Functional abdominal pain is a disorder in which central and peripheral sensitization processes converge, leading to hypersensitivity and allodynia. Differential diagnosis is made with organic digestive, renal, gynecological, endocrine, or neurological diseases. Treatment should be individualized for each patient. In cases of debilitating pain, therapy combining drugs with different mechanisms of action can be initiated, while in less severe cases, therapy with a progressive introduction of drugs based on clinical response is advised. The first line includes general lifestyle advice and antispasmodic substances, like peppermint oil, anticholinergic/antimuscarinic, and calcium channels antagonists. In the second line of treatment, neuromodulating agents are added. Finally, when these measures fail, third-line treatments such as gabapentine and atypical antipsychotics are considered. Psychological interventions should be considered if specialized therapists are available to manage these disorders.
El dolor abdominal funcional es un trastorno en el que confluyen procesos de sensitización central y periférica productores de hipersensibilidad y alodinia. El diagnóstico diferencial se hace con enfermedades orgánicas digestivas, renales, ginecológicas, endocrinas o neurológicas. El tratamiento debe de individualizarse para cada paciente. En casos de dolor invalidante se puede iniciar una terapia combinando fármacos con diferentes mecanismos de acción, mientras en casos menos graves se aconseja una terapia con introducción progresiva de fármacos en función de la respuesta clínica. La primera línea incluye consejos generales de estilo de vida y sustancias espasmolíticas, como el aceite de menta piperita, anticolinérgicos/antimuscarínicos, y antagonistas de los canales de calcio. En la segunda línea de tratamiento se añaden agentes neuromoduladores. Finalmente, cuando fallan estas medidas planteamos tratamientos de tercera línea, como los gabapentinoides y antipsicóticos atípicos. Las intervenciones psicológicas deben de considerarse si se disponen terapeutas especializados en el manejo de estos trastornos.
Functional abdominal pain is pain that occurs in the absence of a causative organic, metabolic or infectious disease. It can occur in isolation, called centrally mediated abdominal pain syndrome (CAPS), or as part of another functional gastrointestinal disorder such as irritable bowel syndrome (IBS) or functional dyspepsia. To diagnose functional abdominal pain, it is necessary to rule out organic diseases that may be causing the pain, and treatment is often a challenge, because of the variable response to the different therapeutic measures. In this document, the Asociación Española de Neurogastroenterologia y Motilidad (ASENEM) [Spanish Association of Neurogastroenterology and Motility] provides an update on the management of patients with functional abdominal pain, with a practical approach based on the available scientific evidence.
Biological bases of visceral painAccording to the International Association for the Study of Pain (IASP), pain is defined as “An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage”.1 Depending on the region of the body affected, pain can be divided into two basic types: somatic and visceral. Somatic pain is pain originating in superficial, somatic structures of the body (skin, muscles, joints), while visceral pain is pain originating in internal organs (viscera), with pain originating in the gastrointestinal tract being of great clinical significance.
Visceral pain of gastrointestinal origin has differential characteristics with respect to somatic pain or pain originating in other viscera (particularly solid viscera): diffuse character (difficult localisation); poor relationship with the disease (or the intensity of the stimulus) that causes it; capacity to produce high intensity autonomic responses; and capacity to generate referred sensations (referred pain). In the gastrointestinal tract, the relationship between tissue damage and pain is not obvious, so relatively mild stimuli (tonic contraction of smooth muscles, mild mucosal inflammation or mild ischaemia) can cause intense pain reactions.
Organisation of sensory pathways in the gastrointestinal tractSensory innervation of the gastrointestinal tract, and thus the generation of nociceptive responses, depends on both extrinsic nerve pathways (which may be of vagal or spinal origin) and the enteric nervous system. In general, the sensory pathways are polymodal and respond to both chemical and mechanical stimuli, with the mechanical stimuli being particularly important in the gastrointestinal tract. From the spinal cord, ascending pathways (dependent on second-order neurons) would enable central integration and the generation of the series of autonomic and behavioural responses which characterise the nociceptive response. Similarly, descending pathways originating in the brain also act as modulators of nociceptive responses2–4 (Fig. 1).
Representation of nerve pathways involved in the processing and integration of visceral nociceptive signals originating in the gastrointestinal tract. In contrast to somatic nerve pathways (for example, from skeletal muscle), which have a clear somatotopic organisation in the central nervous system, pathways of visceral origin terminate at multiple levels in the spinal cord, giving visceral pain (including gastrointestinal) its diffuse character and showing a complex organisation (A). Visceral afferents converge with somatic pathways at the dorsal horn of the spinal cord (viscero-somatic convergence),1 which explains the phenomenon of referred pain. Primary visceral afferents can divide to terminate simultaneously at multiple levels in the spinal cord.2 In the spinal cord, there may also be convergence of afferents from different viscera (viscero-visceral convergence).3 This would explain why gastrointestinal disorders can cause symptoms in remote organs/viscera. Visceral afferent pathways reach the spinal cord with sympathetic nerves, which may produce sympathetic autonomic reflexes associated with visceral stimulation.4 The parasympathetic afferent component (vagus nerve and other parasympathetic nerves)5 normally mediates non-nociceptive physiological reflexes, but its integration at the base of the brain (brainstem) may modulate the activity of descending spinal pain modulatory systems (B-6). The enteric nervous system7 may also be involved in modulating sensory activity and associated autonomic reflexes, which may explain some of the symptoms associated with intestinal pain, such as motor and secretory disturbances. (C) Detail of the sensory afferent endings in the gastrointestinal tract. Nerve endings are located in all layers of the intestine, as well as in blood vessels and mesentery. Intramuscular and intraganglionic endings (4 and 5 in the figure) have no proven role in the transmission of pain stimuli. Source: Modified from: Knowles et al.70; Drewes et al.71; and Martinez.4
Functional gastrointestinal disorders are largely characterised by altered nociceptive mechanisms. Alterations in pain perception are associated with sensitisation of pain-conducting pathways and/or nociceptive signal integration mechanisms leading to states of increased sensitivity (hypersensitivity), which can manifest in two forms: hyperalgesia and allodynia. In sensitised states, it is normal for changes in nociceptive signal processing to be associated with a combination of changes at both the peripheral level (peripheral sensitisation) and the central level (central sensitisation).
