Nonadherence to medication is common in patients with inflammatory bowel disease (IBD) and can result in disease complications, therapy escalation, and the need for corticosteroids. The aim of this study was to assess the adherence to self-administered subcutaneous biologic medications prescribed for IBD and to identify the risk factors for nonadherence.
MethodsA retrospective cohort study on IBD patients initiated on subcutaneous biologic therapy between January 2016 and July 2019 was performed. Medical records were retrospectively reviewed for collection of demographic and IBD data. Medication possession ratios (mMPRs) during the first 12 months of treatment and at the end of the follow-up period (global, 42 months) were calculated. Nonadherence was defined as an mMPR of <90%. Multiple regression analysis was performed to assess the risk factors for nonadherence to therapy.
ResultsA total of 154 patients (84 male and 70 female; mean age at biologic treatment initiation, 36±14 years; Crohn's disease, n=118; ulcerative colitis, n=31; indeterminate colitis, n=5) were included; 121 received adalimumab (ADA) and 33 received ustekinumab (UST); 63% were naive to anti-TNF therapy, while 16.9% previously received more than two biologic treatments. Mean time from IBD diagnosis to subcutaneous biological agent use was 16±10 months. Mean duration of subcutaneous agent use was 17.6 (SD, 11.0) and 17.08 (SD, 6.8) months for ADA and UST, respectively. Global nonadherence (mMPR≤90%) rate was 6.6% for all patients receiving subcutaneous treatment, 6.3% for ADA, and 6.5% for UST. Nonadherence during the first 12 months of treatment (n=98) was 6.1% for all patients, 2.7% for ADA, and 16% for UST. In the multivariate analysis, UST use was independently associated with higher nonadherence only within the first 12 months (OR, 6.7; 95% CI, 1.1–39.5).
ConclusionsHigh global adherence to self-administered subcutaneous biologic treatment was shown in our study, with higher rates of adherence to ADA than to UST within the first 12 months.
La falta de adherencia al tratamiento médico es muy frecuente en los pacientes con enfermad inflamatoria intestinal (EII), puede determinar el desarrollo de complicaciones, el uso de corticoides y la necesidad de escalar tratamientos en estos pacientes. Los objetivos de este estudio son analizar la adherencia al tratamiento biológico de administración subcutánea en pacientes con EII e identificar factores de riesgo para la no-adherencia al tratamiento.
MétodosEstudio unicéntrico retrospectivo de cohorte en pacientes con EII que recibieron tratamiento biológico subcutáneo (adalimumab y ustekinumab) entre enero de 2016 y julio de 2019. Se realizó revisión retrospectiva de la historia clínica para recoger datos demográficos y de la EII. Se calculó el ratio modificado de posesión de la medicación (mMPR) para los primeros 12 meses de tratamiento y para el final del seguimiento (global-42 meses). Se definió como no-adherencia (adherencia inadecuada) si el mMPR era <90%. Se realizó un análisis de regresión logística para evaluar los factores de riesgo asociados con la no-adhesión.
ResultadosSe incluyeron 154 pacientes (84/70; edad media de inicio de tratamiento biológico 36±14 años; enfermedad de Crohn n=118, Colitis Ulcerosa n=31, Colitis Indeterminada n=5). De ellos, 121 (78,6%) recibieron adalimumab (ADA) y 33 (21,4%) ustekinumab (UST); 97/154 (63%) de los pacientes no recibieron tratamiento biológico previo y 26/154 (16,9%) recibieron >2 agentes biológicos antes del tratamiento subcutáneo. El tiempo medio entre el diagnóstico de EII y el uso del biológico subcutáneo fue de 16±10 meses. El tiempo medio de uso de tratamiento subcutáneo se prolongó durante 17,6±11,0 y 17,08±6,8 meses para ADA y UST, respectivamente. La tasa global de no-adherencia al tratamiento fue 6,5% (10/154 pacientes) y específicamente del 6,1% (8/121 pacientes) y del 6,6% (2/33 pacientes) para el uso de ADA y UST, respectivamente. La no-adherencia durante los primeros 12 meses de tratamiento (n=98) fue del 6.1% (6/98pacientes), en todos los pacientes pero diferencialmente del 2,7% (2/73 pacientes) para ADA y del 16% (4/25pacientes) para UST (p=0,017). En el análisis multivariado, el tratamiento con UST mostró un valor predictivo independiente de no-adherencia en los primeros 12 meses del tratamiento (OR 6,7, 95%CI 1,1-39,5).
