Swallowed topical steroids are a mainstay drug therapy for eosinophilic esophagitis (EoE), studies have demonstrated good histologic response, but with enormous discrepancy in clinical and endoscopic improvement. We conducted this meta-analysis to investigate the efficacy of topical steroids in EoE in histological, clinical and endoscopic improvement.
MethodsSeveral databases were searched from inception to August 1, 2019 for randomized controlled trials (RCTs) comparing topical steroids with placebo for EoE in the short-term. The outcomes of interest mainly included basic characteristics of the studies, histologic, clinical, endoscopic response rate and adverse events. The results were pooled together using Reviewer Manager 5.3.5 software, and inconsistency was quantified using I2 statistics.
ResultsNine studies were eventually selected. The results showed that topical steroids were effective in inducing histologic response compared with placebo for both complete (OR 35.82, 95% CI 14.98–85.64, P<0.0001; I2=0, P=0.72) and partial response (OR 28.44, 95% CI 8.56–94.47, P<0.0001; I2=70%, P=0.0009). Moreover, topical steroids were useful in gaining clinical response (OR 2.53, 95% CI 1.14–5.60, P=0.02; I2=60%, P=0.02) and endoscopic response (OR 3.51, 95% CI 1.47–8.36, P=0.005; I2=0, P=0.57). Generally, topical steroids are well tolerated. The most common adverse events are infections and infestations (59 cases).
ConclusionTopical steroids were effective in inducing histological, clinical and endoscopic response in the short-term, and the adverse events were almost tolerable; however, we should interpret the result of clinical and endoscopic response with caution.
Los esteroides tópicos tragados son una terapia farmacológica principal de la esofagitis eosinofílica (EoE). Algunos estudios han demostrado una buena respuesta histológica, aunque con gran discrepancia en la mejora clínica y endoscópica. Hemos realizado este metaanálisis para investigar la eficacia de los esteroides tópicos en EoE en la mejora histológica, clínica y endoscópica.
MétodosSe buscaron varias bases de datos desde el inicio hasta el 1 de agosto de 2019 para ensayos controlados aleatorios comparando esteroides tópicos con placebo para EoE a corto plazo. Los resultados de interés incluyeron principalmente características básicas de los estudios, histológicas, clínicas, tasa de respuesta endoscópica y eventos adversos. Los resultados se agruparon mediante el software de Reviewer Manager 5.3.5, y la incoherencia se cuantificó mediante estadísticas I2.
ResultadosNueve estudios fueron finalmente seleccionados. Los resultados mostraron que los esteroides tópicos fueron eficaces en la inducción de la respuesta histológica en comparación con placebo tanto para la respuesta completa (OR 35,82; IC 95%: 14,98-85,64; p<0,0001; I2 0, p=0,72) como para la parcial (OR 28,44; IC 95%: 8,56-94,47; p<0,0001; I2 70%, p=0,0009). Los esteroides tópicos también fueron útiles en la obtención de respuesta clínica (OR 2,53; IC 95%: 1,14-5,60; p=0,02; I2 60%; p=0,02) y respuesta endoscópica (OR 3,51; IC 95%: 1,47-8,36; p=0,005; I2 0; p=0,57). Los esteroides tópicos suelen ser bien tolerados. Los acontecimientos adversos más frecuentes son infecciones e infestaciones (59 casos).
ConclusiónLos esteroides tópicos fueron eficaces en la inducción de la respuesta histológica, clínica y endoscópica a corto plazo, y los eventos adversos fueron tolerables; sin embargo, debemos interpretar con precaución el resultado de la respuesta clínica y endoscópica.
Eosinophilic esophagitis (EoE) is an immune-mediated chronic inflammatory condition of the esophagus characterized by elevated eosinophils infiltration. The clinical manifestation is mainly esophagus dysfunction, including heart burn, dysphagia and food impaction.1 The prevalence of EoE is not high in Asian countries, but has risen recently, as in western countries.2,3 The diagnosis of EoE is made upon clinical symptoms and histopathologic biopsy results.4 When EoE is suspected, an upper endoscopy with esophageal biopsy is required. The endoscopic features of EoE are similar in children and adults. However, adult patients may exhibit a higher frequency of rings and strictures compared to children, as a consequence of esophageal remodeling due to sustained eosinophilic inflammation in the long term.5 Multiple specimens from at least two levels, targeting area of apparent inflammation are recommended to increase the diagnostic yield.6 The diagnosis of EoE is confirmed if at least one esophageal specimen shows a minimum of 15 eosinophils per high power field (eos/hpf), with the exclusion of other causes of symptoms and/or esophageal eosinophilia.
The short-term goals of treatment include symptom resolution and histologic response, and the long-term management goal is the prevention of dysmotility and strictures.6-8 Swallowed topical steroids are a mainstay of EoE drug therapy as they provide an anti-inflammatory effect, with a secondary function in reducing esophageal remodeling and fibrosis.9 Other formulations, such as oral aerosolized fluticasone propionate and oral viscous budesonide are also frequently prescribed as off-label therapy in EoE. Various studies have evaluated the efficacy of topical steroids, and most studies have shown good histologic response, however, with respect to clinical and endoscopic improvement, the results are conflicting. Murali et al. performed a literature search from inception to 2015 of randomized controlled trials (RCTs) comparing topical steroids with placebo in EoE.10 They selected five studies and performed a meta-analysis, showing that topical steroids are effective in inducing histological response but may not have a similar significant impact on the resolution of clinical symptoms resolution in EoE. Since then, more studies have been published, although the pooled results have not been updated. Moreover, the pooled results on the efficacy of topical steroids in endoscopic improvement have not yet been evaluated. Thus, we conducted this meta-analysis to investigate the efficacy of topical steroids in EoE based on histological, clinical and endoscopic improvement for the induction of remission.
