Alcohol-related liver disease (ARLD) is the most prevalent cause of advanced liver disease and liver cirrhosis in Europe, including Spain. According to the World Health Organization the fraction of liver cirrhosis attributable to alcohol use in Spain is 73.8% among men and 56.3% among women. ARLD includes various stages such as steatohepatitis, cirrhosis and hepatocellular cancer. In addition, patients with underlying ARLD and heavy alcohol intake may develop alcoholic hepatitis, which is associated with high mortality. To date, the only effective treatment to treat ARLD is prolonged withdrawal. There are no specific treatments, and the only treatment that increases life expectancy in alcoholic hepatitis is prednisolone. For patients with alcoholic hepatitis who do not respond to treatment, some centres offer the possibility of an early transplant. These clinical practice guidelines aim to propose recommendations on ARLD taking into account their relevance as a cause of advanced chronic liver disease and liver cirrhosis in our setting. This paper aims to answer the key questions for the clinical practice of Gastroenterology, Hepatology, as well as Internal Medicine and Primary Health Centres, making the most up-to-date information regarding the management and treatment of ARLD available to health professionals. These guidelines provide evidence-based recommendations for the clinical management of this disease.
La Enfermedad hepática alcohólica (EHA) es la causa más prevalente de enfermedad hepática avanzada y cirrosis hepática (CH) en Europa incluyendo a España. De acuerdo con la Organización Mundial de la Salud (OMS) la fracción de CH atribuible al uso de alcohol en España es del 73.8% entre varones y del 56.3% entre mujeres. La EHA incluye diversos estadios como la esteatohepatitis, la cirrosis y el cáncer hepatocelular. Además, enfermos con EHA de base e ingesta abundante de alcohol pueden desarrollar hepatitis alcohólica (HA), que cursa con una elevada mortalidad. Hasta la fecha, el único tratamiento efectivo para tratar la EHA es la abstinencia prolongada. No existen tratamientos específicos, y el único tratamiento que aumenta la esperanza de vida en la HA es la prednisolona. Para enfermos con HA que no responden al tratamiento, algunos centros ofrecen la posibilidad de un trasplante precoz. Estas guías de práctica clínica tienen como objetivo proponer recomendaciones sobre la EHA teniendo en cuenta su relevancia como causa de hepatopatía crónica avanzada y CH en nuestro medio. En el presente trabajo se propone como objetivo responder las preguntas claves para la práctica clínica de Gastroenterología, Hepatología, así como de Medicina Interna y centros de Salud Primaria, poniendo al servicio del profesional de la salud la información más actualizada respecto al manejo y tratamiento de la EHA. Estas guías proporcionan recomendaciones basadas en la evidencia para el manejo clínico de esta enfermedad.
These guidelines have been prepared and updated after the First Consensus Meeting on non-alcoholic fatty liver (NAFLD) and alcoholic liver disease (ALD) held in May 2016 in Seville, under the auspices of the Asociación Española para el Estudio del Hígado [Spanish Association for the Study of the Liver] (AEEH) and with the collaboration of a panel of national and international experts in both diseases. The guidelines have undergone a review process and all authors have declared their potential conflicts of interest.
MethodologyThe evidence and the recommendations in this guideline have been established in accordance with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system. The principles of the GRADE system have already been published.1 The quality of the evidence and recommendations have been established according to levels: A (high), B (moderate), C (low); and grades: strong,1 weak2 (Table 1).
Description and codification of the classification of evidence and strength of recommendations.
| Observations | Grade | |
|---|---|---|
| Quality of evidence | ||
| High | New research is unlikely to change our confidence in estimating the effect | A |
| Moderate | It is likely that new research will have a significant impact on our confidence in the estimation of the effect and therefore could change the estimate | B |
| Low | Additional studies are likely to have a significant impact on the confidence to estimate the effect. Any change in the estimate is uncertain | C |
| Strength of recommendation | ||
| Strong | Factors that influence the strength of the recommendations include the quality of the evidence, potentially important results for patients and the cost | 1 |
| Weak | There is variability in preferences and values, or they are more uncertain. The recommendation is made with less certainty, greater cost and use of resources | 2 |
The initial recommendations after the Consensus Meeting on the degree of scientific evidence were subsequently evaluated by an independent committee of experts to ensure their greatest possible objectivity.
Terminology used in this guideline| Previous terminology | New terminology | Abbreviation |
| Alcoholic | Alcohol use disorder | AUD |
| Alcoholic liver disease | Alcoholic liver disease | ALD |
| Alcoholic cirrhosis | Cirrhosis due to alcoholic liver disease | Cirrhosis due to ALD |
| Alcoholic steatohepatitis | Steatohepatitis due to alcoholic liver disease | ----- |
| Alcoholic fibrosis | Fibrosis due to alcoholic liver disease | Fibrosis due to ALD |
| Alcoholic hepatitis | Alcoholic hepatitis | AH |
ALD is the most prevalent cause of advanced liver disease and liver cirrhosis (LC) in Europe, including Spain.2–4 Along with NAFLD,5,6 they are the most prevalent causes of chronic liver disease. In contrast, due to advances in the prevention and treatment of viral hepatitis B and C, its prevalence has begun to decrease in our setting, especially as a cause of liver disease.7 In countries such as the United States of America, the annual mortality rate due to age-adjusted chronic liver disease has increased among patients with ALD and NAFLD since 2013, coinciding with a fall in the number of patients with hepatitis C virus and to a lesser extent in patients with hepatitis B virus.8 Despite its high prevalence, there are relatively few studies on the early detection, diagnosis and treatment of ALD compared to NAFLD or viral liver disease.9
Natural history and the factors that modify ALD are not well known. Due to the lack of screening and early detection programmes, patients are not diagnosed until late stages of the disease, when they develop jaundice or complications. A recent international study found that ALD is, by far, the cause of liver disease with the lowest percentage of early detection. In Spain, for every 9 patients diagnosed late, one patient is diagnosed early.10
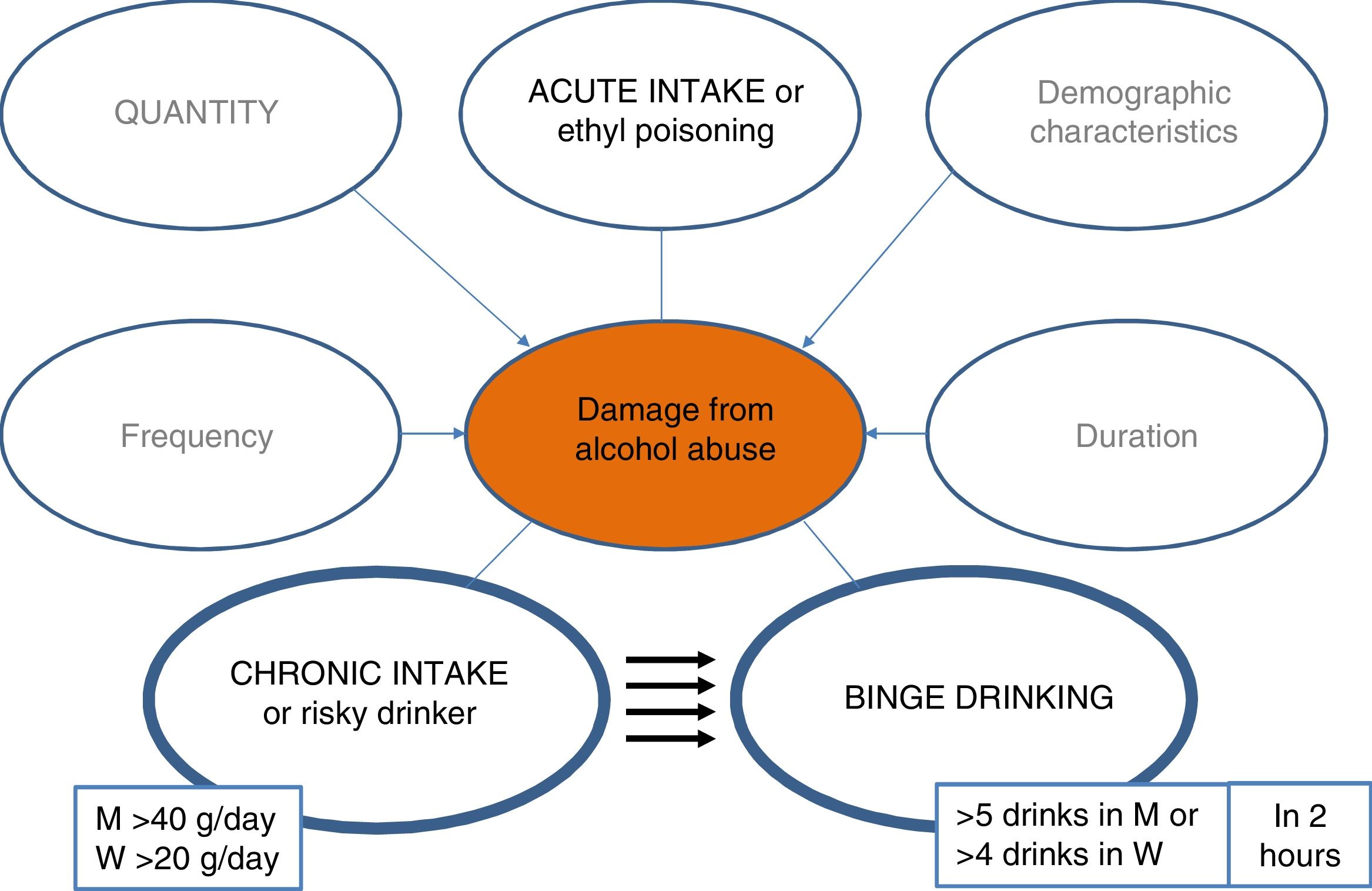
It is therefore essential to launch campaigns to detect this disease in less advanced phases. In this regard, it is vital to quantify alcohol consumption in all patients, and it is recommended to standardise the quantification to grams of alcohol or use the standard unit of alcohol (SUA), whereby each SUA equals 10 g of alcohol.
