free hepatic venous pressure (FHVP). The reduction of HVPG to <12mmHg or by >10–20% of baseline decreases the chances of bleeding.60 Due to its low availability and the small number of patients that are in risk of adverse events (for example, hematoma, leakage, arteriovenous fistula, or Horner's syndrome),61 the use of clinical endpoints has been evaluated, such as using the dose necessary to achieve a target of 50–55bpm, with the most popular objective being a 25% decrease in heart rate. However, there is currently no consensus that defines the correct way to dose and evaluate the response to beta-blockers.62
ConclusionsThe development of refractory ascites not only represents a watershed in the prognosis of patients with cirrhosis, but also is associated with high mortality; therefore, all patients with cirrhosis and refractory ascites are possible candidates for liver transplantation. The key to successful treatment is a personalized therapy with a pathogenetically based approach, taking into consideration the systemic hemodynamics, where the use of beta-blockers may benefit the survival rate of patients beyond the so-called therapeutic window, as has been reviewed here in patients with end-stage cirrhosis.
A non-invasive evaluation of the beta-blockers hemodynamic efficacy is not yet established, which allows the execution of this proposed personalized therapy. More studies are necessary regarding non-invasive evaluation of the hemodynamic response to non-selective beta-blockers, as demonstrated in the study by Téllez et al.,56 where an echocardiogram through the ejection intraventricular pressure difference (EIVPD) estimated systolic function, correlated with sympathetic activation and renal perfusion pressure, in addition to clinical and biochemical markers, such as severe hyponatremia, systolic blood pressure, decreased heart rate and increased serum creatinine.63
Given the heterogeneity in the existing results due to differences in the dose, type of beta-blocker and the duration of treatment prior and during the study,64 a new comparative, randomized and open clinical trial exposing these differences may provide answers to these issues. Finally, it would be interesting to know the benefit that the combination of drugs such as midodrine with non-selective beta-blockers could have, considering it could theoretically improve the deleterious effects of beta-blockers on kidney function. These conclusions can only be reached with the results of ongoing trials (NCT04208776).
Ethical considerationsThis work does not involve the use of human subjects.
FinancingThis research has not received specific aid from public sector agencies, the commercial sector or non-profit entities.
Conflict of interestNone.