MethodsData sources and selection criteriaWe searched the PubMed, Embase, and Cochrane Controlled Register Databases, for the articles published from inception to August 1, 2019. The following search strategy was used [topical steroid OR topical steroids OR budesonide OR fluticasone OR topical corticosteroid OR topical corticosteroids] AND eosinophilic esophagitis.
Two authors selected the articles independently. The inclusion criteria were: (1) RCTs comparing topical steroids with placebo in EoE; (2) histological, clinical and endoscopic improvement were reported; (3) the response rate could be extracted; (4) references from eligible articles or reviews were also assessed. The exclusion criteria were: (a) case reports, editorials, letters, reviews or systematic reviews; (b) the total number of patients was less than 10; (c) the response rate could not be calculated; (d) Jadad scale less than 211; (e) for studies with repeated data, the one of better quality was chosen.
Study selection and data extractionAfter a thorough search, the duplicates were first removed, and then two reviewers scanned the titles and abstracts to identify eligible articles. Later, the full-text was assessed using the inclusion criteria and the exclusion criteria. If any dispute existed, the two reviewers discussed or asked for help from a third reviewer. Two investigators extracted the data from the final selected trials using a standardized form, mainly including the author, country, publication year, study design, information of the patients, name of the drug, the route and dose administered, the histological definition of EoE, complete and/or partial response, clinical and endoscopic response rate, and adverse events.
Statistical analysisReviewer Manager 5.3.5 software (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014) was used to calculate the pooled odds ratios (ORs) and 95% confidence intervals (CIs), and the Mantel-Haenszel type method was used. Heterogeneity among studies was assessed by the I2 statistic. I2>50% was regarded as significant heterogeneity. If I2>50%, the random model was used, otherwise, the fixed model was used. Statistical significance was defined as P<0.05 (two-tailed). Cochrane collaboration tool was used to assess the risk of bias.
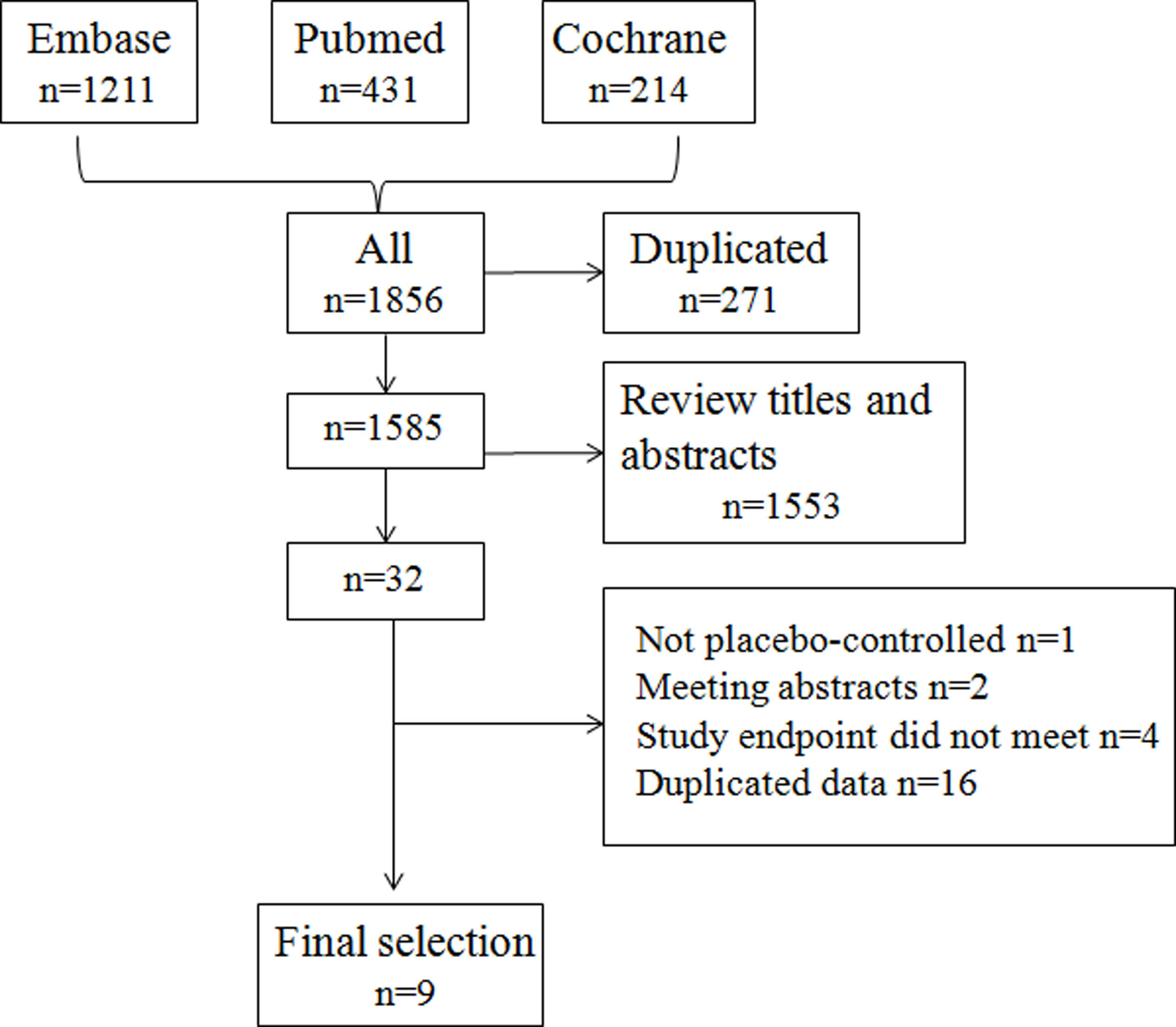
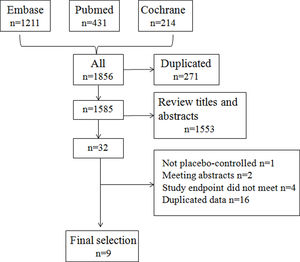
ResultsSearch results and study characteristicsThe initial research identified 1585 articles after removing duplicates, and no new articles were identified by a reference search. Based on the inclusion criteria, nine studies were selected for final analysis.12–20Fig. 1 shows the selection algorithm.
