To describe the epidemiological, analytical and histological characteristics and clinical course of hepatitis B virus (HBV) carriers with negative HBe antigen.
Materials and methodsObservational, retrospective cohort study of HBV carriers with negative HBe antigen (2005–2012), with no other causes of liver disease.
ResultsOne hundred and thirty-eight patients were included, with mean age 40.5±12.2 years; 54% were women, and 38% were of foreign origin; the number of foreign patients significantly increased (p<.001) over the years. Transaminases were normal in nearly 75% and HBV-DNA was <2000IU/mL in 56% of patients at diagnosis. There was a gradual decrease in HBV-DNA levels in inactive carriers over the study period. Fibrosis study was performed in 47% of patients by Fibroscan® or liver biopsy: 55.4% normal histology and 6.1% cirrhosis. Just over three quarters of patients (77.77%) were inactive carriers. Treatment was required in 15.5% of patients (20% because of cirrhosis and 80% HBeAg-negative chronic hepatitis B). Five patients cleared HBsAg (annual rate .94%), all of whom presented HBV-DNA <2000IU/mL at diagnosis. Five patients developed complications (3.6%), 4 of them hepatocellular carcinoma (HCC), of which only 2 had cirrhosis. There was 1 HBV-related death (.72%).
ConclusionAmong HBV carriers with negative HBe antigen, inactive HBs-Ag carriers are predominant. HBV-DNA gradually decreases in the first few years after diagnosis. Morbidity and mortality are low, especially if glutamic pyruvic transaminase (GPT) is normal and HBV-DNA levels are low at diagnosis. Treatment is needed in a considerable number of patients. HCC is the most frequent complication, even in the absence of cirrhosis.
Describir las características epidemiológicas, analíticas, histológicas y evolutivas de pacientes con infección crónica por VHB AgHBe-negativo.
Material y métodosEstudio observacional de cohorte retrospectivo de pacientes diagnosticados de infección crónica VHB AgHBe-negativo (2005-2012) sin otras hepatopatías.
ResultadosSe incluyeron 138 pacientes con edad media de 40,5±12,2 años, de los cuales el 54% eran mujeres. El 38% eran extranjeros, con incremento de estos en los últimos años (p<0,001). Las transaminasas en el momento del diagnóstico eran normales en casi el 75% y el ADN-VHB <2.000UI/ml en el 56%. En los portadores inactivos existe una disminución progresiva de los niveles de ADN-VHB en el periodo de estudio. En el 47% se evaluó la fibrosis hepática por Fibroscan® o biopsia hepática: el 55,4% resultó normal y el 6,1% reportó cirrosis. El 77,77% eran portadores inactivos. Precisaron tratamiento el 15,5% (20% por cirrosis y 80% por HBC AgHBe-negativo). Aclararon el AgHBs 5 pacientes (tasa anual 0,94%), presentando todos al diagnóstico ADN-VHB <2.000UI/ml. Cinco pacientes desarrollaron alguna complicación (3,6%), 4 de ellos carcinoma hepatocelular (CHC) (solo 2 presentaban cirrosis). Hubo un fallecimiento relacionado con el VHB (0,72%).
ConclusiónEntre los enfermos con infección crónica por VHB AgHBe-negativo predominan los portadores inactivos. Se produce un progresivo descenso de ADN-VHB en los primeros años tras el diagnóstico. Desarrollan poca morbimortalidad, especialmente si existe GPT normal y ADN-VHB bajo al diagnóstico. Un número no despreciable de pacientes precisa tratamiento. El CHC es la complicación más frecuente, incluso en pacientes sin cirrosis.