ConclusionesLa adherencia al tratamiento con biológicos por vía subcutánea en pacientes con EII es muy alta en nuestro medio, siendo superior con ADA que con UST en los primeros 12 meses de tratamiento.
Inflammatory bowel disease (IBD) is a chronic disease characterized by inflammation of the digestive tract, causing various symptoms and involving periods of relapse and remission. In North America, the incidence reaches up to 23 new cases per 100,000 inhabitants/year,1 while in Spain, according to the latest information provided by the Spanish Working Group on Crohn's Disease and Ulcerative Colitis (GETECCU), the incidence is 15.6 cases per 100,000 inhabitants/year.2 The upward trend in the incidence of IBD worldwide3 highlights the importance of achieving effective treatments to control intestinal inflammation, slow the natural progression of the disease, and reduce the development of complications and the need for surgery in these patients.4 Current biologic treatment options vary in therapeutic targets and routes of administration, which include anti-tumor necrosis factor (TNF) (infliximab [IFX],5 intravenous [IV], and adalimumab [ADA],6,7 subcutaneous [SC]); selective intestinal anti-integrin (vedolizumab [VEDO],8,9 intravenous (IV)]; and anti-interleukin (IL)-12/IL23 agents (ustekinumab [UST],10 IV for first dose followed by SC); all of these options have demonstrated efficacy in inducing and maintaining a response to achieve clinical remission in IBD patients and, as such, have been incorporated into usual clinical practice. Adherence to treatment is defined as the patient's following of medical recommendations; it includes not only taking the indicated medication at recommended dosages and intervals but also appearing for visits and/or scheduled tests and following advice regarding lifestyle and diet. Nonadherence (inadequate adherence) can affect the effectiveness of treatment and, consequently, aggravate disease symptoms, increase the number of supplementary examinations or new treatments required, and increase the morbidity and mortality of patients.11,12
IBD is associated with a high risk of nonadherence because it is a chronic disease that frequently affects young patients and involves an unpredictable course. Various studies have estimated a 30–40% rate of nonadherence to medical treatment in IBD.13 In these studies, the adherence rates vary depending on study design, treatment monitoring approach, and/or the definition of adherence, and are limited in relation to the type of biologic agents used. The identified risk factors for nonadherence to biologics in both Crohn's disease and ulcerative colitis include the female sex, smoking, and anxiety.13,14 A recent study, which reviewed the use of ADA and certolizumab, with outpatient dispensing and self-administration of drugs, verified that the subcutaneous treatment regimen constitutes, per se, a factor that brings about poorer adherence to medical treatment.15 Currently, data on the adherence to IBD treatment with UST are lacking.
The main objective of the study was, therefore, to compare the adherence to subcutaneous biologic treatment (ADA and UST) in patients with IBD. Additionally, we analyzed the risk factors for nonadherence and the clinical course of patients treated with such biologic agents.
MethodsStudy design and study populationThis was a retrospective observational cohort study performed in a tertiary level hospital with a reference population of approximately 500,000 inhabitants. The study included patients with a diagnosis of IBD who had received induction or maintenance treatment with biologic agents such as ADA (anti-TNF agent) or UST (anti-IL12/23 agent) by subcutaneous administration, between January 2016 and September 2019.