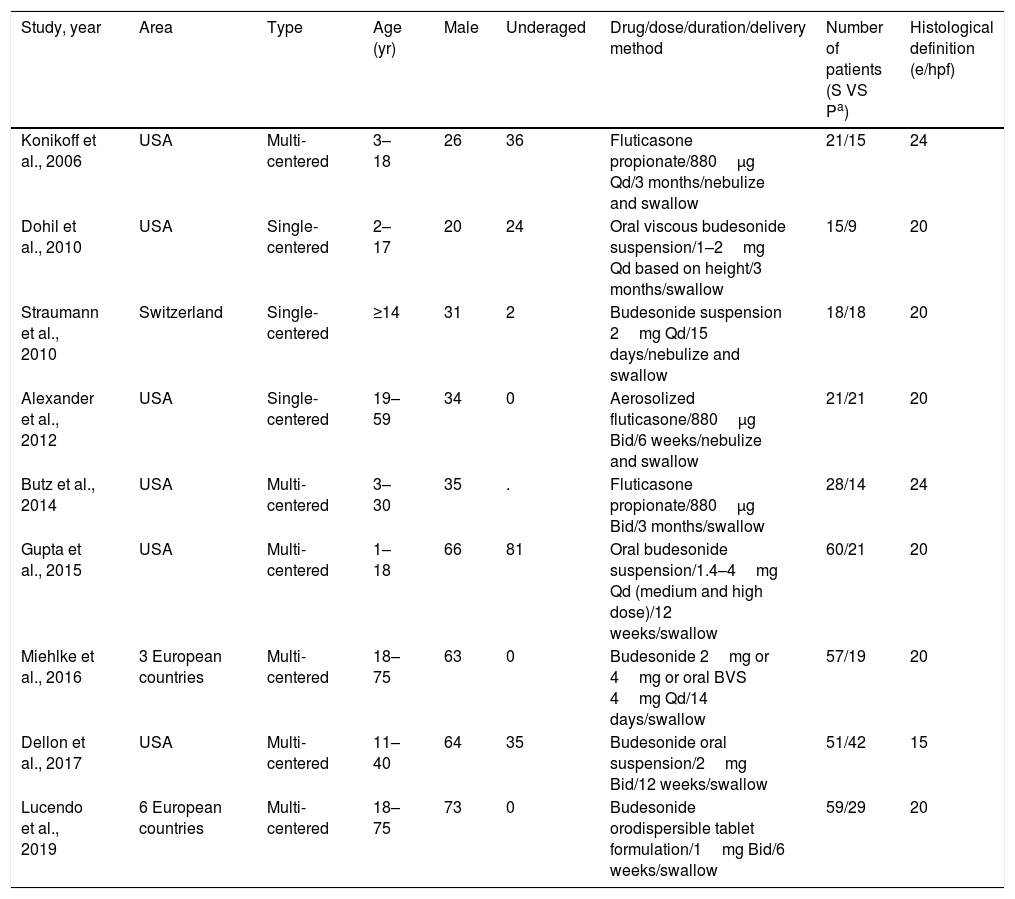
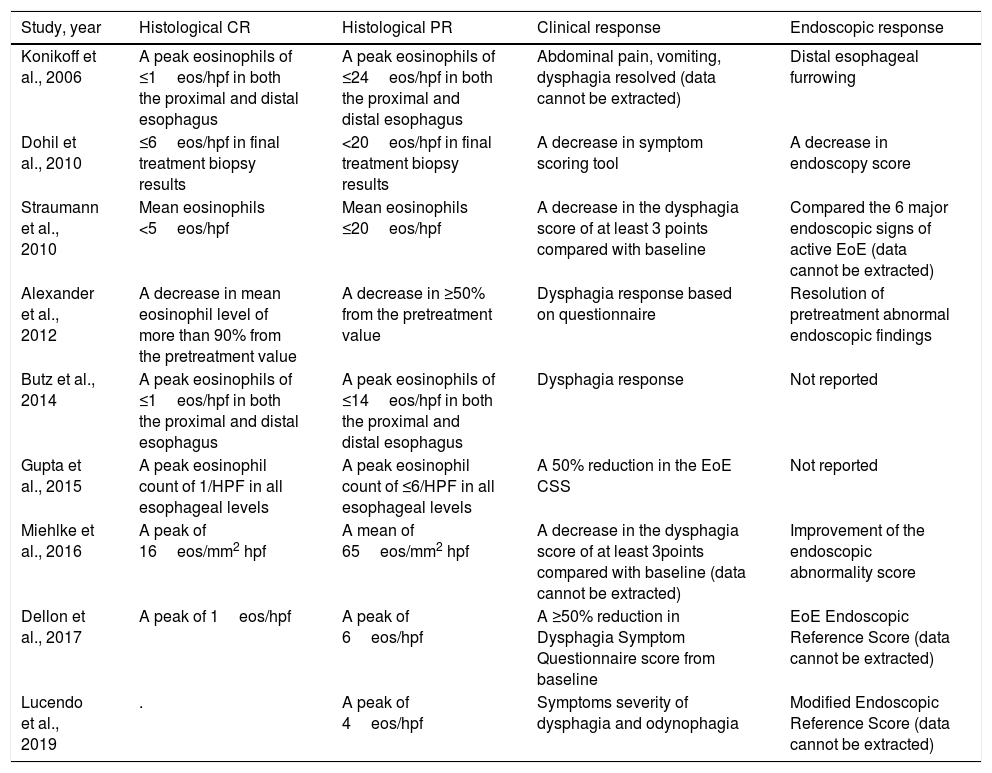
The basic characteristics of the studies are shown in Table 1. Altogether, 518 patients were enrolled in the nine studies. In the study by Gupta et al.,17 we analyzed only the patients who received the medium and high doses, excluding the patients who received the low dose (other studies also used the same doses, but no study used the low dose as in Gupta et al.). The cut-offs of histological enrollment definition were different in these studies, ranging from 15 to 24 (Table 2 shows the detail). All the studies evaluated the histological response; the clinical response was also evaluated in the 9 studies, but in 2 studies, the data cannot be extracted to perform the meta-analysis; 7 studies reported endoscopic response, the data of only 3 studies which used endoscopic score assessment could be used. The various definitions of histological, clinical and endoscopic outcome of the selected studies were displayed in Table 2.