Overall, the visceral hypersensitivity that characterises functional disorders is probably generated by the interaction between four mechanisms: a) sensitisation of sensory afferents (peripheral sensitisation); b) sensitisation at the level of the dorsal horn of the spinal cord (central sensitisation); c) alterations in descending modulatory systems (facilitators and inhibitors); and d) cognitive/emotional alterations (including the response to stress states) which alter the central interpretation of peripheral sensory signals.4
The molecular mechanisms involved in visceral nociceptive responses and sensitisation processes are not fully understood, but appear to rely on highly redundant systems involving multiple mediators: ion channels (such as voltage-gated/dependent sodium channels, transient receptor potential vanilloid [TRPV] ion channels or purinergic receptors); kinins; biogenic amines (histamine or serotonin); prostanoids (PGE2); growth factors (nerve growth factor [NGF] or glial-derived neurotrophic factor [GDNF]); proteases; cytokines; chemokines; adenosine triphosphate (ATP); endocannabinoids; and endogenous opioids4–7 (Fig. 2). A large amount of clinical and experimental evidence also suggests that the microbiota may play an important role in modulating nociceptive mechanisms.8
Mechanisms involved in the generation of visceral nociceptive signals in a sensory afferent. The diagram shows the mechanisms generating nociceptive signals under normal conditions, and the mechanisms of peripheral sensitisation. In the intestinal epithelium, sensory mechanisms generating nociceptive signals depend on complex interactions involving multiple levels of signalling, in many cases with two-directional actions and feedback mechanisms. Under normal conditions, the generation of nociceptive signals depends on the activation of different ion channels (for example, Na+ channels, transient receptor potential [TRP] channels, acid-sensing ion channels [ASIC], P2X). The activity of these channels increases in states of sensitisation, and contributes to the changes in excitability that characterise these states. Sensitisation mechanisms involve the activation of membrane receptors (usually G protein-coupled receptors [GPCR], such as receptors for bradykinins, prostaglandins, protease-activated receptors [PAR], histamine, serotonin, nerve growth factor [NGF] neurotrophins, substance P or calcitonin gene-related peptide [CGRP]) by specific ligands. This process depends on neuroimmune interactions involving immune cells (mast cells, lymphocytes, dendritic cells, macrophages and neutrophils), the enteric nervous system (enteric neurons and enteric glia) and the intestinal epithelium (epithelial cells and enteroendocrine cells, mainly responding to chemical and mechanical stimuli). The microbiota has recently been added to this system of interactions as an active component. Microbial derivatives can therefore penetrate the epithelium and act as direct receptor ligands or modify local neuroimmune activity. Under certain circumstances, persistent phenotypic changes can occur in sensory neurons. These changes maintain sensitised states in the long term and can affect synaptic activity in the spinal cord, and may generate sensitised states at a central (spinal cord) level. Together these processes appear to be particularly important in mediating the sensitisation that accompanies inflammatory or infectious states. Source: Modified from: Knowles et al.70 and Martinez.4
Chronic abdominal pain, as a symptom, is challenging to diagnose given its high frequency, low specificity and broad differential diagnosis, which also includes organs outside the gastrointestinal sphere (genito-urinary). All of this makes it difficult to establish the functional origin of the condition, leading to multiple medical visits, tests and even surgery, in an attempt to define the cause of the pain.9 An accurate differential diagnosis, starting with a detailed medical history and thorough physical examination, allows early diagnosis and a targeted therapeutic approach.
Table 1 lists causes of chronic abdominal pain based on the visceral or somatic origin of the pain and its relation to systemic disorders.