Chronic hepatitis B virus (HBV) infection is a global public health problem. An estimated 350–400million people worldwide have chronic HBV infection,1 and around 620,000 people die annually from HBV-related causes.2
Chronic HBV infection can present as hepatitis B e antigen (HBeAg) positive (+) or negative (−). HBeAg(−) chronic infection occurs after HBeAg seroconversion. In 75% of cases, the patient seroconverts from an HBeAg(+) chronic hepatitis state to an inactive HBV carrier (IC) state, characterised by persistently normal transaminases and low HBV deoxyribonucleic acid (DNA) (generally <2000IU/mL), with little histological damage. In the remaining 25%, HBeAg seroconversion gives rise to HBeAg(−) chronic hepatitis, either directly, mostly as a result of mutations that occur in the precore and/or core promoter region, or secondary to reactivation from the IC state, in which there are periods of reactivation with a pattern of fluctuating HBV DNA (HBV-DNA) and aminotransferase levels, and active hepatitis.3
The HBeAg(−) chronic infection phase was first described in Mediterranean countries,4 but has now been reported worldwide.5 Studies from Europe, Asia and the United States describe an increase in the prevalence of these types of patients,6 mainly due to ageing of the infected population, and it is the most common form of chronic hepatitis B not only in Europe, but worldwide,7 currently affecting 85–90% of patients. In our published series of patients with chronic HBV infection,8 those with HBeAg(−) chronic infection represented the largest group (87.61%), similar to other studies recently published in Spain,9,10 other Mediterranean countries,11 and more remote regions, such as Asia12 and South America.13 Since HBeAg(−) patients are the largest HBV infection group, we set out to study the characteristics and clinical course of this patient group in our healthcare area.
Materials and methodsObservational, retrospective cohort study of patients diagnosed with HBeAg(−) chronic HBV infection at the time of diagnosis in our healthcare area from June 2005 to July 2011, with follow-up through December 2012.
All patients over the age of 13 years, HBsAg(+) for more than 6 months, with HBeAg(−) and Anti-HBeAg(+) were included. Exclusion criteria were: Patients coinfected with human immunodeficiency virus (HIV), hepatitis C virus (HCV) and/or hepatitis D virus (HDV); presence of advanced renal failure (creatinine clearance <30mL/min and/or dialysis) (since they are monitored by the nephrology department), other associated liver diseases (study of thyroid hormones, autoimmunity, iron profile [haemochromatosis gene if transferrin saturation was greater than 45% in 2 tests], urinary porphyrins, alpha-1 antitrypsin, blood copper, urinary copper and ceruloplasmin; also excluded were patients with obesity (body mass index [BMI]≥35kg/m2), steatohepatitis findings on liver biopsy, no definitive diagnosis of cirrhosis-induced chronic HBV infection and alcohol consumption ≥40g/day, as well as patients who had received previous treatment for HBV infection.
Sociodemographic characteristics, epidemiological and clinical data at diagnosis and the reason chronic HBV infection was diagnosed were recorded. During the course of the study, analytical and serological variables, HBV-DNA measured by polymerase chain reaction (PCR), as well as hospital admissions and their reasons, were recorded every 6±2 months. Cirrhotic patients had ultrasound follow-up every 6 months and non-cirrhotic patients every 1 or 2 years.
A liver fibrosis study was performed by liver biopsy (according to the METAVIR classification14), as per the indications of the current clinical guidelines. The following categories were established for this study: normal or minimal changes (A0-F0), different grades of fibrosis, and cirrhosis (F4). If there was no liver biopsy, but FibroScan® had been carried out, fibrosis was based the results of the scan, using the system presented by Viganò et al.15 as a reference, where: F0-1 (from 2.5 to 6.2kPa), normal; F2-3 (from 6.2 to 13.1kPa), different grades of inflammation-fibrosis; and F4 (>13.1kPa), cirrhosis; which correspond to the fibrosis values in the METAVIR system used for the liver biopsies. Patients diagnosed with liver cirrhosis using clinical-analytical data or imaging tests, in whom liver biopsy or FibroScan® were not required to reach the diagnosis, were also categorised as F4 for the purposes of the study.