Basic characteristics of the selected studies.
| Study, year | Area | Type | Age (yr) | Male | Underaged | Drug/dose/duration/delivery method | Number of patients (S VS Pa) | Histological definition (e/hpf) |
|---|---|---|---|---|---|---|---|---|
| Konikoff et al., 2006 | USA | Multi-centered | 3–18 | 26 | 36 | Fluticasone propionate/880μg Qd/3 months/nebulize and swallow | 21/15 | 24 |
| Dohil et al., 2010 | USA | Single-centered | 2–17 | 20 | 24 | Oral viscous budesonide suspension/1–2mg Qd based on height/3 months/swallow | 15/9 | 20 |
| Straumann et al., 2010 | Switzerland | Single-centered | ≥14 | 31 | 2 | Budesonide suspension 2mg Qd/15 days/nebulize and swallow | 18/18 | 20 |
| Alexander et al., 2012 | USA | Single-centered | 19–59 | 34 | 0 | Aerosolized fluticasone/880μg Bid/6 weeks/nebulize and swallow | 21/21 | 20 |
| Butz et al., 2014 | USA | Multi-centered | 3–30 | 35 | . | Fluticasone propionate/880μg Bid/3 months/swallow | 28/14 | 24 |
| Gupta et al., 2015 | USA | Multi-centered | 1–18 | 66 | 81 | Oral budesonide suspension/1.4–4mg Qd (medium and high dose)/12 weeks/swallow | 60/21 | 20 |
| Miehlke et al., 2016 | 3 European countries | Multi-centered | 18–75 | 63 | 0 | Budesonide 2mg or 4mg or oral BVS 4mg Qd/14 days/swallow | 57/19 | 20 |
| Dellon et al., 2017 | USA | Multi-centered | 11–40 | 64 | 35 | Budesonide oral suspension/2mg Bid/12 weeks/swallow | 51/42 | 15 |
| Lucendo et al., 2019 | 6 European countries | Multi-centered | 18–75 | 73 | 0 | Budesonide orodispersible tablet formulation/1mg Bid/6 weeks/swallow | 59/29 | 20 |
Definitions of outcome of the selected studies.
| Study, year | Histological CR | Histological PR | Clinical response | Endoscopic response |
|---|---|---|---|---|
| Konikoff et al., 2006 | A peak eosinophils of ≤1eos/hpf in both the proximal and distal esophagus | A peak eosinophils of ≤24eos/hpf in both the proximal and distal esophagus | Abdominal pain, vomiting, dysphagia resolved (data cannot be extracted) | Distal esophageal furrowing |
| Dohil et al., 2010 | ≤6eos/hpf in final treatment biopsy results | <20eos/hpf in final treatment biopsy results | A decrease in symptom scoring tool | A decrease in endoscopy score |
| Straumann et al., 2010 | Mean eosinophils <5eos/hpf | Mean eosinophils ≤20eos/hpf | A decrease in the dysphagia score of at least 3 points compared with baseline | Compared the 6 major endoscopic signs of active EoE (data cannot be extracted) |
| Alexander et al., 2012 | A decrease in mean eosinophil level of more than 90% from the pretreatment value | A decrease in ≥50% from the pretreatment value | Dysphagia response based on questionnaire | Resolution of pretreatment abnormal endoscopic findings |
| Butz et al., 2014 | A peak eosinophils of ≤1eos/hpf in both the proximal and distal esophagus | A peak eosinophils of ≤14eos/hpf in both the proximal and distal esophagus | Dysphagia response | Not reported |
| Gupta et al., 2015 | A peak eosinophil count of 1/HPF in all esophageal levels | A peak eosinophil count of ≤6/HPF in all esophageal levels | A 50% reduction in the EoE CSS | Not reported |
| Miehlke et al., 2016 | A peak of 16eos/mm2 hpf | A mean of 65eos/mm2 hpf | A decrease in the dysphagia score of at least 3points compared with baseline (data cannot be extracted) | Improvement of the endoscopic abnormality score |
| Dellon et al., 2017 | A peak of 1eos/hpf | A peak of 6eos/hpf | A ≥50% reduction in Dysphagia Symptom Questionnaire score from baseline | EoE Endoscopic Reference Score (data cannot be extracted) |
| Lucendo et al., 2019 | . | A peak of 4eos/hpf | Symptoms severity of dysphagia and odynophagia | Modified Endoscopic Reference Score (data cannot be extracted) |
CR, complete response; PR, partial response; eos/hpf, eosinophils per high power field.