Rome IV diagnostic criteria for the diagnosis of centrally mediated abdominal pain syndrome (CAPS) and differential diagnosis of chronic abdominal pain.
| Rome IV Diagnostic Criteria for CAPS |
|---|
| Meeting criteria within the last 3 months with onset of symptoms at least 6 months prior to diagnosis. Should include all of the following:1. Constant or nearly constant abdominal pain.2. No or only occasional relationship of pain with physiological events (for example, eating, moving bowels, menstruation), although there may be some degree of gastrointestinal dysfunction.3. Pain limits some aspect of daily functioning (disruption of work, intimate relationships, social life and leisure, family life, and caring for oneself or others).4. Pain is not feigned.5. The pain is not explained by another structural or functional gastrointestinal disorder or other medical condition.CAPS is often associated with psychiatric comorbidity, but there is no specific profile that can be used for diagnosis. |
| Differential diagnosis: other causes of chronic abdominal pain | |
|---|---|
| Visceral origin | Pancreatic disease (chronic pancreatitis, cysts, adenocarcinoma)Hepato-biliary disease (lithiasis, tumour)Kidney disease (lithiasis, tumour)Gastroduodenal disease (peptic disease, tumour)GastroparesisPostcholecystectomy syndromeInflammatory bowel diseaseAbdominal adhesion syndrome (occlusive/sub-occlusive conditions)Fitz-Hugh-Curtis syndrome (chronic perihepatitis due to pelvic inflammatory disease)69Actinic enterocolitisDiverticular diseaseAbdominal herniasRelated to vascular structures:- Coeliac artery (or arcuate ligament) compression syndrome- Superior mesenteric artery syndrome- Chronic mesenteric ischaemiaGynaecological origin:- Endometriosis- Pelvic inflammatory disease (PID) |
| Somatic origin | Abdominal wall:- Anterior cutaneous nerve entrapment syndrome (Carnett sign)- Postherpetic neuralgiaCostal:- Intercostal or segmental neuralgia- Twelfth rib syndrome- Costochondritis |
| Systemic diseases | Familial Mediterranean feverPorphyria |
| Functional disease | Irritable bowel syndrome (associated with change in bowel habit, pain is not the predominant symptom)Functional dyspepsia-type epigastric pain (related to ingestion, associated with other dyspeptic symptoms)Gallbladder or sphincter of Oddi dysfunction (relation to ingestion, biliary characteristics)Narcotic bowel syndrome or opioid-induced hyperalgesia |
CAPS: Centrally mediated abdominal pain syndrome.
Functional abdominal pain may be present in isolation, or as a symptom of other functional gastrointestinal disorders. Isolated functional abdominal pain, currently referred to as CAPS, is characterised by abdominal pain as the dominant symptom, of at least six months’ duration, poorly localised, often severe, recurrent, occurring almost daily in the last three months, rarely related to physiological stimuli such as menstruation, ingestion or defaecation and affecting the individual’s activities in the personal, work and/or family sphere.10 Conversely, when the pain is related to changes in bowel habit, or to changes in stool frequency and consistency, we refer to it as functional pain associated with IBS. To establish the diagnosis of functional abdominal pain, structural and metabolic causes must be ruled out, when suspected, by available diagnostic methods. The Rome IV criteria for the diagnosis of CAPS are listed in Table 1.11
The following aspects should be taken into account in order to guide diagnosis:
- -
Associated comorbidity: conditions related to functional gastrointestinal disorders such as fibromyalgia or chronic fatigue syndrome are common in this context, as well as psychiatric illnesses of various kinds.10 A history of abdominal surgery may lead to the misdiagnosis of adhesions, which are more likely to cause occlusive/sub-occlusive symptoms than isolated abdominal pain, so the diagnosis of CAPS should be considered in these cases.12 Gynaecological comorbidities should be specifically assessed in women (for example, pelvic inflammatory disease or endometriosis) and urological comorbidities in men (such as prostatitis).
- -
Drugs, especially non-steroidal anti-inflammatory drugs (NSAID) and opioids (opioid-induced hyperalgesia).
- -
Characteristics of pain, such as location, onset and changes over time, and precipitating, aggravating or relieving factors.13 Functional abdominal pain can have a number of characteristics which, although not specific, can facilitate diagnosis; it is usually generalised in location and expressed in emotional terms (verbal and non-verbal), with emphasis on its chronic and recurrent nature, even from childhood. Patients often ask for further tests, demanding complete pain relief, and they are often not aware of the involvement of psychosocial factors in the development of their problem.10
- -
Symptoms and warning signs: onset of pain after the age of 60; hyporexia; weight loss; signs of gastrointestinal bleeding or iron deficiency anaemia; persistent vomiting; and family history of colorectal cancer, gastric cancer or inflammatory bowel disease.13
- -
Other gastrointestinal and systemic symptoms: help to orientate the origin of the pain, in order to rule out other functional gastrointestinal disorders and systemic diseases that cause constant pain (Table 1).
- -
Psychosocial status and impact on quality of life: unresolved trauma, depression, histrionic personality14 or history of sexual/physical abuse may be triggers for functional abdominal pain and cause a worse response to treatment.15 The possibility of feigned pain or opioid dependence should be addressed.
Diagnostic tests are not always necessary. In general, blood tests including complete blood count, basic blood screen, iron, liver function, CRP and anti-tissue transglutaminase antibodies are condoned.16 In cases where the psychosocial situation, comorbidities and pain characteristics are suggestive of CAPS, further diagnostic tests can be avoided. Occasionally, the intensity of the pain and/or patient demand makes it necessary to complete the investigations, always after fostering the doctor/patient relationship and raising awareness of the possible causes of the pain.