The patients were identified through a list provided by the central pharmacy service of the hospital. The diagnosis of IBD was based on clinical, endoscopic, and histological criteria established at least 3 months prior to treatment initiation. The inclusion criteria were as follows: age≥18 years, induction of treatment with ADA or UST during the study period, duration of treatment>2 months, follow-up in the IBD-Digestive outpatient clinics of the University Hospital of the Canary Islands, and dispensing of treatment according to pharmacy consultation. The study was approved by the clinical research committee of the University Hospital of the Canary Islands (LRL-ADA-2019-01).
Variables and objectivesTreatment adherence was determined by the modified medication possession ratio (mMPR)16 which was calculated by comparing the amount of theoretical supply of drug during the follow-up period against the actual amount of drug refilled at the outpatient pharmacy service. The indications for biologic treatment type, dose, and need for intensification, were made based on the clinical judgment of the responsible physician. Medication was refilled monthly at the hospital pharmacy, with the use of the prescription signed by the regular doctor, which was then validated by the dispensing pharmacist considering a validity period of 6 months. The ADA treatment regimen included an initial 160mg SC dose, followed by an 80mg SC dose at week 2 (induction dose), and subsequently, 40mg SC doses every 2 weeks (maintenance dose), such that the three boxes of medication (with two vials each) were expected to be dispensed on two occasions during the first 2 months of treatment, with a total of 12 pharmacy consultations for the dispensing of medication in 1 year. UST therapy involved an initial IV dose administrated at the Day Hospital of approximately 6mg/kg, an SC dose of 90mg at week 8 (induction dose), and subsequent maintenance doses every 8–12 weeks as indicated by the responsible physician; attendance to a total of six pharmacy consultations were expected in 1 year if the regimen was maintained every 8 weeks. To calculate the mMPR, indications for a maintenance regimen and treatment intensification (40mg ADA weekly or 90mg UST every 4 weeks) were reviewed. In addition, possible delays in the dispensing of medication (infectious disease, hospital admission, surgical intervention, or medical indication) were determined based on a clinical history review.
The age, sex, and family history of IBD were recorded, as were the clinical variables of IBD, including the type of IBD (a diagnosis of indeterminate colitis during the final evaluation was considered as ulcerative colitis), the time from diagnosis to the use of subcutaneous biologic agents, and history of biologic treatment or surgery either during the course of the disease or in the year preceding the study.
During the follow-up period, the clinical course of the patient with subcutaneous biologic treatment was reviewed, by recording any incidences of IBD flare-ups, which were defined as the need for treatment intensification, systemic corticosteroid use, emergency consultation, hospital admission, or surgical intervention.
The main objective of the study was to assess the rates of adherence to subcutaneous biologic treatment (ADA and UST) in patients with IBD, which were reviewed during all follow-up time points (global) and at 12 months (first year of treatment). Patients presenting with a mMPR of ≤90% were considered to have inadequate adherence (IA). The secondary objective was to review the predictive variables of adherence to subcutaneous biologic treatment and to relate the clinical course of patients with their adherence to treatment.
Statistical analysisContinuous variables are expressed as means and standard deviations, while qualitative variables are expressed as frequencies. Univariate analysis and multivariate Cox regression were performed to determine the possible predictive factors for adherence. Results of the logistic regression are presented as odds ratio (OR) alongside the 95% confidence interval (CI). The rates of emergency room visits, hospitalizations, and surgical interventions as a function of adherence to treatment were analyzed using the chi-square test. The probability of an IBD flare-up during the follow-up period was analyzed using Kaplan–Maier survival curves. p-Values<0.05 were considered statistically significant. All statistical analyses were carried out using SPSS software v16.