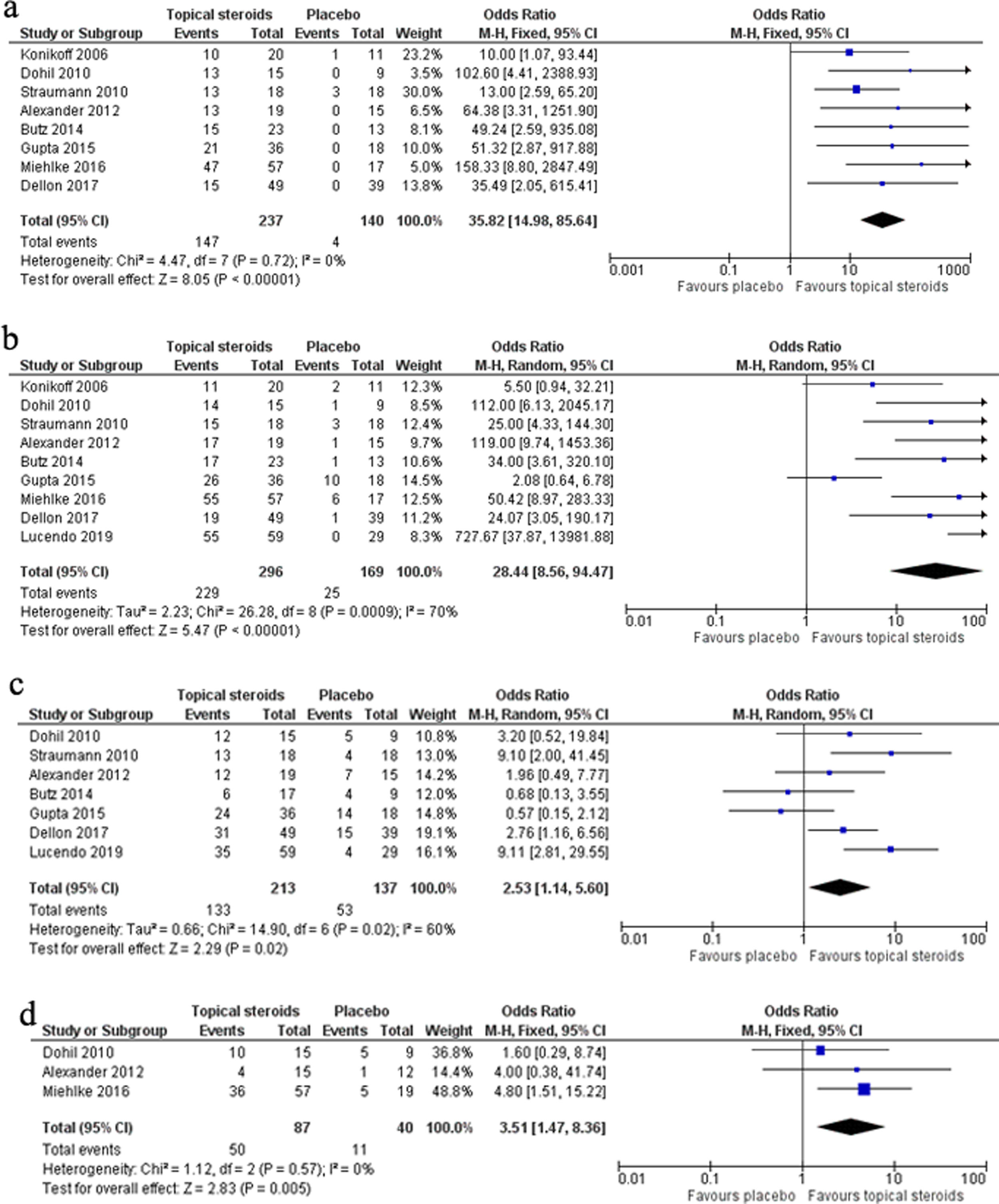
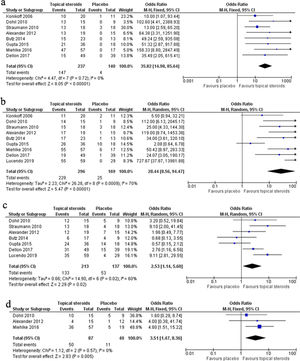
The meta-analysis of 8 RCTs including 377 patients showed remarkable efficacy of topical steroids in inducing complete response (CR) compared with placebo (OR 35.82, 95% CI 14.98–85.64, P<0.0001). No significant heterogeneity was found among the studies (I2=0, P=0.72). The pooled CR rate of topical steroids and placebo was 62.03% and 2.86%, respectively (Fig. 2a).
The meta-analysis of nine RCTs including 465 patients showed marked efficacy of topical steroids in gaining partial response (PR) compared with placebo (OR 28.44, 95% CI 8.56–94.47, P<0.0001), but significant heterogeneity was found among the studies (I2=70%, P=0.0009). The pooled PR rate of topical steroids and placebo was 77.36% and 14.79%, respectively (Fig. 2b).
The meta-analysis of seven RCTs including 350 patients showed significant efficacy of topical steroids in obtaining clinical response compared with placebo (OR 2.53, 95% CI 1.14–5.60, P=0.02), but significant heterogeneity was found among the studies (I2=60%, P=0.02). The pooled clinical response rate of topical steroids and placebo was 62.44% and 38.69%, respectively (Fig. 2c).