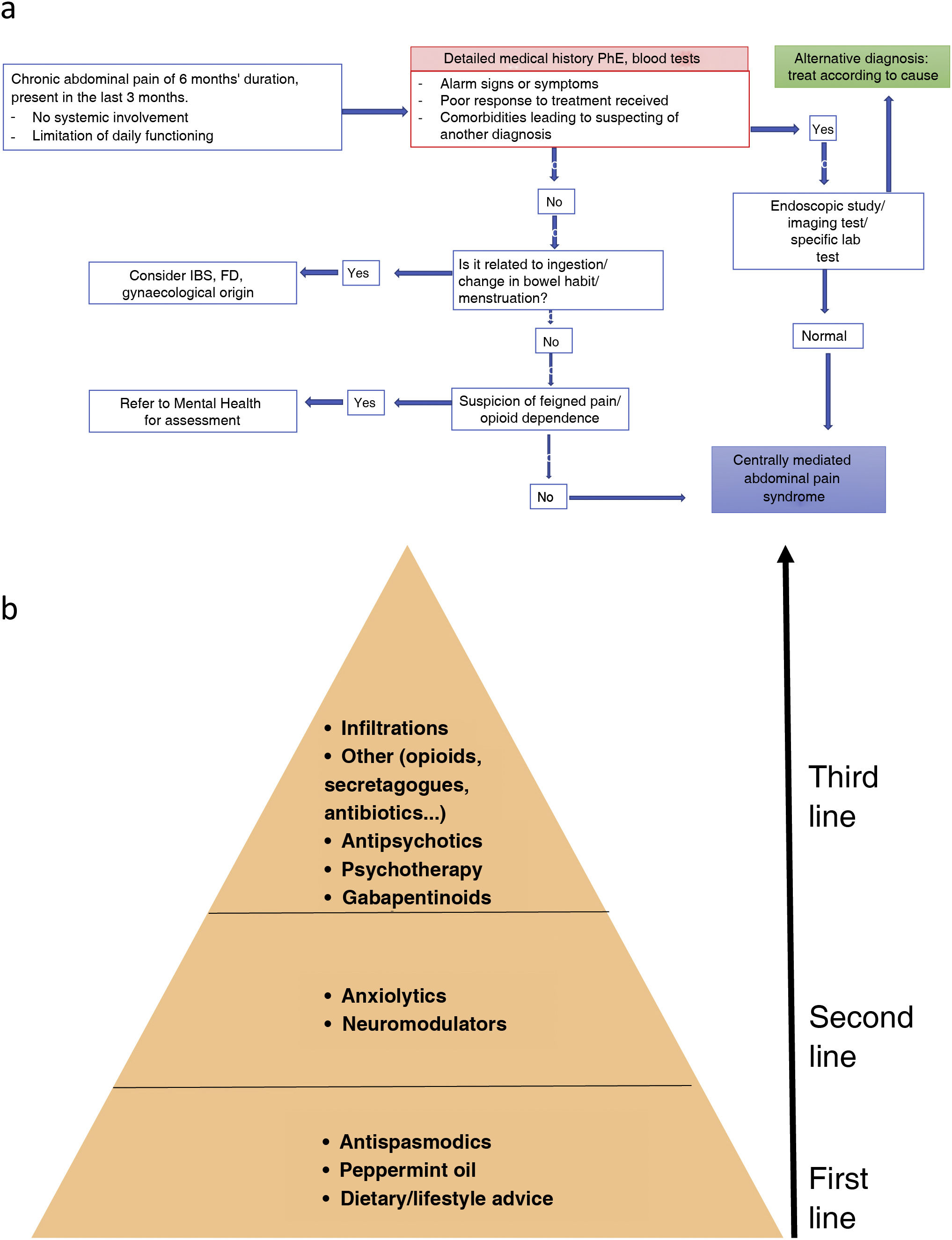
Endoscopic examination and imaging (ultrasound, CT or MRI depending on the suspected diagnosis) should be indicated in cases with alarm symptoms/signs, poor response to treatment and severe symptoms limiting daily functioning.16 The diagnostic algorithm is shown in Fig. 3A.
(A) Diagnostic algorithm for CAPS. FD: functional dyspepsia; IBS: irritable bowel syndrome; PhE: physical examination. * Determination of complete blood count, basic blood screen, iron, liver, CRP and anti-tissue transglutaminase antibodies. Source: adapted from Setia et al.72 (B) Management of functional abdominal pain. First-line treatment includes review of dietary and lifestyle measures, and treatment with peppermint oil and antispasmodics. If this fails, neuromodulators such as tricyclic antidepressants should be included. The third line of treatment includes gabapentinoids, psychological interventions, atypical antipsychotics or other drugs with indirect action on pain. In cases of disabling pain, a combination treatment with drugs of different levels can be initiated. Also, if psychotherapy is available, it can be provided in the early stages of treatment.