Clinical follow-up of patients continued until the last check-up at the hepatology clinic. The analytical study included all tests up to the last follow-up laboratory test with HBV serology ordered by the hepatology unit. Only patients with 3 or more tests during follow-up were included in the analysis. In patients who required medical treatment for chronic HBV infection, the clinical follow-up was evaluated until the date of the visit at which treatment was started, and the analytical follow-up until the last test prior to this.
ICs were defined as all patients with persistently normal alanine transaminase (ALT) (<40mU/mL, and low HBV-DNA, discriminating among those with persistent HBV-DNA <2000IU/mL (pure ICs), and those with occasional HBV-DNA between 2000 and 20,000IU/mL. Indeterminate state was defined as a patient presenting persistently normal or fluctuating ALT with slight elevations (generally <2 upper limit of normal [ULN]) and occasionally peak HBV-DNA >20,000IU/mL. Reactivation of an IC to HBeAg(−) chronic hepatitis B (CHB) was defined as any patient in whom the first 3 ALT results were normal (<40mU/mL) and HBV-DNA was low (<20,000IU/mL) but subsequently presented elevated ALT values above 40mU/mL together with HBV-DNA >20,000IU/mL. HBeAg(−) CHB phase at diagnosis was defined as any patient who, from the first laboratory tests, persistently presented ALT >40mU/mL and HBV-DNA >20,000IU/mL.
Data from patients who presented cirrhosis during the clinical course, together with associated complications (hepatic encephalopathy [HE]), variceal gastrointestinal bleeding (VGIB), oedematous ascitic decompensation (OAD) or hepatocellular carcinoma (HCC) were recorded. HCC was diagnosed as space-occupying lesions >1cm in patients with liver cirrhosis, where at least one imaging test (tomography and/or contrast magnetic resonance imaging) showed a typical HCC pattern (enhancement in the arterial phase and washout in the portal or late phase). A histological study was performed in patients with atypical or suspicious radiological patterns, and in those with no liver cirrhosis. Adverse events during the study were defined as: presentation of cirrhosis, any of its complications, HCC in a non-cirrhotic liver, and/or need for treatment during the clinical course.
The study was approved by the hospital ethics committee.
Statistical analysisStatistical analysis was performed using SPSS software, v. 20.0 (IBM Corporation, 2011). Categorical variables are described as using the count (percentage). Quantitative variables are described using the mean±standard deviation if distribution is normal, and using the median (interquartile range [IQR]) if this hypothesis is not met.
The distribution between dichotomous and categorical variables was compared using contingency tables with the Chi-square or Fisher's test, as applicable. Comparison of means between qualitative variables was performed using Student's t-test when 2 means were compared, or one-factor analysis of variance (ANOVA) when more than 2 means were compared, if normality criteria were met. If the variable of interest did not meet the assumption of normality, we used the non-parametric Mann–Whitney U test or the Kruskal–Wallis H test to compare the equality of the distribution of the quantitative variable in 2 groups, or in more than 2 groups, respectively.
Patient evolution was studied using tests for dependent samples, such as the Student's t-test for related variables or ANOVA for repeated measures, if the variable had a normal distribution, or the Wilcoxon or Friedman test if not. A p value<0.05 was considered significant.