The meta-analysis of three RCTs including 127 patients showed evident efficacy of topical steroids in having endoscopic response compared with placebo (OR 3.51, 95% CI 1.47–8.36, P=0.005). No significant heterogeneity was found among the studies (I2=0, P=0.57). The pooled endoscopic response rate of topical steroids and placebo was 57.47% and 27.5%, respectively (Fig. 2d).
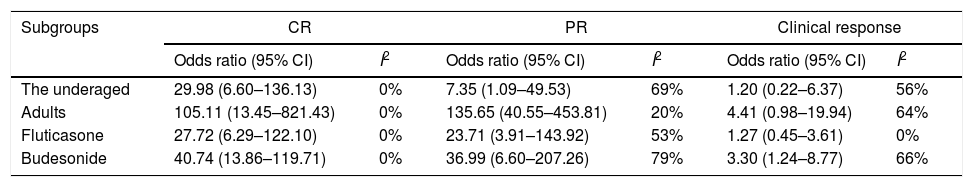
Subgroup analysisThe underaged and the adultsThe underaged patientsIn three studies, altogether including 109 patients, the histological response rate of topical steroids in the underaged patients could be extracted for meta-analysis. The result showed that topical steroids were more effective at acquiring CR and PR compared with placebo in the underaged (Table 3). The pooled CR rate of topical steroids and placebo was 61.97% and 2.63%, and the pooled PR rate was 71.83% and 34.21%, respectively.
Subgroup analysis results.
| Subgroups | CR | PR | Clinical response | |||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | I2 | Odds ratio (95% CI) | I2 | Odds ratio (95% CI) | I2 | |
| The underaged | 29.98 (6.60–136.13) | 0% | 7.35 (1.09–49.53) | 69% | 1.20 (0.22–6.37) | 56% |
| Adults | 105.11 (13.45–821.43) | 0% | 135.65 (40.55–453.81) | 20% | 4.41 (0.98–19.94) | 64% |
| Fluticasone | 27.72 (6.29–122.10) | 0% | 23.71 (3.91–143.92) | 53% | 1.27 (0.45–3.61) | 0% |
| Budesonide | 40.74 (13.86–119.71) | 0% | 36.99 (6.60–207.26) | 79% | 3.30 (1.24–8.77) | 66% |
CR, complete response; PR, partial response.
In two studies, altogether including 78 patients, the clinical response rate of topical steroids in the underaged patients could be extracted for meta-analysis. The results showed that the clinical response rate had no significant difference between topical steroids and placebo in the underaged (Table 3). The pooled clinical response rate of topical steroids and placebo was 70.59% and 70.37%, respectively.
The adult patientsIn two studies, altogether including 109 patients, the complete histological response rate of topical steroids in the adults could be extracted for meta-analysis. The results showed that topical steroids were more effective at inducing CR compared with placebo in the adults (Table 3). The pooled CR rate of topical steroids and placebo was 78.95% and 0%, respectively. In 3 studies, altogether 196 patients, the partial histological response rate of topical steroids in the adults could be extracted for meta-analysis. The result showed that topical steroids were effective in inducing PR compared with placebo in the adults (Table 3). The pooled PR rate of topical steroids and placebo was 94.07% and 11.48%, respectively.
In two studies, altogether including 122 patients, the clinical response rate of topical steroids in the adult patients could be extracted for meta-analysis. The results showed that the clinical response rate had no significant difference between topical steroids and placebo in adult patients (Table 3). The pooled clinical response rate of topical steroids and placebo was 60.26% and 25%, respectively.
Different kinds of topical steroidsFluticasoneIn three studies, altogether including 101 patients, the complete histological response rate of fluticasone could be extracted for meta-analysis. The result showed that fluticasone was more effective at inducing CR compared with placebo (Table 3). The pooled CR rate of fluticasone and placebo was 61.29% and 2.56%, respectively. In three studies, altogether including 101 patients, the partial histological response rate of fluticasone could be extracted for meta-analysis. The results showed that fluticasone was more effective at inducing PR compared with placebo (Table 3). The pooled PR rate of fluticasone and placebo was 72.58% and 10.26%, respectively.
In two studies, altogether including 60 patients, the clinical response rate of fluticasone could be extracted for meta-analysis. The results showed that the clinical response rate had no significant difference between fluticasone and the placebo (Table 3). The pooled clinical response rate of fluticasone and placebo was 50.00% and 45.83%, respectively.
BudesonideIn five studies, altogether including 276 patients, the complete histological response rate of budesonide could be extracted for meta-analysis. The results showed that budesonide was more effective at inducing CR compared with placebo (Table 3). The pooled CR rate of budesonide and placebo was 62.29% and 2.97%, respectively. In six studies, altogether including 374 patients, the partial histological response rate of budesonide could be extracted for meta-analysis. The results showed that budesonide was more effective at inducing PR compared with placebo (Table 3). The pooled PR rate of budesonide and placebo was 78.63% and 15.00%, respectively.
In five studies, altogether including 290 patients, the clinical response rate of budesonide could be extracted for meta-analysis. The results showed that budesonide was more effective at inducing clinical response compared with placebo (Table 3). The pooled clinical response rate of budesonide and placebo was 64.97% and 37.17%, respectively.