ALD encompasses a wide range of stages ranging from simple hepatic steatosis, alcoholic steatohepatitis with or without fibrosis, cirrhosis and hepatocellular carcinoma (HCC). Compared to NAFLD, ALD is associated with more aggressive liver fibrosis, which is the major determinant of long-term mortality.11 In addition, patients with underlying ALD who continue to drink alcohol may develop a very serious clinical condition called alcoholic hepatitis (AH). AH is characterised by rapid elevation of bilirubin and the onset of decompensations.12 Its prevalence is not well known and sometimes manifests in young patients. Severe AH is associated with the development of bacterial infections, multiorgan failure and high short-term mortality.13 To date, the only treatment that slightly increases short-term survival in AH is prednisolone.14 There is an urgent need to develop new therapies for this severe form of liver disease.
Liver transplantation is the only curative treatment for advanced liver diseases that do not improve after abstinence from alcohol. The evaluation of these patients is complex and includes an assessment of the risk of relapse. In recent years, some centres have started to offer early transplantation to a select group of patients with severe AH who do not respond to standard treatment.
PrevalenceWhat is the prevalence of ALD and the pattern of alcohol use in Spain?The prevalence of ALD has progressively increased over the years, although only by a small percentage in industrialised countries, and has remained stable over the last decade.15–17 Even so, estimated figures of 2% in the general population are significant and make ALD a public health problem. In addition, data from different neighbouring countries, such as the United Kingdom, indicate that despite the general trend towards lower mortality from common causes (e.g., ischaemic heart disease, diabetes, respiratory or cerebrovascular causes) there was an alarming increase in mortality between 1970 and 2010 attributed to liver causes, two thirds of which were attributable to alcohol. The liver-related mortality rate is also increasing in other countries such as Finland and Ireland, while remaining stable in Norway and Sweden and falling in France and Italy. In Spain, the proportion of LC attributable to alcohol use in 2016 was 73.8% among men and 56.3% among women.18
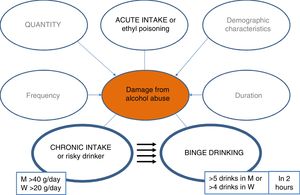
In Spain, according to the World Health Organisation, average pure alcohol consumption per capita in people over 15 years of age between 2015 and 2017 was 10 litres, having fallen by 0.5 l compared to the period of 2009–2011.18 Notable changes in the pattern of use are observed among young people, especially among women.18 It should be noted that 28% of the population between 15 and 19 years of age engage in episodes of excessive alcohol intake (≥ 60 g of alcohol in one sitting at least once per month).18 In addition, in the last decade the pattern of binge drinking has increased threefold (5 drinks of 14 g/unit or 4 in women in 2 h), and the pattern of high-risk use has decreased (>20 g/day for women, >40 g/day for men).19,20 It is important to mention that the impact that the new patterns of use will have on the mortality rate due to alcoholic liver disease is unknown, and new studies are needed to clarify this issue.21–23 This consumption behaviour, which is increasing in all industrialised countries, is associated with negative effects in the area of safety and health and is likely to also have negative repercussions on the liver.
Conclusion- •
ALD is the most common cause of LC in Spain, making it a major public health problem. (A1).
- •
Given the 2% prevalence of ALD, together with the change in the pattern of alcohol use in Spain, it is necessary to implement preventive measures at different levels to prevent abusive alcohol consumption. (A1).
In ALD, different biochemical markers can support clinical suspicion based on adequate history and physical examination. Although no biochemical marker is able to determine chronic alcohol use by itself, the combination of classic markers, such as aspartate aminotransferase (AST) elevation (sensitivity 43–68 % and specificity 56–95 %), mean corpuscular volume (sensitivity 24–75 % and specificity 56–96 %) or gamma-glutamyltransferase (sensitivity 42–86 % and specificity 40–84 %), is useful for establishing clinical suspicion.24–26
Other markers such as the determination of carbohydrate-deficient transferrin can help in the screening of a chronic alcohol consumption with a sensitivity of 88%.27,28
Recommendation- •
Classic markers, such as elevated mean corpuscular volume, AST and gamma-glutamyltransferase, along with others such as the determination of carbohydrate-deficient transferrin, can help in the screening of harmful alcohol consumption in patients in whom this clinical suspicion has been established. (B2).
LC is the leading cause of alcohol-related mortality in Europe.29,30 However, the trend has been declining in most European countries including Spain since the late 1970s, with the exception of some Nordic countries, the United Kingdom, Ireland and Eastern countries where the pattern of episodic use prevails. In Spain, as mentioned in the previous section, the proportion of LC attributable to alcohol use in 2016 was 73.8% among men and 56.3% among women, representing an 8.6% increase among men and a 7.6% decrease among women.18 In Europe, deaths attributable to abusive alcohol consumption are estimated at 6%, with LC being the main cause of alcohol mortality, although these figures fluctuate between the different countries of the European Union.29–31
Even so, in Spain, according to the 2013 Health Indicators report, the mortality rate due to chronic liver disease was 15.2 for men and 5.6 for women per 100,000 population.31
In another study it was estimated that, in Spain, among the population segment from 15 to 64 years of age, 12.3% of all causes of death in men and 8.4% in women were attributable to alcohol.
In addition, it is estimated that one in eight men and one in 12 women die prematurely before age 65, due to excessive alcohol consumption.31
Conclusion- •
Excessive alcohol consumption is one of the leading causes of mortality due to LC in the world, including Europe and Spain. It is expected that mortality from other liver diseases (viral and non-alcoholic steatohepatitis [NASH]) will change in the coming years, and that the impact of ALD on liver-related mortality will be higher. (A1).
- •
The impact that the changing pattern of consumption may have on liver-related mortality in Spain is unknown, but current evidence suggests the need for studies that evaluate this association. (B1).