As with any functional gastrointestinal disorder, it is important to recommend an ordered lifestyle, such as keeping a regular timetable and sleep habits, with a balanced diet, trying to avoid stressful situations. In many cases patients will report that their pain may be exacerbated by ingestion of food or drink. In these cases it is important to advise the patient to exclude only those foods that clearly cause their symptoms, avoiding a spiral of increasing food restriction that ends up generating unwanted nutritional deficits. In some patients, stricter dietary control by a nutritionist is recommended to avoid complications arising from restrictive diets.17,18
AntispasmodicsAntispasmodics are a heterogeneous group of drugs used in the treatment of abdominal pain in patients with gut-brain axis disorders. Overall, they act by inhibiting intestinal motility and, although they often have multiple mechanisms of action, depending on their mechanism, they can be classified as: direct smooth muscle relaxants (mebeverine); anticholinergic/antimuscarinic (hyoscine, hyoscyamine, dicyclomine); and calcium channel blockers (peppermint essential oil, otilonium, pinaverium, alverine citrate, trimebutine).19
Several meta-analyses have demonstrated therapeutic benefit of antispasmodics over placebo in relieving functional abdominal pain.20–24 A recent meta-analysis of 51 randomised clinical trials (RCT) on 4,644 patients compared soluble fibres, antispasmodics and neuromodulators24 and concluded that peppermint oil, tricyclic antidepressants (TAD) and antispasmodics were more effective than placebo for both overall symptoms and abdominal pain in IBS, with peppermint oil first for overall symptoms and TAD first for abdominal pain. However, TAD caused the most adverse effects and so generally the advice is to reserve them for second-line treatment. A Cochrane review22 also found no statistically significant benefit over placebo for antimuscarinics and mebeverine. However, a more recently published systematic review25 that included six RCT analysing the effect of mebeverine on abdominal pain showed a positive effect of mebeverine over placebo. It is important to note that most clinical trials with antispasmodics are old and limited by their suboptimal methodology and considerable heterogeneity, having been conducted in many cases prior to the publication of the Rome recommendations.26 They are generally well tolerated and have a good safety profile with the most common adverse effects being those resulting from the anticholinergic action, such as dry mouth, dizziness and blurred vision27 (Table 2).
Dosage, adverse effects and contraindications of antispasmodics marketed in Spain.
| Antispasmodic | Predominant mechanism of action | Recommended dose | Most common adverse effects | Contraindications |
|---|---|---|---|---|
| Peppermint oil (Colpermin®) | Calcium channel blockers | 187mg/1–2 caps 3 times a day at least 30min before meals | Heartburn, belching, headache | None known. Caution in patients with liver disease |
| Butylscopolamine bromide (Buscapine®) | Anticholinergic | 10mg 3 times a day | Dry mouth, blurred vision, urinary retention | Urinary retention, intestinal obstruction, myasthenia gravis, angle-closure glaucoma |
| Mebeverine (Duspatalin®) | Direct smooth muscle relaxanta | 135mg 3 times a day 20min before meals | Dizziness, dry mouth, nausea, headache | Intestinal obstruction |
| Otilonium bromide (Spasmoctyl®) | Calcium channel blockersa | 40mg 2 or 3 times a day 2min before meals | Dry mouth, nausea | Intestinal obstruction |
| Pinaverium bromide (Eldicet®) | Calcium channel blockersa | 50mg 3 times a day during meals | Dizziness, nausea, abdominal discomfort, increased blood pressure | None known. Probably intestinal obstruction |
| Trimebutin (Polibutin®) | Calcium channel blockersa | 100mg 2 or 3 times a day | Dizziness, dry mouth, nausea, abdominal discomfort, trembling of the hands | Urinary retention, intestinal obstruction, angle-closure glaucoma |
Peppermint essential oil is a herbal agent containing menthol as its main constituent.28 It acts as an antispasmodic by antagonising calcium channels in the gastrointestinal tract.29 It also has an analgesic effect through modulation of visceral nociception mediated by transient receptor potential (TRP) channels,30,31 kappa opioid receptor agonism32 and inhibition of 5-HT3 receptors.33 It has also been suggested to have anti-inflammatory,34 antibacterial35 and carminative properties, which may reduce intestinal gas.36 In Spain, it is available as enteric-coated capsules containing 187mg of peppermint essential oil, with prolonged release from the small intestine and throughout the colon. Systematic reviews and meta-analyses available to date show benefit of peppermint oil over placebo in improving abdominal pain with the number needed to treat (NNT) varying between 4 and 7.37–39 Reported adverse effects are mild and transient, the most common being heartburn and belching due to lower oesophageal sphincter relaxation (Table 2). The product is generally well tolerated, and it is recommended to start with the full dose (1–2 capsules 3 times/day). In patients with poor tolerance, progressive dose titration during the first week of treatment has been reported to improve tolerance.40
Second-line treatment: neuromodulatorsThe use of psychotropic drugs or neuromodulators in patients with abdominal pain due to disorders of gut-brain interaction is not widespread and should be reserved for patients with moderate to severe involvement. The first point to take into account is that the patient should be informed about the reason for their use, their interactions, their utility in altering the brain/gut axis, their side effects, and that the therapeutic response will be delayed.
In patients with chronic abdominal pain, particularly centrally mediated, the most studied and used drugs are TAD followed by serotonin and noradrenaline reuptake inhibitors (SNRI). Both increase norepinephrine levels and have analgesic properties. There is more experience with the use of TAD. The preferred TAD are their secondary forms such as desipramine,41,42 which have fewer side effects than amitriptyline or imipramine. To avoid side effects, it is recommended to start treatment with low doses, 10–25mg, and increase the dose gradually to achieve an adequate therapeutic response and avoid patient frustration.41,42 The onset of the analgesic effect is after 1–8 weeks and the antidepressant effect is later at 8–12 weeks. Trazodone is a heterocyclic antidepressant (HTC) which also has an analgesic effect, with marked sedative and sleep-inducing action, also useful in pain management.