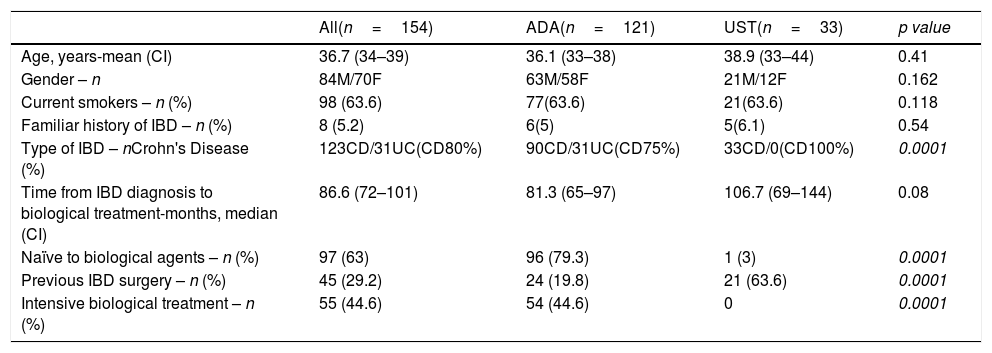
ResultsPopulation dataThis study included 154 patients, with a mean age of 36.7±14.2 years (interquartile range, 34–39 years), and 84 (54.5%) of them were men. Table 1 describes the characteristics of the patients.
Characteristics of study cohort (n=154).
| All(n=154) | ADA(n=121) | UST(n=33) | p value | |
|---|---|---|---|---|
| Age, years-mean (CI) | 36.7 (34–39) | 36.1 (33–38) | 38.9 (33–44) | 0.41 |
| Gender – n | 84M/70F | 63M/58F | 21M/12F | 0.162 |
| Current smokers – n (%) | 98 (63.6) | 77(63.6) | 21(63.6) | 0.118 |
| Familiar history of IBD – n (%) | 8 (5.2) | 6(5) | 5(6.1) | 0.54 |
| Type of IBD – nCrohn's Disease (%) | 123CD/31UC(CD80%) | 90CD/31UC(CD75%) | 33CD/0(CD100%) | 0.0001 |
| Time from IBD diagnosis to biological treatment-months, median (CI) | 86.6 (72–101) | 81.3 (65–97) | 106.7 (69–144) | 0.08 |
| Naïve to biological agents – n (%) | 97 (63) | 96 (79.3) | 1 (3) | 0.0001 |
| Previous IBD surgery – n (%) | 45 (29.2) | 24 (19.8) | 21 (63.6) | 0.0001 |
| Intensive biological treatment – n (%) | 55 (44.6) | 54 (44.6) | 0 | 0.0001 |
Of the total patients, 121 (78.6%) received ADA and 33 (21.4%) received UST; 63% (97/154) were naïve to the use of biologic agents, and 26 (16.9%) had received more than two biologic agents prior to the study.
Comparison of the patient characteristics according to treatment type revealed that those treated with UST were largely patients with Crohn's disease (p<0.001), with a longer disease course (p=0.08), prior biologic use (p<0.001), and a history of surgery related to IBD (p<0.001). All UST patients required the maintenance regimen every 8 weeks, while none required treatment intensification every 4 weeks.
Mean time from IBD diagnosis to the use of subcutaneous biologic agent was 16±10 months. The mean treatment duration was prolonged for 17.6±11.0 and 17.0±6.8 months for ADA and UST, respectively.