Excessive alcohol consumption is a risk factor for LC and has a much greater impact on cirrhosis mortality32 than morbidity. In addition, the same average intake is associated with a higher risk of LC in women than in men. It is possible that this is due to differences in alcohol metabolisation, which is lower in women.33 There is a dose-response relationship between quantity of alcohol and cirrhosis due to ALD. Daily amounts of 20−30 g of alcohol increase the risk of developing LC.34 More recent data suggest that drinking daily seems to increase the risk of cirrhosis due to ALD, regardless of the amount of alcohol, especially among men.35 The onset of alcohol use at an early age is the most important risk factor for developing cirrhosis.36,37
Recommendation- •
Alcohol consumption greater than 30 g/day increases the risk of LC, especially if consumption begins at an early age. The medical history should place special emphasis on questions about alcohol use, including quantification and frequency. There should be insistence and advice on an intake below the level of risk. (A1).
In patients who have suffered an episode of AH, alcohol abstinence is the most important factor that influences medium- and long-term mortality.38 Moderate intake (14 SUA/week for women and 21 SUA for men) is associated with higher mortality.38 Therefore, the main objective with these patients is to achieve total abstinence. These results reinforce the importance of promoting and supporting abstinence in this group of patients from the time of their admission. Multidisciplinary treatment combined with addiction units to help acquire dependency awareness and to work on relapse prevention are essential for sustained abstinence.38
Recommendation- •
After an episode of AH, complete alcohol abstinence is recommended. Moderate intake is associated with higher mortality. It is recommended to evaluate and treat the problem of alcohol from admission by multidisciplinary cooperation with addiction units. (A2).
ALD includes the development of steatosis (usually microvesicular) along with liver damage (ballooning and development of Mallory–Denk bodies) and inflammatory infiltrate (typically pro neutrophils). These lesions, similar to NASH, usually manifest with pericellular fibrosis and can progress to LC and HCC (Fig. 1).39 Due to its silent course, few patients with early ALD are diagnosed and treated in Spain.40 This early form of the disease is not well characterised in humans, and there is an urgent need to define the natural history and prognostic factors, as well as to develop biomarkers for early detection. Although the histological characteristics that define ALD do not differ from those that define NASH, the lesions of alcoholic steatohepatitis are usually more severe. A recent study shows a high prevalence of advanced fibrosis among patients with ALD, which has a negative impact on long-term mortality.41 There are few studies on the natural history of ALD. 90% of chronic drinkers develop simple steatosis and only a minority progress to steatohepatitis,42 with between 10% and 20% of cases developing LC.
A unique feature of ALD is the possible development of AH episode(s), characterised by jaundice and decompensations. Most episodes of AH occur in patients with advanced fibrosis or established LC, but can occur at any stage of the disease.43
In Spain, AH has a short-term mortality of 20–30 % and 50% at one year (ABIC). The biggest determinant of medium- and long-term survival is abstinence from alcohol.38
Patients with alcoholic cirrhosis are usually diagnosed with a high MELD score and their natural course and survival are highly conditioned by alcohol abstinence.38 Although the incidence of HCC in alcoholic cirrhosis is lower than in other aetiologies, it is diagnosed in more advanced stages and is usually associated with high mortality.44
Conclusions- •
Most chronic drinkers develop simple steatosis, although only 10–20 % will end up developing alcoholic steatohepatitis, LC and HCC. (A1).
- •
The development of advanced fibrosis is the most important histological determinant of mortality in patients with ALD. (A2).
- •
It is recommended to focus particularly on the diagnosis of ALD during the early stages. This diagnosis must be made through the medical history, physical examination in search of signs or symptoms of alcohol use and biochemical markers given the silent course. (A2).
- •
In patients with risky alcohol use who present with hepatic decompensation, AH should be considered as part of the differential diagnosis. (A2).
Individual susceptibility to developing different ALD phenotypes depends on the interaction of behavioural, genetic and environmental factors.45 A possible correlation between the degree of alcohol intake and liver fibrosis has been suggested, but the histological lesions caused by alcohol exhibit great interindividual variability.46 In general, an intake greater than 30 g/day in women and 40 g/day in men predispose to the development of ALD. There is much debate over whether drinking a single drink a day, especially wine, could protect against liver fibrosis.47 A large recent study shows that even the intake of a single alcoholic drink per day can be detrimental to health, as it increases the risk of cancer.48 It has also been indicated that the alcohol intake pattern, especially binge drinking, may be a risk factor for the development of advanced ALD, especially among the population with metabolic syndrome.21
However, not all studies are concordant and the study populations are very diverse.22 The main environmental factors involved in the progression of ALD include obesity, an independent risk factor for fibrosis49 and smoking, which favours the progression to cirrhosis.50
There are genetic factors that influence individual susceptibility to developing ALD, as studies in twins have shown.51 Genetic susceptibility impacts multiple levels, influencing alcohol intake and its metabolism in the development of cirrhosis.
Genetic variations related to neurotransmitters52 and modifiers of alcohol metabolism, such as alcohol dehydrogenase (ADH) and aldehyde dehydrogenase predispose to the development of alcoholism.45 The second group of genes modifies the natural history of ALD through different mechanisms such as oxidative stress and by modifying the activity of endotoxins and cytokines. Variations of patatin-like phospholipase domain-containing protein 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2) and membrane-bound O-acyltransferase domain 7 (MBOAT7) predispose to cirrhosis due to ALD.53–58 The fact that these genes also influence NASH suggests that both diseases share pathogenic mechanisms.
Conclusion- •
ALD has a dose-response relationship with the amount of alcohol ingested but genetic and environmental factors are also influential. (A1).
- •
Permanent abstinence from alcohol consumption is the only factor capable of modifying the natural history of ALD. Obesity and alcohol have an additive effect. (A1).
- •
During the evaluation of ALD, permanent alcohol abstinence should be promoted and advised, and other factors such as smoking or obesity should also be taken into account. (A1).
The socio-economic costs of alcohol abuse include accidents, violence, hospital expenses and low productivity. In 2007, it was estimated to be more than ten billion four hundred and sixty-three million (10,463) euros in Spain (1% of gross domestic product).59
Although this figure includes direct health costs (hospitalisations) and indirect costs (attributable to mortality and missed work due to hospitalisation) in general attributable to alcohol, the percentage due to liver disease is not specifically known.
Because ALD is the most common cause of decompensated cirrhosis in Spain, the cost to the healthcare system is very high. Other Western countries show an increase in economic costs of up to 40% in just one decade, accounting for 1% of the gross domestic product, similar to in Spain.16,60
Recommendation- •
Given the high economic burden of excessive alcohol use on the development of advanced liver diseases, the development of active policies that prevent alcohol abuse is essential. (A1).
In 2010, alcohol abuse caused 4.9 deaths per 100,000 population attributable to cancer (0.15% of total deaths). In addition to increasing the risk of oropharyngeal, oesophageal and pancreatic cancer, alcohol abuse increases the incidence of HCC. The daily amount of alcohol consumed impacts the risk of developing HCC.61
Alcohol consumed in excess is a liver carcinogen that has a direct and synergistic impact with other factors such as obesity or viral hepatitis.62–64 As such, the consumption of more than three drinks per day in patients with a body mass index (BMI) >30 increases the incidence of HCC.62 Furthermore, alcohol use is a common co-factor in more than half of patients with hepatitis C who develop HCC.
The relative risk of HCC for patients with ALD is 4.06, which is lower than in viral hepatitis and NASH.61,62 In recent years there has been much debate regarding the cost-effectiveness of screening for HCC among individuals with LC due to ALD. Some population studies in Denmark and the United Kingdom had reported an annual risk of 1%, below the 1.5% threshold at which screening is recommended.65,66 However, a recent prospective study has shown an annual incidence of HCC among this population of 2.9%,67 justifying screening.68
It is important to note that HCC is diagnosed in patients with ALD in more advanced stages, in whom the cure rate is much lower, and it therefore leads to higher mortality than in patients with other types of liver disease.18 It is therefore essential to recommend screening in alcoholic cirrhosis and to motivate and support patients to ensure proper monitoring of the disease.