Of the selective amine reuptake inhibitors, selective serotonin reuptake inhibitors (SSRI) and SNRI are important. The SNRI have a dual effect, inhibiting the reuptake of both serotonin and noradrenaline. These drugs are easily dosed and have fewer side effects and are therefore safer. However, their analgesic effect is less than that of TAD. In psychiatry, they are first-line drugs for the treatment of associated psychopathology. Based on the hypothesis that depression and anxiety are correlated with a deficit of monoamines,43 noradrenaline and dopamine, antidepressants would exert their action by increasing the levels of these monoamines. The most commonly used SSRI are citalopram, escitalopram, fluoxetine, sertraline and paroxetine, which improve depression, anxiety and hypervigilance. Their most common gastrointestinal side effect is diarrhoea, so they are most commonly used for the management of abdominal pain in patients whose predominant bowel habit is constipation.44 Fluoxetine has a more stimulant action, so it is useful in patients with depression and pain. It has a longer half-life of up to nine days, so it can be withdrawn without titrating; the other SSRI have a half-life of approximately 20hours. Paroxetine has more anticholinergic effect and causes more sedation and constipation. The usual recommended doses of SSRI are, for citalopram 20–40mg/day, escitalopram 10–20mg/day, sertraline 50–100mg/day, fluoxetine 50–100mg/day and paroxetine 20–40mg/day. Among the SNRI, the recommended dose for venlafaxine ranges from 75 to 300mg/day and for duloxetine from 60 to 120mg/day. To achieve noradrenergic analgesic doses, duloxetine doses of 90–120mg/day and venlafaxine doses of 225–300mg/day are required42 (Table 3).
Posology, adverse effects and contraindications of neuromodulators used in functional abdominal pain.
| Tricyclic antidepressants (TAD) | Serotonin reuptake inhibitors (SRI) | Serotonin and norepinephrine reuptake inhibitors (SNRI) | |
|---|---|---|---|
| Drug | Amitriptyline 10–75mg/dNortriptyline 10–50mg/dClomipramine 10–75mg/d | Paroxetine 20–40mg/dEscitalopram 10–20mg/dSertraline 50–100mg/dFluoxetine 20–40mg/dCitalopram 20–40mg/d | Duloxetine 60–120mg/dVenlafaxine 75–300mg/d |
| Effect | Improvement of pain +++Reduces visceral sensitivityDecreases colon transit | Improvement of pain +Improvement in state of well-beingIncreases colon transit | Improvement of pain +++ |
| Side effects | DrowsinessConstipationWeight gainDry eyes/mouthSexual dysfunction | InsomniaDiarrhoea/sensitivityAgitationWeight lossHeadache | NauseaAgitationDrowsinessConstipationDizzinessImpaired liver function |
| Effect on sleep | +++ | + | ++ |
| Psychiatric comorbidity | Second line at high doses +++ | ++ | +++ |
| Onset of effect | 2–6 weeks | 4–6 weeks | 4–6 weeks |
| Efficacy | Good | Moderate | Moderate |
| Dose readjustment | Yes | No | Yes |
Gabapentinoids are used as anticonvulsants, in psychiatry for their anxiolytic effect and for the management of neuropathic pain or in some conditions such as fibromyalgia. Four studies included in the most recent meta-analysis45 evaluated the effect of gabapentinoids on pain management in IBS, with partially satisfactory results. Lee et al.46 concluded that gabapentin improved rectal hypersensitivity (measured by rectal barostat) in patients with IBS. Studies evaluating pregabalin47,48 showed similar results in the assessment of hypersensitivity that correlated with an improvement in clinical scales. However, Iturrino et al.49 did not identify differences in rectal sensitivity in patients receiving pregabalin.
Atypical antipsychoticsQuetiapine and olanzapine are new antipsychotics with the advantage of not having the extrapyramidal effects typical of classic antipsychotics, as in addition to their dopamine blocking action, they act on various serotonergic receptors that counteract these effects. These drugs can be used in combination with other neuromodulators such as antidepressants, when the effects of antidepressants alone are not sufficient to control the pain. Due to the side effects of these drugs, it is advisable to use them in a multidisciplinary treatment context with the support of psychiatrists familiar with the management of visceral pain and functional gastrointestinal disorders.42 Levosulpiride is an atypical antipsychotic which, in addition to its antiemetic effects, has been shown to reduce visceral sensitivity in patients with functional dyspepsia.50
Drugs with indirect action on painPeripherally acting opioidsIn Spain there are two opioids approved for the management of IBS-D: eluxalodin and loperamide. Eluxadoline is a mixed mu and kappa opioid receptor agonist and delta opioid receptor antagonist. The IBS-300151 study evaluated the use of 75–100mg eluxadoline per day in patients with IBS-D, defining improvement in a composite variable consisting of improvement in pain and stool consistency as the primary endpoint. Results against placebo were favourable for eluxadoline, but there was no difference in pain control in isolation between the two groups. Loperamide is a mu opioid receptor agonist, present in the enteric nervous system, promoting a decrease in peristalsis and intestinal secretion. Hovdenak et al.52 evaluated the effect of loperamide 4mg in patients with IBS; patients with IBS-D or IBS-M had improved stool number and consistency, but no effect on abdominal pain.