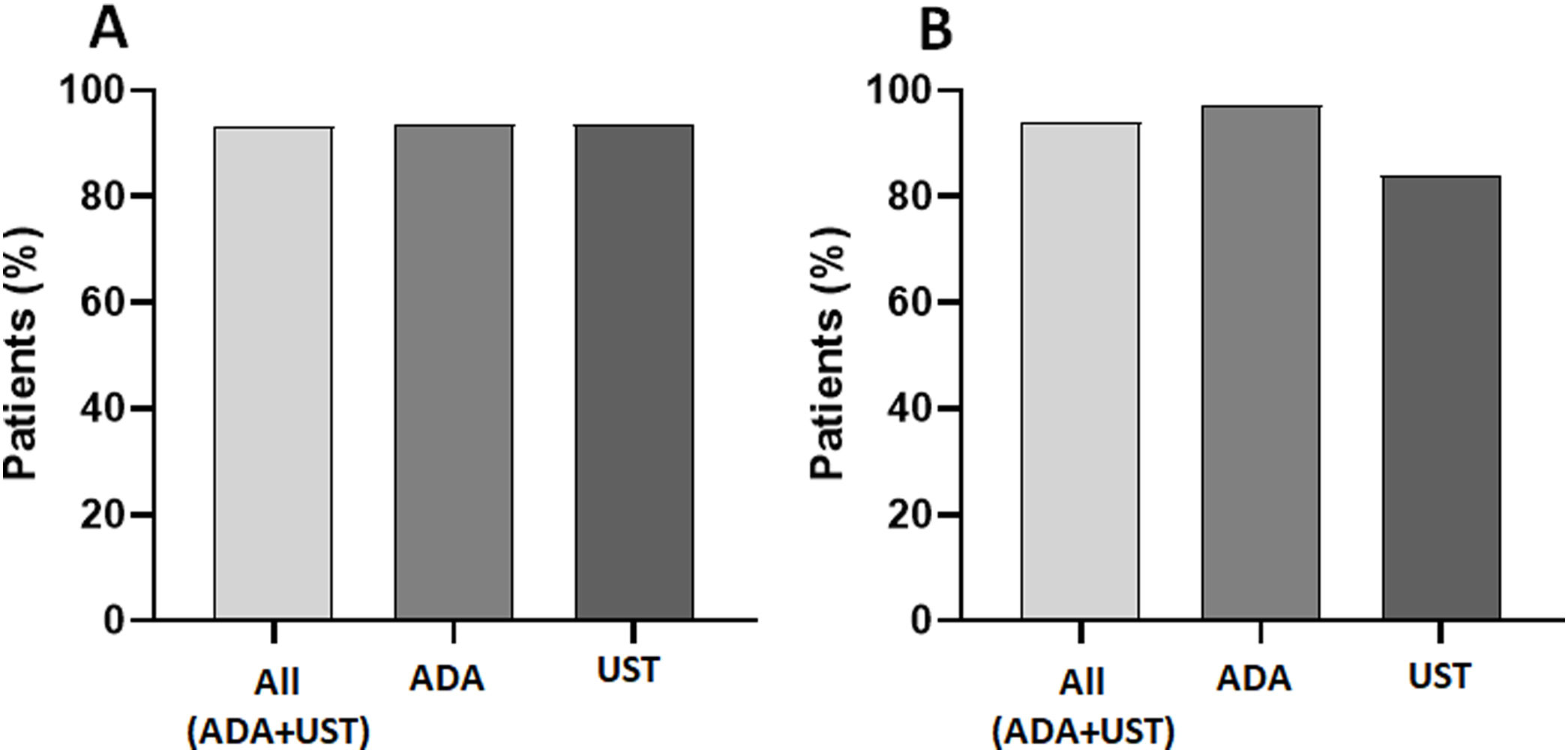
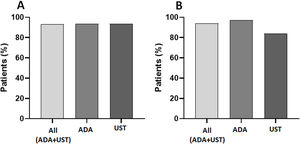
Adherence to subcutaneous biologic treatmentGlobal adherence to treatment was 93.4% (144/154 patients), and no significant differences were observed between the treatment types, with 93.7% (113/121 patients) and 93.5% (31/33 patients) for ADA and UST, respectively (Fig. 1A). The 12-month adherence was 93.9% (92/98 patients), and at this endpoint, the IA rate varied at 2.7% (2/73 patients) for ADA and 16% (4/25 patients) for UST (p<0.02) (Fig. 1B).