Recommendations- •
Cirrhosis due to ALD is a risk factor for the development of HCC and an ultrasound scan is recommended every six months for early detection. (A2).
- •
The coexistence of alcohol abuse, viral hepatitis and obesity increases the risk of HCC. The identification and treatment of multiple aetiologies is recommended in all patients with cirrhosis. (A1).
Yes. High-risk alcohol use (>4–5 SUA/day in men [or >21 SUA per week] and >3 SUA/day in women [or >14 SUA per week]) has been found to beassociated with being overweight or obese.69 However, no association has been found with consumption below these amounts. Some consumption patterns such as “binge drinking” or increased use (e.g. >7 times/week)70 seem to promote weight gain and obesity.71
Recommendation- •
High-risk alcohol use (>4–5 SUA/day in men [or >21 SUA per week] and >3 SUA/day in women [or >14 SUA per week]) should be advised against given that an association with being overweight and obese has been shown. (B1).
Yes, data from population cross-sectional studies in the United States of America have shown a positive association between alcohol use and obesity. In overweight (BMI 25- <30 kg/m2) and obese (BMI > 30 kg/m2) patients, abnormally high levels of transaminases should be assessed as a possible surrogate marker of alcoholic liver disease, since the consumption of more than two drinks/day among overweight patients and one drink/day in obese patients increases the risk of AST elevation. However, it should be kept in mind that obese patients also have a higher risk ratio for exhibiting high levels of alanine aminotransferase (ALT) even when they do not use alcohol or consume only one drink per day.72 The results of the 3rd National Health and Nutrition Examination Survey (NHANES III) showed that the prevalence of elevated transaminases increases as BMI increases, from 4.4% in subjects with normal weight to 7.3% in overweight subjects and 12% in obese subjects.73,74
Recommendation- •
There is a positive association between alcohol use, BMI and elevated transaminase levels. It is recommended to treat both factors in patients with chronic liver disease. (B1).
Yes. Results of cross-sectional studies that included high-risk drinkers (>50 g/day) have shown an independent association between BMI and >10% steatosis in liver biopsy.71 In patients with alcoholism and high-risk alcohol use, a history of being overweight in the last 10 years has been associated with cirrhosis and steatohepatitis.74,75 In addition, it has been found that the adipose tissue of patients with AH expresses elevated levels of proinflammatory cytokines (e.g., TNF-a and interleukin 10) and that these are correlated with the degree of pathological findings in the liver.76
Recommendation- •
There is an association between BMI and the degree of steatosis. In addition, in patients with high-risk alcohol use, a history of being overweight has been associated with cirrhosis and steatohepatitis, so it is recommended to address the patient's weight as part of the treatment. (B1).
Yes. Data from the latest meta-analysis of observational studies including more than 1,000,000 individuals has shown a synergistic and risk effect in those who consume more than 63 g/day of alcohol and the risk of developing diabetes. To date, there are no long-term longitudinal studies assessing the prevalence of insulin resistance or diabetes specifically in patients with alcoholic liver disease.77 However, there are numerous cross-sectional studies associating the risk of diabetic complications (e.g., diabetic cardiomyopathy, diabetic nephropathy, diabetic neuropathy and diabetic retinopathy) with high-risk alcohol use.
Recommendations- •
Because there is a synergistic effect between high-risk alcohol use and the development of diabetes, it is recommended to evaluate the presence of diabetes and insulin resistance. (B1).
We do not have accurate information on moderate alcohol consumption.78 With the available evidence, alcohol consumption greater than 2 SUA/day cannot be recommended as safe in overweight or obese patients, or in patients with diabetes.79 In this group of patients it is prudent to avoid daily and/or frequent alcohol use, as well as "binge drinking". Finally, it is likely that sporadic alcohol consumption in moderate amounts may be acceptable in obese or diabetic patients without evidence of fatty liver.
Recommendation- •
Currently, alcohol consumption greater than 2 SUA/day cannot be recommended as safe in obese or diabetic patients. It is likely that sporadic alcohol consumption in moderate amounts may be acceptable in patients with obesity and/or diabetes without evidence of fatty liver. (B1).
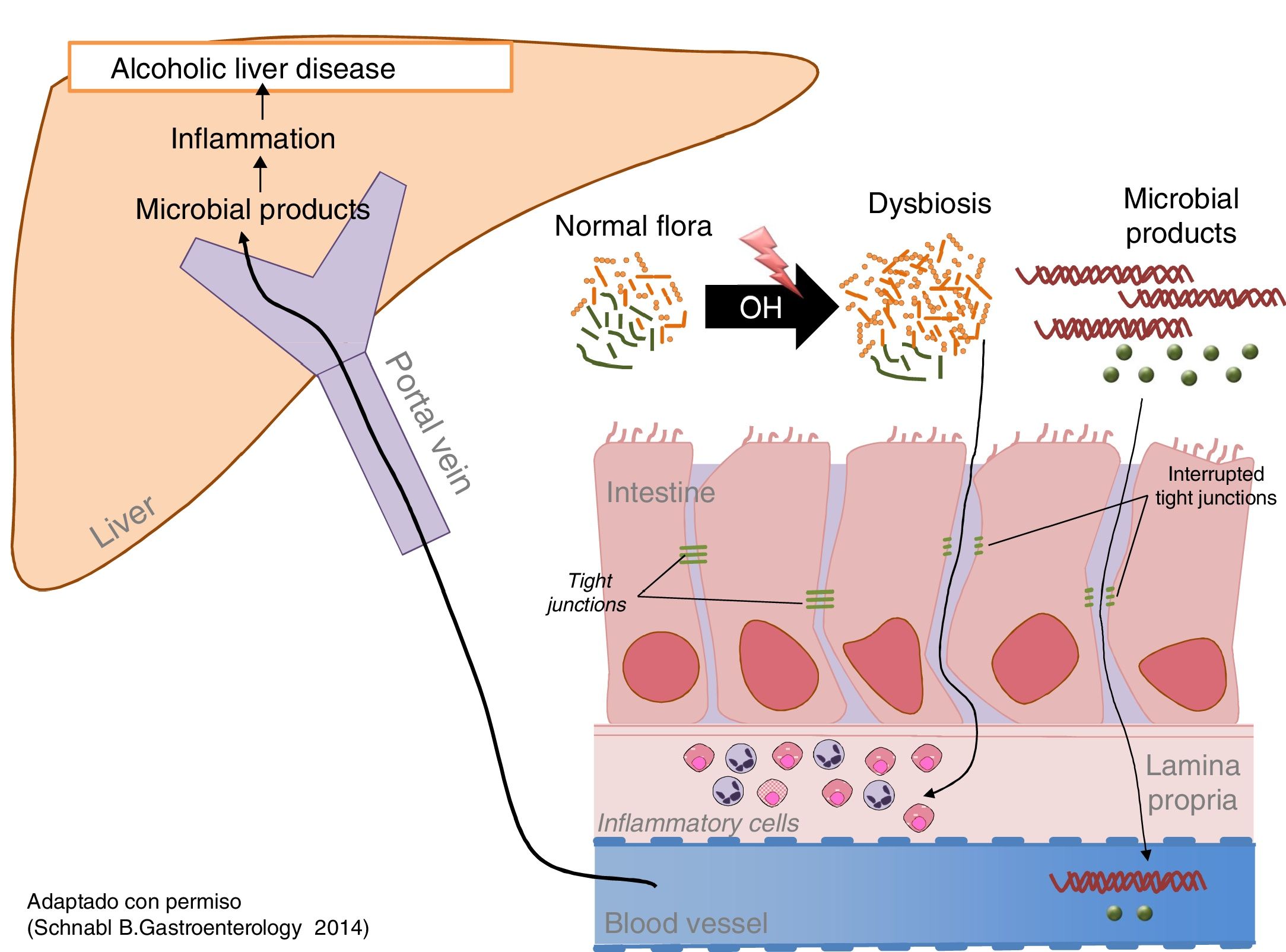
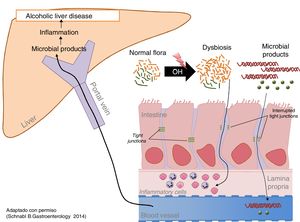
Excessive alcohol intake is associated with an increase in bacterial load (overgrowth) and intestinal dysbiosis.80 Both aerobic and anaerobic bacteria increase throughout the intestine, but more markedly in the proximal small intestine. Prolonged and excessive alcohol intake also causes intestinal dysbiosis. The most constant finding is the relative increase in the phyla Bacteroidetes and Proteobacteria to the detriment of Firmicutes. More specifically, a decrease in beneficial commensal species such as Lactobacillus spp. is observed, while potentially pathogenic commensal species, such as Enterobacteriaceae, increase (Fig. 2).81
Conclusions- •
Chronic alcohol intake increases the intestinal bacteria content and modifies its composition. (B1). Does alcohol damage the intestinal barrier? Alcohol damages the intestinal barrier and increases intestinal permeability, facilitating the passage of bacterial products found only in the intestinal lumen, such as endotoxins, to the circulatory system and the organs.82 Direct measurement of these products is the best way to identify increased intestinal permeability. Intestinal inflammation and ethanol toxicity in intestinal epithelial cells are both mediators of damage. Subclinical intestinal inflammation is identified by an increase in TNF-α-producing monocytes/macrophages in the lamina propria of the small intestine.83 In addition, ethanol and acetaldehyde damage the junctions between epithelial cells (tight junctions), causing redistribution and phosphorylation of their proteins.