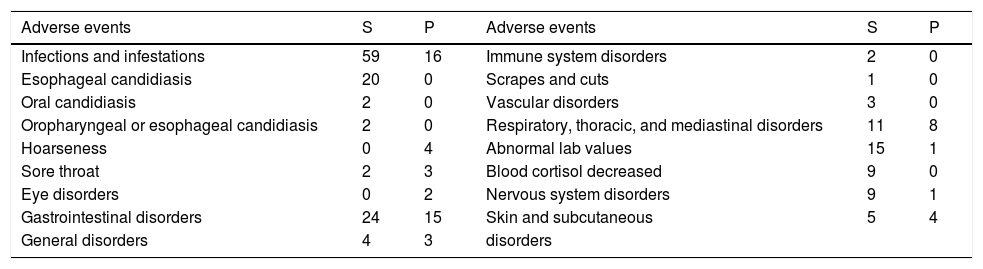
Adverse eventsGenerally, topical steroids are well tolerated. The most common adverse event was infections and infestations (59 cases), and oropharyngeal or esophageal candidiasis was the most common infection (24 cases). Other common adverse events include gastrointestinal disorders (24 cases), abnormal laboratory values (15 cases, and 9 were decreased blood cortisol), respiratory, thoracic, and mediastinal disorders (11 cases), and nervous system disorders (9 cases). Other adverse events were sore throat (2 cases), general disorders (4 cases), immune system disorders (2 cases), scrapes and cuts (1 case), vascular disorders (3 cases) and skin and subcutaneous disorders (5 cases). Table 4 shows the adverse events in each group.
Adverse events in each group.
| Adverse events | S | P | Adverse events | S | P |
|---|---|---|---|---|---|
| Infections and infestations | 59 | 16 | Immune system disorders | 2 | 0 |
| Esophageal candidiasis | 20 | 0 | Scrapes and cuts | 1 | 0 |
| Oral candidiasis | 2 | 0 | Vascular disorders | 3 | 0 |
| Oropharyngeal or esophageal candidiasis | 2 | 0 | Respiratory, thoracic, and mediastinal disorders | 11 | 8 |
| Hoarseness | 0 | 4 | Abnormal lab values | 15 | 1 |
| Sore throat | 2 | 3 | Blood cortisol decreased | 9 | 0 |
| Eye disorders | 0 | 2 | Nervous system disorders | 9 | 1 |
| Gastrointestinal disorders | 24 | 15 | Skin and subcutaneous | 5 | 4 |
| General disorders | 4 | 3 | disorders |
S, topical steroids; P, placebo.
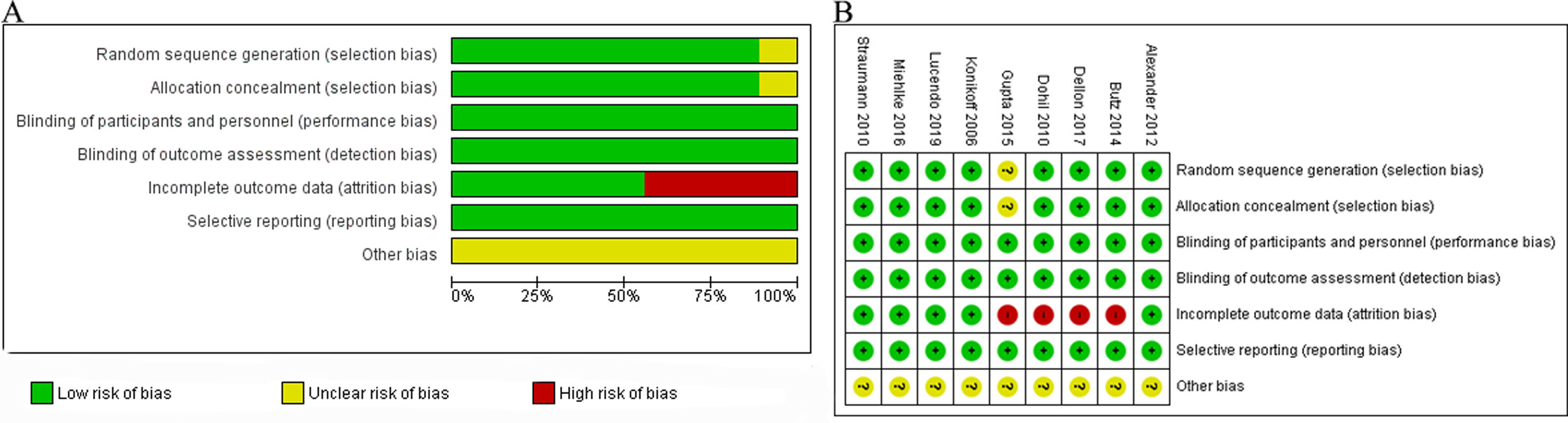
The risk of bias mainly lies in incomplete outcome data as shown in Fig. 3. Patients withdrawn from trials in four studies13,16,17,19 might influence the result, as there were patients withdrawn due to adverse events, so the case of adverse events may be underestimated. Since the number of studies in each analysis group was less than 10, it is difficult to draw the funnel plot and reveal the publication bias. We tried our best to minimize the publication bias by searching the PubMed, Embase, and Cochrane Controlled Register Databases and the references exhaustively.