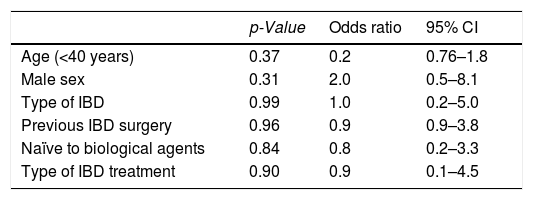
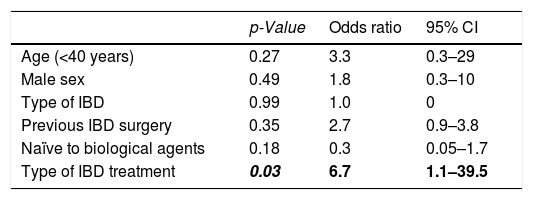
Risk factors associated with treatment adherenceLogistic regression analysis was used to identify predictors of IA to treatment, which included gender, young age (< 40 years), family history of IBD, type of IBD (ulcerative colitis or Crohn's disease), history of surgery related to IBD, prior use of biologic treatment before the use of the subcutaneous treatment under study, and the type of subcutaneous biological treatment received (ADA or UST). None of these variables presented as an independent predictive factor for IA to maintenance treatment (Table 2). Treatment with UST showed an independent predictive value for IA within the first 12 months of treatment (OR, 6.7; 95% CI, 1.1–39.5); however, the rest of the factors evaluated did not show statistical significance (Table 3).
Analysis of clinical factors associated with inadequate adherence to subcutaneous biologic treatment during the follow-up period (global).
| p-Value | Odds ratio | 95% CI | |
|---|---|---|---|
| Age (<40 years) | 0.37 | 0.2 | 0.76–1.8 |
| Male sex | 0.31 | 2.0 | 0.5–8.1 |
| Type of IBD | 0.99 | 1.0 | 0.2–5.0 |
| Previous IBD surgery | 0.96 | 0.9 | 0.9–3.8 |
| Naïve to biological agents | 0.84 | 0.8 | 0.2–3.3 |
| Type of IBD treatment | 0.90 | 0.9 | 0.1–4.5 |
Analysis of clinical factors associated with inadequate adherence to subcutaneous biologic treatment within the first 12 months.
| p-Value | Odds ratio | 95% CI | |
|---|---|---|---|
| Age (<40 years) | 0.27 | 3.3 | 0.3–29 |
| Male sex | 0.49 | 1.8 | 0.3–10 |
| Type of IBD | 0.99 | 1.0 | 0 |
| Previous IBD surgery | 0.35 | 2.7 | 0.9–3.8 |
| Naïve to biological agents | 0.18 | 0.3 | 0.05–1.7 |
| Type of IBD treatment | 0.03 | 6.7 | 1.1–39.5 |
During the follow-up period (mean, 17.5±10.1 months; median, 16.5 months), there were 14 (9.1%) visits to the emergency room, 16 (10.4%) hospitalizations, and 9 (5.9%) surgical interventions, but no significant differences were observed in relation to adherence to subcutaneous ADA or UST treatment: emergency care (12 [8.3%] and 2 [20%] for good adherence and IA, respectively; p=0.22); hospitalizations (14 [9.7%] and 2 [20%], respectively; p=0.27); surgical interventions (7 [4.9%] and 2 [20%], respectively; p=0.10).
A total of 81 (52.6%) patients presented with inflammatory flare-ups during the follow-up period, without any significant differences based on adherence to treatment (78 [54.2%] and 3 [30%] for good adherence and IA, respectively; p=0.19).
DiscussionThe results of this study show that adherence to treatment with subcutaneous biologics in patients with IBD is higher than 90% overall during the follow-up period, and adherence was only higher for the use of ADA than for UST within the first 12 months of treatment. Our study is the first to evaluate the adherence to ustekinumab and to focus on subcutaneous biologic treatment in IBD patients.
To assess the adherence to treatment, mMPR was used in the present study, since previous studies have shown its usefulness and relationship with the level of adherence and clinical course of the patients.12,16 The choice of mMPR≥90% was validated by Gionani et al.,12 who assessed the adherence to subcutaneous treatment with adalimumab and certolizumab in patients with IBD. In these patients, the use of ADA with mMPR≥0.86 had a 25% lower risk of developing inflammatory flare-ups (hazard ratio [HR], 0.75; 95% CI, 0.67–0.83; p<0.01).12 Thus, this cut-off point was used in our study to ensure optimum therapeutic levels of the drug and clinical benefit in our patients.