- •
Alcohol damages the intestinal barrier and increases intestinal permeability, facilitating the passage of bacterial products into the blood circulation; this factor must be taken into account when conducting studies in patients with ALD (B2).
Microbial products exert a synergistic effect with alcohol on the progression of liver disease. The increase in bacterial load and intestinal dysbiosis generates an excess of PAMP (pathogen-associated molecular pattern), such as endotoxins, which reach the liver through the portal blood, where they stimulate pattern recognition receptors, such as toll-like receptors, promoting inflammation and hepatic fibrogenesis. Alcoholic liver disease in experimental models improves with intestinal decontamination and using mutants that express non-functional molecules of the toll-like receptor signalling pathway.83
Recommendation- •
Microbial products exert a synergistic effect with alcohol on the progression of liver disease. Alcoholic liver disease in experimental models improves with intestinal decontamination (B2).
There is currently no recommendation on intestinal decontamination outside clinical trials.
New contributions to the diagnosis of alcoholic liver diseaseHow can liver steatosis be evaluated by imaging techniques in patients with ALD?Ultrasound is a simple and inexpensive technique but with low sensitivity to detect mild steatosis. Computed tomography (CT) is similar to ultrasound but more expensive; the major drawback of CT is the radiation to which the patient is exposed during the procedure. The controlled attenuation parameter is a non-invasive method that has the advantage of being associated with transient elastography and simultaneously assesses fibrosis. It also boasts higher sensitivity and can quantify the degree of steatosis.84 The controlled attenuation parameter evaluates hepatic steatosis using software that interprets the ultrasound waves. Both magnetic resonance spectroscopy (MRS) (1H-MRS) and MRI with different methods that analyse the proton density fat fraction have a high sensitivity and diagnostic capacity.85 1H-MRS is considered the best technique to quantify liver fat but it is very expensive.86
Recommendations- •
Abdominal ultrasound is a good imaging technique for the initial evaluation of patients with suspected liver disease with steatosis, and can also provide additional information on other parameters (A1).
- •
The controlled attenuation parameter can be used simultaneously with transient elastography for the evaluation of steatosis (detection and quantification), especially in high prevalence populations (obese, diabetic). (B1).
- •
MRI techniques (imaging and spectroscopy) have a high diagnostic capacity and can precisely quantify fat content. They are very useful for clinical studies and therapeutic trials. (A1).
Serological fibrosis panels generally have a high negative predictive value for the diagnosis of advanced fibrosis and are useful for ruling out advanced disease, but less useful in early stages of fibrosis.87 Specific tests for ALD such as the AST to Platelet Ratio Index (APRI) have limited utility in subjects with high levels of AST, and patented panels require validation. These panels can predict liver-related mortality in this group of patients.
Recommendations- •
Biomarker-based serological fibrosis panels are useful for ruling out advanced fibrosis and may negate the need for liver biopsy. (B2).
- •
Although most of the information on steatosis detection by imaging techniques comes from studies on NAFLD, it is likely that the data can be extrapolated to ALD. (B2).
Inflammation associated with steatosis identifies patients with ALD who are at a higher risk of fibrosis and disease progression. Fibrosis is an indirect sign of inflammation.87 Simple steatosis cannot be distinguished from steatohepatitis with the usual imaging techniques (ultrasound, CT, MRI, elastography).
Recommendation- •
There is no imaging technique to identify and monitor those patients with ALD who present with inflammation or steatohepatitis. Therefore, it is not recommended to use imaging tests for this purpose. (C2).
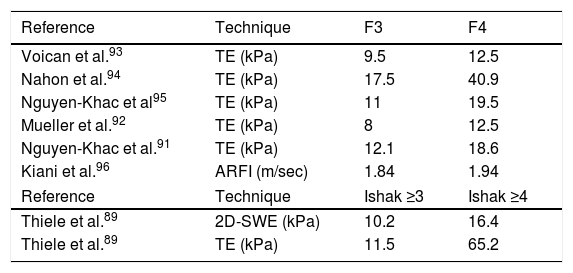
Conventional imaging techniques (ultrasound, CT, MRI) are not able to quantify the stage of fibrosis. Only elastography provides a clinically useful estimate of the stage of fibrosis and is useful for monitoring its progression.88 In general, elastography has more diagnostic accuracy for advanced stages of fibrosis (F3-F4) than for initial stages (F1-F2), and is better overall for ruling out advanced fibrosis (negative predictive value) than for confirming it (positive predictive value).88–91 Recent alcohol use, the existence of a marked steatohepatitis (AST > 200 U/l) and bilirubin levels can increase elasticity values, so they should be taken into account.91,92 These factors, together with the pre-test prevalence of the disease, make it difficult to establish a definitive cut-off point. The most accepted cut-off points for the staging of liver fibrosis in ALD are detailed in Table 2.
The most widely accepted cut-off points for the staging of liver fibrosis in ALD.
| Reference | Technique | F3 | F4 |
|---|---|---|---|
| Voican et al.93 | TE (kPa) | 9.5 | 12.5 |
| Nahon et al.94 | TE (kPa) | 17.5 | 40.9 |
| Nguyen-Khac et al95 | TE (kPa) | 11 | 19.5 |
| Mueller et al.92 | TE (kPa) | 8 | 12.5 |
| Nguyen-Khac et al.91 | TE (kPa) | 12.1 | 18.6 |
| Kiani et al.96 | ARFI (m/sec) | 1.84 | 1.94 |
| Reference | Technique | Ishak ≥3 | Ishak ≥4 |
| Thiele et al.89 | 2D-SWE (kPa) | 10.2 | 16.4 |
| Thiele et al.89 | TE (kPa) | 11.5 | 65.2 |
ARFI: Acoustic Radiation Force Impulse; TE: Transient Elastography; 2D-SWE: Two Dimension Shear Wave Elastography.
The frequency of the assessments is not defined but it seems advisable to perform periodic assessments of liver stiffness annually in patients in more advanced phases (≥F3) and every three years in patients in earlier phases.
- •
Transient elastography is recommended as a tool to identify patients with ALD with a very low risk of developing advanced fibrosis (F3) and LC (F4). (A1).
- •
The other ultrasound-based elastographic techniques have similar performance to transient elastography and can be used for the same purpose. (B2).