DiscussionThe incidence of EoE has risen in recent years, it has been described in patients of various ethnic groups, and is more prevalent in males than in females.13,16,17,19 The clinical manifestations may vary from children to adults, as feeding refusal or intolerance is a common symptom in children who are perhaps too young to express the feeling of dysphagia, while for the adults, the most common symptom is dysphagia. Other symptoms include gastroesophageal reflux disease (GERD)-like symptoms, food impaction, and so on.21 The endoscopic findings include longitudinal furrowing, edema, increased fragility, exudates, white specks, strictures, and the other,22 but none of the features can be regarded as pathognomonic for EoE. However, with clinical manifestations, the presence of more than one of these findings is strongly suggestive of EoE.22 Some studies have even reported a normal appearance of the mucosa; hence, biopsy is of great importance. Multiple biopsy specimens should not only be obtained from different esophageal locations, but also from the stomach and duodenum to rule out other diseases such as eosinophilic gastroenteritis and inflammatory bowel disease.23,24
Glucocorticoids are known to be effective in the treatment of EoE, nevertheless, long-term administration is often necessary for EoE, which may subsequently increase the risk of adverse events.25 To minimize adverse events, topical glucocorticoids including budesonide and fluticasone have been investigated.26,27 These agents are known to be directly delivered to the esophageal mucosa and quickly degraded by the liver.23,28 However, considerable discrepancy exists regarding corticosteroid efficacy in different studies due to the wide variability in methodology, drugs, doses, duration and outcome parameters. In the present meta-analysis, we compared the histological, clinical and endoscopic response of topical steroids with placebo in patients with EoE.
Histologic remission is one of the treatment end points in EoE.23 Our findings demonstrated excellent complete histologic remission with topical steroids, and subgroup analysis further pointed out that, whether in the underaged or the adult patients, and whether budesonide or fluticasone was used, the effectiveness was certain, and without heterogeneity among the studies. When it comes to partial remission, the result was positive, but heterogeneity existed among the studies. Our findings are similar to previously published results.10 The reason for this heterogeneity might lie in that the definition of CR and PR, which is different in each study. For CR, the definition is stricter, so the range is small, which might have helped in reducing heterogeneity. Nevertheless, for PR, the scope is much larger among different studies, which might explain the cause of heterogeneity.
In our study, the clinical response with topical steroids was statistically better than the placebo, but great heterogeneity was observed. Regarding further subgroup analysis, we did not observe superiority in the clinical response neither in the underaged nor the adult patients, but budesonide might be more effective in inducing a clinical response. The reasons for heterogeneity might be, firstly, that most of the studies did not use validated measure tools to evaluate clinical symptoms in EoE, probably because had not been developed at the time the studies were performed. Secondly, some studies mainly evaluated dysphagia,14,19 while two others also evaluated additional symptoms, such as abdominal pain, and food impaction and the like,13,20 however, even if we excluded these two studies, the heterogeneity was still considerable (I2=58%). Thirdly, the clinical symptom are associated with other complications, for example, if fibrotic stricture appeared, it is difficult to have clinical response to medication, and at that time, and endoscopic dilation is needed.29,30 Fourthly, the clinical response does not always mirror the histological response, as tissue remodeling and strictures might need some time to respond9,30; the duration of the studies varied greatly, which might influence the result. Our results are different from those of Murali et al.’s,10 the reason may be that, in recent years, 2 more studies with much larger sample sizes and positive results have been published, which influenced the weight of the previous results.
As for endoscopic response, our study shows that topical steroids were superior to placebo, and Murali et al. did not assess the endoscopic response between them. In the selected studies, Dohil et al. and Miehlke et al.13,18 used similar endoscopic scoring methods to evaluate the improvement in endoscopic characteristics, mainly including white exudates, furrows, edema, fixed rings, crepe paper sign, and stricture. In 2013, Hirano et al. developed a novel endoscopic assessment classification and grading system, i.e. the EREFS, therefore, inaccessible for older studies. The EREFS incorp31 orates both major (exudates, fixed rings, edema, furrows, stricture) and minor features (crepe paper esophagus), and this system has shown good interobserver agreement among gastroenterologists. Recently, Schoepfer et al.32 developed and validated three EREFS-based scoring systems, and they concluded that the new scores were no better than a simple EREFS score; they suggested that a simple EREFS score should be used in short-term clinical trials.
Histologic, clinical and endoscopic outcomes have traditionally been used to assess EoE activity. Ma et al.32 tried to investigate the heterogeneity in these outcome measures in clinical trials of EoE, and identified substantial heterogeneity in the outcome definitions in this field. We also found different outcome definitions in the selected studies, especially regarding clinical and endoscopic endpoints. Choosing appropriate endpoints is extremely important in clinical trial design, so as to reduce heterogeneity in outcome reporting and facilitate the comparison of different trials.33
Topical steroids might be useful for EoE in the short term, but with its chronic nature, long-term treatment is needed. There is limited literature on the long-term use of steroids in EoE. For over 50 weeks, Straumann et al. followed up patients who received low-dose budesonide (0.25mg twice daily) or placebo, and concluded that the former was more effective in maintaining histologic and clinical remission, and that signs of esophageal remodeling tended to be normalized. Additionally, long-term administration of topical corticosteroids was well tolerated.34 Andreae et al. also demonstrated that, swallowed fluticasone was appropriate as a long-term maintenance therapy for up to 5.7 years in pediatric patients, without growth retardation or serious adverse events.35 A low-dose of budesonide was recommended by the Swiss EoE cohort, but there is concern that the high rates of treatment failure may actually represent inadequate dosing.1 Hence, in the Mayo Clinic, for patients with frequent symptoms and those with significant esophageal narrowing are maintained on a reduced dose (budesonide 1.0–1.5mg or fluticasone 880mcg daily) or without any reduction.1 However, some studies have pointed out that the treated EoE was rarely continually symptomatic, which required mainly proton pump inhibitor (PPI)-based therapies.36,37
ConclusionTopical steroids are effective in inducing histological, clinical and endoscopic response in the short-term, and the adverse events were mostly tolerable. However, we should interpret the results on clinical and endoscopic response with caution, as more studies with larger sample sizes are still needed to uniformly assess and validate clinical and endoscopic improvement in EoE. Long-term follow-up treatment results should be evaluated.
Conflict of interestNone declared.