Wentworth et al.15 evaluated the lack of adherence to biologic treatment for IBD by collecting data for infliximab, vedolizumab, ADA, and certolizumab. They determined that the subcutaneously administered drugs, ADA and certolizumab, were associated with a lower adherence rate at 57% and 50%, respectively, during the 24-month follow-up and with 100% mMPR. The availability of non-commercial medical insurance was a risk factor for the lack of adherence to biological treatment. Similarly, van der Have et al.17 showed a 57% adherence rate to ADA treatment, and the use of this drug was, by itself, an independent risk factor for the lack of adherence (OR, 10.1; 95% CI, 2.62–40.00). Spain has a national health system with universal coverage; therefore, the prescription of biologic treatment in IBD patients, both its indication and the type and form of administration of the drug, depends on the established clinical guidelines and not on the insurance of individual patients. This characteristic of the health system in which this study was carried out may explain the differences with respect to previous studies.
In our study, we obtained an overall adherence rate of >90% for both ADA and UST, although when assessing the 12-month adherence rate, with longer treatment duration, there was an advantage of ADA therapy over treatment with UST. This difference is similar to that observed in the study that compared ADA with certolizumab, with adherence rates of 86.5% and 74.4%, respectively.12 In this study, data on the use of both subcutaneous biologic drugs over a period of 5 years were collected, and a lower adherence to certolizumab was confirmed, with a more spaced schedule of administration (every 4 weeks during maintenance) compared to ADA (every 2 weeks). Both studies showed that drugs with less frequent administration are at risk of lower adherence, and strategies have to, therefore, be established to remind patients of their administration. This decrease in adherence over time for drugs with less frequent administration could be attributed to the clinical benefit and improvement of symptoms perceived by the patient, which often lead to him or her forgetting or delaying the refilling and administering of these subcutaneously administered drugs, which requires the patient to take the initiative to collect them at the hospital pharmacy in order to use it. In our study, only few patients with inadequate adherence to treatment (mMPR<90%) were present, and, therefore, we could not establish a relationship between adherence and clinical course (development of inflammatory flare-ups) in our patients.
The main strength of this study is that it is the first to assess and compare the adherence to subcutaneous UST therapy with ADA therapy over a prolonged period of time in IBD patients. In addition, we consider it an advantage of the study to utilize information provided by mMPR values, with retrospective and “real” reviewing of the patients, without the patients having a perception of vigilance regarding treatment adherence.
There were several limitations to our study. First, given that it was a single-center study, the number of patients included, particularly in the UST group, was small; the results should, therefore, be interpreted with caution. Second, we did not determine the drug's levels to be able to correlate it with the pharmacy filling rate (mMPR) or assess its possible relationship with the clinical course of the disease. Third, we did not use the eight-item questionnaire to assess the adherence to treatment (Morinsky Mediation Adherence Scale-8 [MMAS-8])18 validated in IBD patients. However, this limitation is relative because the use of these questionnaires has been related to the so-called Hawthorne effect,19 whereby the patient, upon perceiving the surveillance or monitoring of their adherence to treatment in the context of a controlled study, can modify their behavior by habitually increasing their adherence to treatment.
In conclusion, this is the first study to assess adherence to UST therapy in IBD. Overall, the adherence to treatment with subcutaneous biologic agents is good (>90%), but the risk of loss of adherence increases with prolonged use of drugs in regimens involving less frequent administrations, as with that of UST (every 8 weeks). Therefore, strategies should be developed to encourage patients to remember administering and refilling outpatient medications such as these to maintain adherence and favor optimal disease management with less risk of flare-ups and loss of treatment efficacy.
IRB approval statusCómite Etico Hospital Universitario de Canarias (LRL-ADA-2019-01).
Funding sourcesNone.
Conflicts of interestLR: Educational and travel grants, speaker fees: MSD, Pfizer, Abbvie, Takeda, Janssen, Shire Pharmaceuticals, Ferring, and Dr. Falk Pharma.
EQ: Advisor to Sysmex España SL.
The rest of the authors declare no conflict of interest relating to this study.