- •
The use of elastography is recommended as a tool for the follow-up of patients with ALD, repeating the tests every 1–3 years depending on the degree of baseline fibrosis. (C2).
The presence of LC with portal hypertension or compensated advanced chronic liver disease in patients with NAFLD or ALD has significant prognostic implications and screening recommendations. Imaging tests (ultrasound, CT and MRI) can indicate LC when detecting a liver with a nodular surface, splenomegaly and collateral circulation. Collateral circulation detection is an unequivocal sign of clinically significant portal hypertension.97
In patients with compensated ALD who have >150,000 platelets and liver stiffness <20 kPa, diagnostic endoscopy is not necessary due to the low risk of having large varicose veins. This criterion is more validated for advanced compensated liver disease of viral aetiology, but is also applicable to patients with ALD.98
Recommendations- •
LC with portal hypertension should be suspected in patients with NAFLD or with ALD upon the finding of indirect signs (nodular liver, splenomegaly, collateral circulation) in imaging tests (ultrasound, CT, MRI). (B2).
- •
The existence of clinically significant portal hypertension should be suspected in a patient with NAFLD or ALD with abdominal collateral circulation. (B2).
- •
Screening endoscopy of varicose veins can be avoided in patients with compensated advanced liver disease caused by NAFLD or by ALD who have >150,000 platelets and liver stiffness <20 kPa. (B1).
AH is a clinical entity characterised by the rapid development of jaundice (total bilirubin >3 mg/dl) and the elevation of AST > ALT in patients with excessive and active alcohol consumption. The biopsy usually shows steatohepatitis with macrovesicular steatosis and is usually accompanied by at least one of the following findings: neutrophilic infiltrate, hepatocyte injury (ballooning degeneration) or Mallory–Denk bodies. In addition, these patients present with fibrosis in the form of pericellular fibrosis and bilirubinostasis. Lastly, it should be noted that most patients with severe AH have cirrhosis at diagnosis.42 The amount of alcohol that can cause an individual to develop AH is unknown; however, a minimum average intake of at least three alcoholic beverages (∼40 g) in women and four alcoholic beverages (∼60 g) in men is considered necessary to be able to diagnose AH. It is common for individuals who develop AH to have consumed alcohol excessively for a period of more than five years, which may include periods of abstinence. To be able to diagnosis AH, the individual is expected to have consumed excessive quantities of alcohol for a minimum of six months prior to the diagnosis, with fewer than 60 days of abstinence before the onset of jaundice. Patients with AH usually have painful hepatomegaly at diagnosis, as well as poor general condition. In addition, AH is usually accompanied by hepatic decompensations (encephalopathy, ascites, gastrointestinal bleeding due to varicose veins and bacterial infections).
Lab test results reveal such patients to have total bilirubin >3 mg/dl, AST (>50 IU/ml) with a AST/ALT ratio >1.5, and AST and ALT levels usually do not exceed 400 IU/ml. An imaging test should be performed to rule out biliary tract disease. Lastly, viral hepatitis, autoimmune hepatitis and Wilson disease should be ruled out with the corresponding tests.99 Liver biopsy can be useful to confirm the diagnosis, especially when it is doubtful, and also due to its prognostic utility.42,100
Not all patients with alcoholic cirrhosis and jaundice and/or decompensation suffer from AH. For example, patients with alcoholic cirrhosis and sepsis may have acute-on-chronic liver failure (ACLF) that does not correspond to AH. In cases of diagnostic uncertainty, transjugular liver biopsy may also be useful.
Recommendation- •
In the absence of confounding factors (e.g., suspicion of drug-induced liver toxicity, ischaemic hepatitis, sepsis upon admission, doubts regarding active alcoholism, etc.), AH can be diagnosed with clinical-analytical criteria. However, definitive diagnosis requires a liver biopsy, since there are no validated non-invasive markers. In patients with confounding factors, the diagnosis requires a transjugular liver biopsy. (C3).
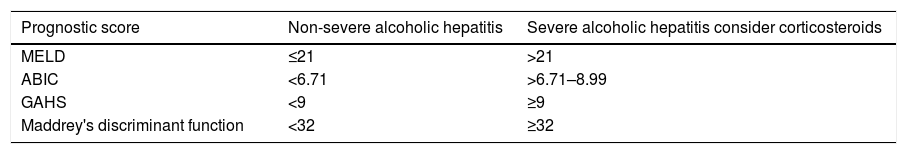
There are numerous prognostic indices (Table 3) based on data obtained at admission that allow patients with severe AH who need specific therapy (prednisolone in most cases) to be identified.101 The most commonly used index is Maddrey's Discriminant Function (DF > 32).102 However, there are data that suggest that the MELD score (>21) (Louvet, 2015 # 1199) and the ABIC score (>6.7) (Dominguez, 2008 # 615) better identify patients with severe AH.103 In addition, the calculation of Maddrey's Discriminant Function requires prothrombin time in seconds, while in most tests the information is collected as INR.104
Cut-off points of the most widely used prognostic scores.
| Prognostic score | Non-severe alcoholic hepatitis | Severe alcoholic hepatitis consider corticosteroids |
|---|---|---|
| MELD | ≤21 | >21 |
| ABIC | <6.71 | >6.71–8.99 |
| GAHS | <9 | ≥9 |
| Maddrey's discriminant function | <32 | ≥32 |
ABIC: age, bilirubin, international normalised ratio, creatinine score; GAHS: Glasgow alcoholic hepatitis score; MELD: Model for end stage liver disease score.
- •
It is recommended to use the MELD score (>21) and/or the ABIC score (>6.7) for the identification of patients with severe AH in which the administration of prednisolone should be considered. (B2)
Controlled studies and meta-analyses of individual data suggest that prednisolone (40 mg/day for four weeks) improves short-term survival in patients with severe AH compared to pentoxifylline or placebo.14,105,106 The recent STOPAH study found that this effect is only evident in the first month.107 Although initial studies suggested that pentoxifylline may be effective in preventing renal failure, these results have not been reproduced, so there is no clear evidence to recommend the use of pentoxifylline.
Recommendation- •
In patients with severe AH (MELD ≥21 or with an ABIC score ≥6.7), it is recommended to start prednisolone 40 mg/day for four weeks, after which the treatment can be discontinued abruptly or the dose tapered over the course of one week. If there is sepsis upon admission, it is recommended to start this treatment once the infection is controlled. There is no clear evidence that pentoxifylline increases survival in these patients. (A1).
There is evidence that the decrease in serum bilirubin levels during the first week of treatment with prednisolone makes it possible to evaluate its efficacy (Lille model).108,109 In the absence of a significant decrease in bilirubin (Lille score >0.45), there is no evidence that prolongation of treatment is associated with an improvement in survival. For non-responders to prednisolone treatment, there is no effective rescue drug therapy (e.g., pentoxifylline).110 The only treatment that has shown efficacy is "early" transplantation in a highly selective group of patients. This indication, although increasingly accepted, should be considered with caution in each centre until there are studies in Spain that help identify the precise indications and an adequate selection of patients.111–113
Recommendation- •
Patients receiving prednisolone should be evaluated within one week using the Lille score. In the absence of a significant decrease in bilirubin (Lille score ≥0.45), prednisolone should be withdrawn. It is not recommended to add any other pharmacological treatment for non-responders. (B2).
- •
For non-responders to prednisolone treatment, and depending on the policy of each centre, possible candidacy for a liver transplant may be considered in the absence of prolonged withdrawal. In the event that this indication is approved in a transplant centre, a thorough evaluation is recommended by a multidisciplinary team composed of hepatologists, surgeons, addiction specialists and social workers. In highly selected cases, liver transplantation increases the survival of AH patients who are not responsive to prednisolone. (A1).