LinaclotideLinaclotide is a secretagogue which acts on cyclic GMP to promote intestinal secretion. In addition to this effect, studies in experimental animals have shown a reduction in the transmission of peripheral nociceptive stimuli to the spinal cord, thereby reducing visceral sensitivity.53 Randomised clinical trials have shown that linaclotide reduces abdominal pain in patients with constipation-predominant IBS.54,55 However, in patients without constipation their use is limited as they easily cause diarrhoea.
RifaximinRifaximin is an antibiotic derived from rifampicin, active against Gram-positive and Gram-negative microorganisms and protozoa. Its pharmacokinetics are unique in that it has minimal systemic absorption, allowing its effects to be limited to the gastrointestinal tract.56 Those effects are based on its antibiotic, eubiotic and possibly anti-inflammatory/immunomodulatory actions. Its effects on abdominal pain have been associated with a decrease in intestinal fermentation as a consequence of a reduction in the number of fermenting bacteria. The role of rifaximin in IBS-D-related abdominal pain was evaluated in two phase III, double-blind, placebo-controlled studies, TARGET 1 and TARGET 2, in 2011.57 The use of rifaximin 550mg three times daily/for two weeks improved abdominal pain in 40.8% of patients, compared to 31.7% of patients on placebo (p<0.001). Subsequently, re-treatment of patients who responded to a first course of rifaximin was evaluated, with good results in improving abdominal pain.58
Intestinal mucosal barrier protectorsThis group of drugs creates a protective film (physical barrier) on the intestinal mucosa, preventing contact with food antigens or pro-inflammatory molecules. Gelsectan® is commercially available in Spain; a recent study by Trifan et al.59 demonstrated its superiority over placebo in a double-blind study in patients with IBS-D in the number of bowel movements, abdominal pain, abdominal distension and quality of life. In addition, a Spanish multicentre study in real-life clinical practice showed efficacy and safety of this compound on abdominal pain.60
Other drugsEbastine is a histamine H1-receptor antagonist that has been shown to reduce abdominal pain in patients with IBS.61,62 Other drugs such as ibodutant63 and tenapanor64 have shown good effects on abdominal pain in clinical trials, but these drugs are not currently on the Spanish market. Different trials with cannabinoids have given mixed results on their effect on abdominal pain.17
PsychotherapyBrain-gut behaviour therapy (BGBT) was defined by the Rome Group as a non-pharmacological intervention delivered by a trained practitioner with the aim of improving gastrointestinal symptoms.65 This definition includes cognitive behavioural therapy (CBT), hypnosis and mindfulness-based stress reduction therapies. A recent meta-analysis found that the most effective therapies in reducing symptoms were CBT and hypnosis.66 In five studies involving 278 patients comparing hypnosis with a control group (usually consisting of patients who remained on the waiting list for treatment), it was shown that hypnosis improved gastrointestinal symptoms, including abdominal pain (relative risk [RR] of no improvement 0.74, 95% CI 0.63–0.87).67 Although these treatments have been shown to be effective in reducing gastrointestinal symptoms of IBS, there is little evidence of their isolated effect on abdominal pain. However, although widely validated by the scientific community, poor accessibility to these therapies in our healthcare setting limits the use of this treatment option. The creation of multidisciplinary teams incorporating psychologists with special training in this type of disorder should be a priority in order to alleviate these deficits.
Nerve root blockCoeliac plexus block has been used successfully for the relief of abdominal pain, especially in patients with pancreatic disease.68 This technique is reserved for patients with refractory pain which does not respond to other treatments, and is usually indicated within multidisciplinary teams and specialised pain management units.
Intensification of therapyIn general, most patients present with mild abdominal pain, either in isolation or in the context of another functional gastrointestinal disorder, and treatment may be initiated with first-line measures, and escalated to neuromodulatory drugs when these measures are not sufficient. However, some patients will not respond well to this strategy, especially when they present with more severe, sometimes disabling pain, and in these cases treatment should be intensified.
In general, when a patient presents with moderate/severe pain, we recommend starting treatment with neuromodulators, either as monotherapy or in combination with antispasmodics. In the case of poor response, international guidelines advise in the first instance an increase in dose, and if side effects occur, a switch to another type of neuromodulator.42 For example, if a patient has adverse effects to a TAD, they may be switched to an SNRI. When these measures are not sufficient, combined therapy is advised. Depending on the severity of pain and availability at each centre, psychological intervention with cognitive-behavioural therapy may be started, or gabapentinoids and/or atypical antipsychotics such as quetiapine may be added, with dose increases if the response is not as expected. The ideal in these cases is to combine drugs with different mechanisms of action. Finally, if the response to these measures is unsatisfactory, nerve root injection may be considered.
ConclusionsFunctional abdominal pain is a very common symptom, which can occur either in isolation, such as CAPS, or accompanied by other gastrointestinal symptoms, such as IBS. The first step in the management of these patients is to make a differential diagnosis with organic causes of chronic abdominal pain. In this regard, anamnesis and physical examination are of paramount importance to assess associated symptoms and signs suggestive of other diseases. In some cases it will be necessary to perform additional tests to rule out other causes of chronic abdominal pain.