Isolated studies have shown that treatment combining prednisolone with N-acetylcysteine114 or with granulocyte colony-stimulating factor (G-CSF) improves survival of patients with AH.115 However, these results need to be confirmed by larger studies. Another antioxidant drug which is the subject of clinical research is S-adenosylmethionine. New therapies are being assessed with various nutritional supplements, such as zinc in combination with other drugs, metadoxine116,117 or bovine colostrum. The gut–liver axis is key in the development of ALD and AH, which is why this is the therapeutic target of various treatments. There are several ongoing studies assessing the safety and efficacy of probiotics such as Lactobacillus rhamnosus118 and antibiotics such as rifaximin, amoxicillin-clavulanic acid and ciprofloxacin. Last of all, other studies have assessed the efficacy of faecal transplant, with promising results.119 Other drugs under investigation are selective farnesoid X-receptor agonists, specifically obeticholic acid. Mediators of inflammation such as anakinra (an interleukin-1 receptor) have also shown promising results and will be tested in combination with zinc in a clinical trial with several arms. Another group of drugs under investigation are liver regeneration modulators, including stimulators of bone marrow-derived circulating pluripotent stem cell colonies,115 emricasan (a pan-caspase inhibitor) and interleukin-22 alone or in combination with F-652 (a recombinant protein formed by interleukin-22 and immunoglobulin G2). Finally, it is worth highlighting the studies carried out to date with bioartificial livers (e.g. ELAD).101,120
Recommendation- •
Although there are promising studies suggesting that different pharmacological modalities (e.g. N-acetylcysteine or G-CSF) could increase the beneficial effects of prednisolone, none of these treatments can be recommended until the clinical studies have been widely confirmed. (C2).
There is little information to adequately answer the question of whether acute-on-chronic liver failure (ACLF) due to cirrhosis caused by alcoholic liver disease (ALD) has a different course from ACLF due to cirrhosis from other causes.121 The available data suggest that both the type of organs affected and the degree of ACLF and its prognosis are similar no matter the cause of the ACLF.122
Conclusions- •
The prognosis of ALD-ACLF does not appear to be different from that due to non-alcoholic disease. (B2).
There are two potential advantages of performing a liver biopsy on patients with suspected ALD-ACLF. The first is that it can identify histological data compatible with alcoholic hepatitis (AH). This is important, as it can guide specific treatment and provide prognostic information.42 Secondly, it allows us to rule out a previously unrecognised cause of liver disease (toxic, autoimmune, etc.).123 It is important to underline that not all patients with ALD and jaundice are having an episode of AH.
Recommendation- •
A liver biopsy is recommended for patients with ALD-ACLF to confirm the diagnosis of acute alcoholic hepatitis and rule out other causes. This recommendation is subject to the availability of and experience with the transjugular biopsy technique if necessary. (B2).
To date, no effective specific treatment is available either for ACLF in general or for ALD-ACLF, except for corticosteroids if there is severe AH.106 The current therapeutic approach is based on treatment and prevention of complications, whenever possible, and support for multiorgan failure. Recent studies suggest that administration of G-CSF115 may be effective in ACLF and reduce mortality rates. However, the available studies only include small series of patients and exclude patients with severe ACLF. Therefore, new studies in larger series of patients with ALD-ACLF are required before the use of G-CSF can be recommended in clinical practice.115
Recommendation- •
The only treatment known to be effective in treating ALD-ACLF is corticosteroids if the patient has severe AH. (B2)
Liver transplant is theoretically a very effective therapeutic procedure in patients with ACLF due to the high mortality rate associated with this condition. However, the indication of transplant in ALD-ACLF has special implications, particularly for patients whose alcoholism has not been assessed in a protocolised manner before the onset of the ACLF. Rapid decision making on the potential course of the alcoholism after transplantation in the context of the severity of the ACLF is risky and involves ethical problems. In addition, the indication of transplantation in these patients goes against the rule in many countries of a six-month period of abstinence before transplantation. Some studies have shown good post-transplant outcomes and low incidence of alcohol relapse if restrictive selection of patients with severe AH (who mostly have ACLF) is made based on good family support, lack of comorbidities and a clear commitment to alcohol abstinence post-transplant.121 The proportion of patients who might benefit from this approach is less than 5%.124
Recommendation- •
Liver transplantation is indicated in patients with ALD-ACLF whose alcoholism has no contraindications for transplantation. Transplantation is not indicated in patients with severe AH or in those with ACLF due to another trigger in whom alcoholism has not been adequately assessed. (B2).
Benzodiazepines (BZD) are the treatment of choice for alcohol withdrawal syndrome due to their effectiveness in reducing the withdrawal symptoms, the risk of seizures and/or "delirium tremens".125 Long-acting BZD (diazepam, chlordiazepoxide) provide greater protection against seizures and delirium, but the short- and intermediate-acting BZD (e.g. lorazepam, oxazepam) are safer in older patients.126 Neuroleptics should not be used alone as they do not reduce delirium and they increase seizures. They can be considered in combination with BZD for marked agitation or hallucinations. However, there are no available studies on the safety of the use of BZD in patients with cirrhosis caused by ALD or AH. These drugs have to be used with caution due to the risk of encephalopathy and aspiration.
Recommendation- •
BZD are the drugs of choice for withdrawal, reducing the risk of seizures and "delirium tremens". The intermediate-acting BZD are safest in older people. Neuroleptics can be considered in combination with BZD for marked agitation or hallucinations. BZD should be used with caution in patients with cirrhosis or AH as they can induce encephalopathy. (B2).
There are few studies on the efficacy and safety of these drugs in patients with advanced liver disease. A number of drugs are being assessed which can reduce alcohol "craving" and so hopefully increase abstinence and prevent relapse.127,128 Disulfiram, naltrexone and nalmefene should be used with extreme caution in liver disease and are contraindicated in severe liver failure according to their respective summaries of product characteristics. Acamprosate, topiramate and baclofen, with less liver metabolisation, seem to be more highly recommended. Baclofen is the only one of these drugs which has been shown to be useful and safe in patients with alcoholic cirrhosis.129 The recommended dose is from 30 to 60 mg/day with a maximum dose of 100 mg/day, and administration for prolonged periods is safe.
Recommendation- •
There are few data on the efficacy and safety of drugs for reducing alcohol craving in patients with advanced liver disease. Disulfiram, naltrexone and nalmefene are contraindicated in severe liver disease. Baclofen is the only drug proven to be safe and effective in patients with cirrhosis caused by ALD. (B2).
A period of pre-transplant abstinence as sole tool for predicting lasting abstinence is insufficient.130 However, it should be borne in mind that pre-transplant abstinence is important as, due to the reversibility of liver dysfunction and improvement in function, in some cases it can reverse the candidate's indication for transplant.131 In addition to the duration of abstinence, predicting lasting abstinence after transplantation is influenced by other factors such as disease awareness, psychiatric comorbidities or other addictions, repeated attempts at stopping drinking and socio-family support. A thorough and comprehensive assessment of all these factors by a multidisciplinary team including addiction specialists and psychiatrists is important.132–134
Recommendation- •
All transplant candidates with ALD must have a full psychosocial assessment. The suitability of transplantation must be established based on the degree of alcohol dependence, and the existence of promising factors for lasting abstinence. (A1).
A multidisciplinary approach with the participation of different specialists such as hepatologists, addiction specialists, social workers, etc. is essential to adequately address the problem of alcohol relapse.135 This approach helps to prevent relapse, to better interpret the different relapse behaviours and to provide adequate treatment.136,137
Recommendation- •
In the management of patients with alcohol use disorder, working closely with addiction units and specialists helps reduce alcohol relapse and mortality rates. (B2).
See page 29 for the answer and for the specific recommendation.