Once the diagnosis of functional pain has been established, treatment should be personalised for each patient. In cases of disabling pain, a combination therapy with different mechanisms of action can be initiated, while in less severe cases, a stepwise introduction of drugs according to the clinical response is recommended (Fig. 3B). This should firstly be through general lifestyle advice and using antispasmodic substances, such as peppermint oil, anticholinergics/antimuscarinics or calcium channel blockers. If the patient’s pain persists despite this treatment, the next step is to add neuromodulatory agents, such as TAD or SSRI or SNRI. Finally, when all these measures fail, we need to consider third-line treatments, such as gabapentinoids and atypical antipsychotics, as well as psychological interventions such as cognitive behavioural therapy. In the case of very severe pain refractory to all types of treatment, nerve blocks could rescue these patients.
FundingThis document has been prepared by the Asociación Española de Neurogastroenterologia [Spanish Association of Neurogastroenterology] with funding from Tillotts Pharma. Tillotts Pharma has not been involved in or influenced the preparation of the document in any way.
Conflicts of interestJS: Research grants from Bayer and Salvat Laboratories. Consultant/speaker with Menarini, Casen Recordati, Reckit Benkiser and Norgine. CCR: Consultant/speaker with Medtronic. AA, EB, LGP, VM and BSF have no conflict of interest.












![Mechanisms involved in the generation of visceral nociceptive signals in a sensory afferent. The diagram shows the mechanisms generating nociceptive signals under normal conditions, and the mechanisms of peripheral sensitisation. In the intestinal epithelium, sensory mechanisms generating nociceptive signals depend on complex interactions involving multiple levels of signalling, in many cases with two-directional actions and feedback mechanisms. Under normal conditions, the generation of nociceptive signals depends on the activation of different ion channels (for example, Na+ channels, transient receptor potential [TRP] channels, acid-sensing ion channels [ASIC], P2X). The activity of these channels increases in states of sensitisation, and contributes to the changes in excitability that characterise these states. Sensitisation mechanisms involve the activation of membrane receptors (usually G protein-coupled receptors [GPCR], such as receptors for bradykinins, prostaglandins, protease-activated receptors [PAR], histamine, serotonin, nerve growth factor [NGF] neurotrophins, substance P or calcitonin gene-related peptide [CGRP]) by specific ligands. This process depends on neuroimmune interactions involving immune cells (mast cells, lymphocytes, dendritic cells, macrophages and neutrophils), the enteric nervous system (enteric neurons and enteric glia) and the intestinal epithelium (epithelial cells and enteroendocrine cells, mainly responding to chemical and mechanical stimuli). The microbiota has recently been added to this system of interactions as an active component. Microbial derivatives can therefore penetrate the epithelium and act as direct receptor ligands or modify local neuroimmune activity. Under certain circumstances, persistent phenotypic changes can occur in sensory neurons. These changes maintain sensitised states in the long term and can affect synaptic activity in the spinal cord, and may generate sensitised states at a central (spinal cord) level. Together these processes appear to be particularly important in mediating the sensitisation that accompanies inflammatory or infectious states. Source: Modified from: Knowles et al.70 and Martinez.4 Mechanisms involved in the generation of visceral nociceptive signals in a sensory afferent. The diagram shows the mechanisms generating nociceptive signals under normal conditions, and the mechanisms of peripheral sensitisation. In the intestinal epithelium, sensory mechanisms generating nociceptive signals depend on complex interactions involving multiple levels of signalling, in many cases with two-directional actions and feedback mechanisms. Under normal conditions, the generation of nociceptive signals depends on the activation of different ion channels (for example, Na+ channels, transient receptor potential [TRP] channels, acid-sensing ion channels [ASIC], P2X). The activity of these channels increases in states of sensitisation, and contributes to the changes in excitability that characterise these states. Sensitisation mechanisms involve the activation of membrane receptors (usually G protein-coupled receptors [GPCR], such as receptors for bradykinins, prostaglandins, protease-activated receptors [PAR], histamine, serotonin, nerve growth factor [NGF] neurotrophins, substance P or calcitonin gene-related peptide [CGRP]) by specific ligands. This process depends on neuroimmune interactions involving immune cells (mast cells, lymphocytes, dendritic cells, macrophages and neutrophils), the enteric nervous system (enteric neurons and enteric glia) and the intestinal epithelium (epithelial cells and enteroendocrine cells, mainly responding to chemical and mechanical stimuli). The microbiota has recently been added to this system of interactions as an active component. Microbial derivatives can therefore penetrate the epithelium and act as direct receptor ligands or modify local neuroimmune activity. Under certain circumstances, persistent phenotypic changes can occur in sensory neurons. These changes maintain sensitised states in the long term and can affect synaptic activity in the spinal cord, and may generate sensitised states at a central (spinal cord) level. Together these processes appear to be particularly important in mediating the sensitisation that accompanies inflammatory or infectious states. Source: Modified from: Knowles et al.70 and Martinez.4](https://static.elsevier.es/multimedia/24443824/0000004700000008/v1_202410061348/S244438242400186X/v1_202410061348/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