What measures should be implemented during follow-up for ALD-related transplant to reduce mortality rates?The most important measure is to prevent recurrence of alcohol abuse. Depending on the definition of recurrence, its prevalence among patients is 20–40 %.138,139 Recurrence is not associated with higher mortality rates.140,141 However, it does have negative effects on the graft and causes an increase in other alcohol-induced disorders. It is therefore important to detect recurrence early and treat it with the help of a multidisciplinary team. The use of biomarkers which detect recent use of alcohol (e.g. ethyl glucuronide in urine) can be useful.142
Patients transplanted because of ALD have a higher cardiovascular risk than with other causes and they are particularly predisposed to developing head and neck and lung cancer. Risk factors, especially those which are modifiable, such as smoking, obesity and sedentary lifestyle, should therefore be avoided.143–145
Recommendations- •
Of great importance in all ALD-related transplant patients are prevention, early diagnosis and treatment of alcohol relapse. In cases of severe relapse, the chances of survival of the graft and the patient decrease. (A1).
- •
Particular attention should be given to cardiovascular risk factors, specifically to smoking and alcohol use, and to the development of de novo head and neck and gastrointestinal cancer in patients with alcohol use disorder. (A1).
ALD is the most prevalent cause of LC in Europe, including Spain.29–31 Most chronic drinkers develop simple steatosis, but 10–20 % will end up developing alcoholic steatohepatitis, LC and HCC.42,43,61 Sustained abstinence from alcohol is the only factor which can improve long-term survival.70 Obesity and metabolic syndrome have an additive effect71 which can increase the risk of cirrhosis and HCC.60,62 Therefore, as these are potentially avoidable conditions, active preventive policies need to be introduced.
The estimated prevalence of ALD is 2%. That, added to the change in the pattern of alcohol use,15,19 means that ALD has become a public health problem requiring the implementation of preventive measures at different levels. The impact of changing patterns of consumption ("binge" drinking and use among adolescents) on liver-related death here in Spain19 has yet to be determined, but based on the available evidence, it would be advisable to study this association.20
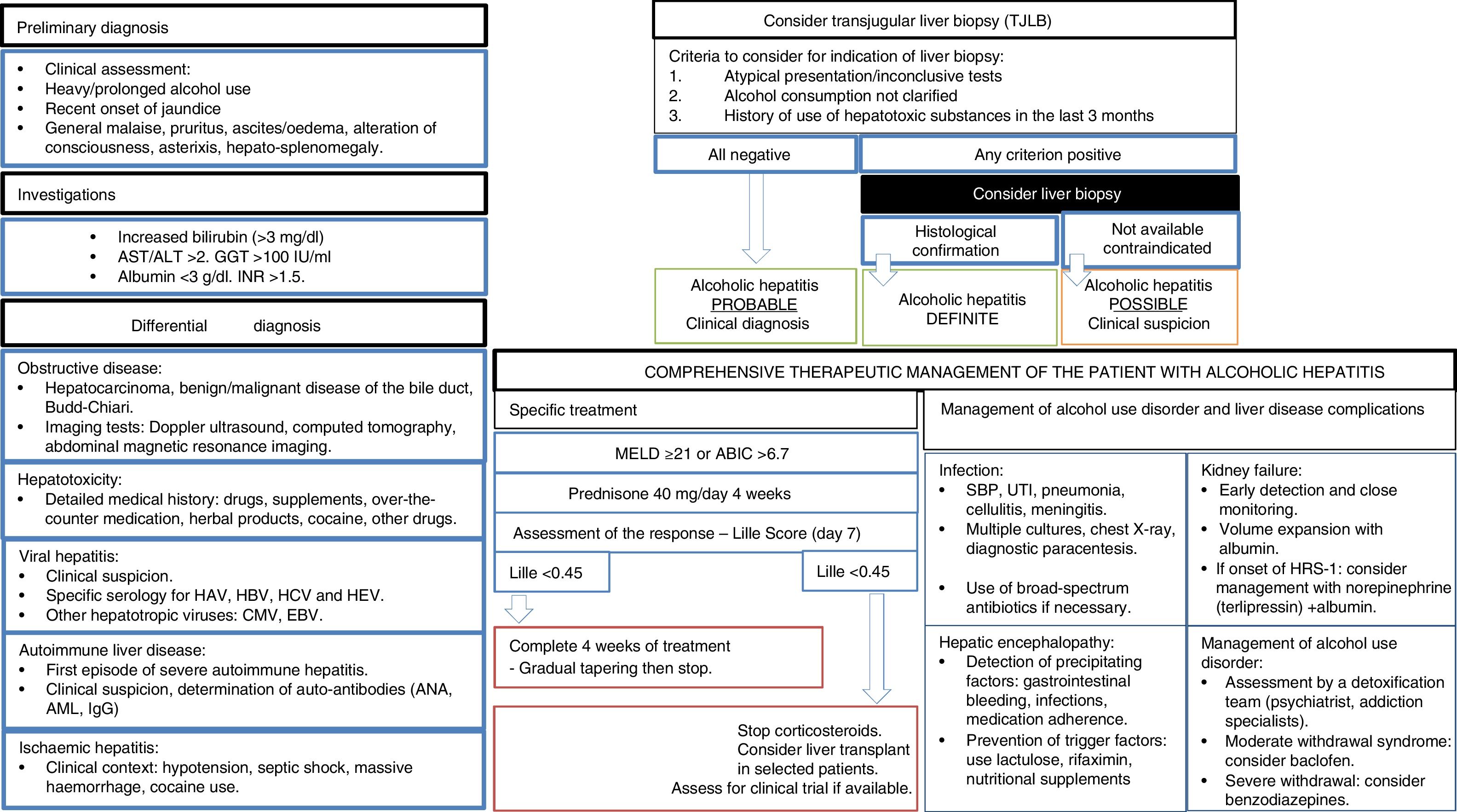
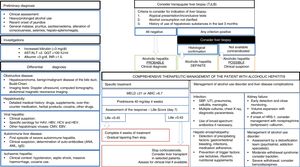
From a clinical point of view, the majority of patients are identified in late stages of the disease, so there is an urgent need for the development of early detection campaigns. In terms of clinical research, AH is the medical condition to have received the most attention.99 In patients with severe AH, starting them on prednisolone is recommended.105,106 There is no evidence that pentoxifylline increases survival in these patients.146 An increasing number of centres are offering the chance of an early liver transplant in highly selected cases, although prospective studies are required to identify patients with a lower risk of relapse.111 Absolute abstinence should be encouraged in patients with previous AH, as moderate intake is associated with higher mortality rates.135 A summary of the diagnostic and therapeutic management of AH is shown in Fig. 3.
Comprehensive management algorithm for patients with alcoholic hepatitis. Diagnostic evaluation, specific treatment, hepatic complications and treatment of alcohol consumption disorder. AST: aspartate-transaminase. ALT: alanin-transaminase.GGT: ganmagglutamyl-transferase. INR: internationa normalized ratio. HAV: Hepatitis A virus. HBV: Hepatitis B virus. HCV: hepatitis C virus. HCV: hepatitis E virus. CMV: cytomegalovirus. EBV: Epsteinbar Virus. ANA: antinuclear antibodies. AML: smooth muscle antibodies. MEDL: model of end stage liver disease. ABIC: Age, bilirubin, INR, creatinine score. PBE: spontaneous peritonitisbacterial. UTI: urinary tract infection. SHR: hepatorenal syndrome.
Multidisciplinary treatment combined with addiction units to help acquire dependency awareness and working on relapse prevention are hugely important elements for sustained abstinence. However, there is less certainty about the benefits of these measures in patients with advanced liver disease.136,137 All transplant candidates with ALD must have a full psychosocial assessment. The suitability of transplantation must be established based on the degree of alcohol dependence and the existence of promising factors for lasting abstinence.136
In conclusion, the current clinical practice guidelines on ALD provide the best available evidence for the appropriate management and treatment of our patients.
Conflicts of interestRamón Bataller has received fees from Echosens for seminars.
The remaining authors declare that they have no conflicts of interest.
Please cite this article as: Bataller R, Cabezas J, Aller R, Ventura-Cots M, Abad J, Albillos A, et al. Enfermedad hepática por alcohol. Guías de práctica clínica. Documento de consenso auspiciado por la AEEH. Gastroenterol Hepatol. 2019. https://doi.org/10.1016/j.gastrohep.2019.09.006