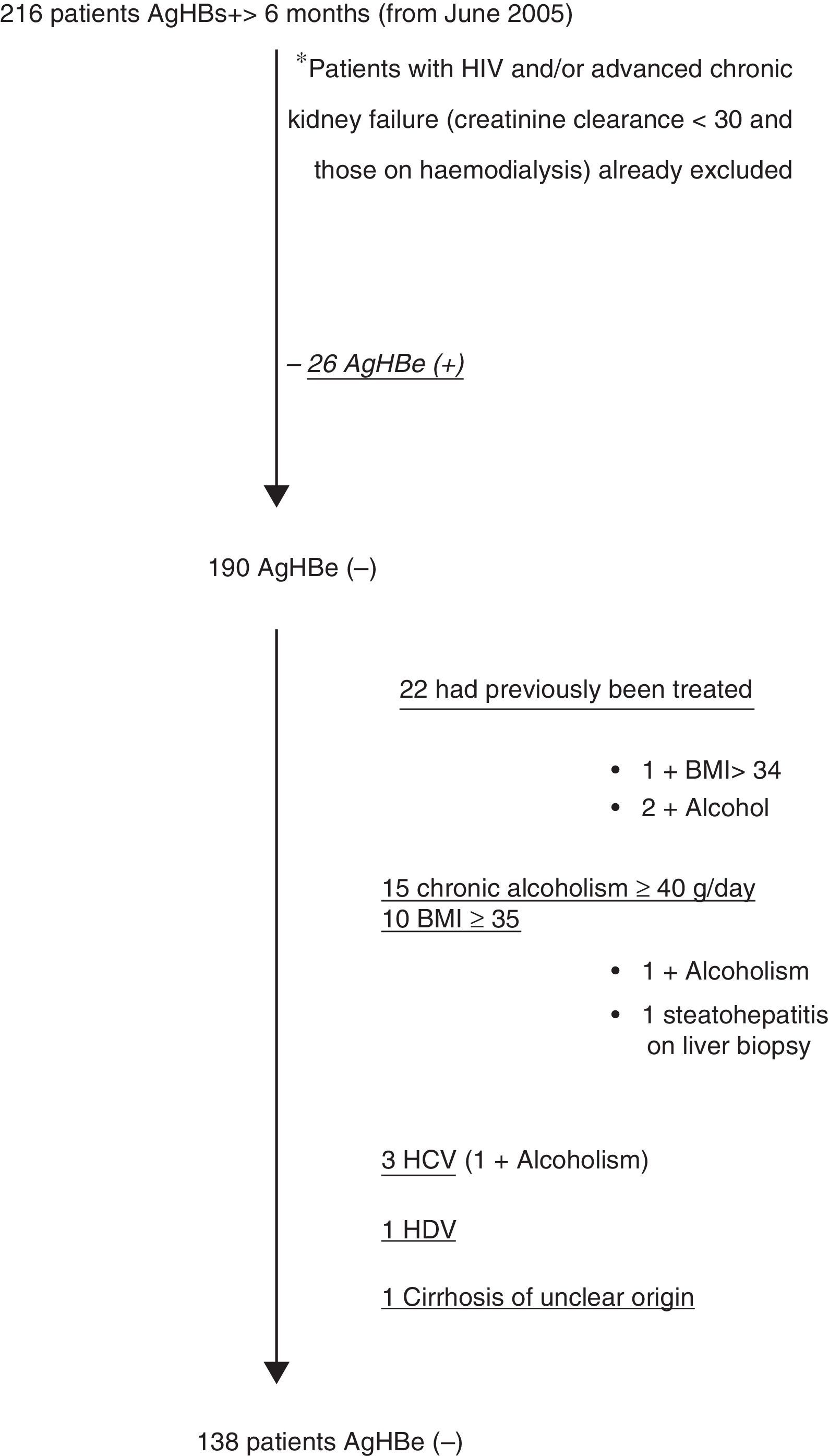
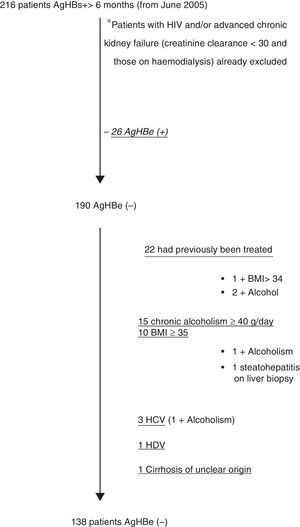
ResultsFig. 1 shows the selection flow chart for HBsAg(+) patients during the study period who met all the inclusion and none of the exclusion criteria; 138 patients were included, 126 of whom had analytical follow-up.
The mean age at diagnosis was 40.5±12.2 years (56.5% of patients diagnosed before age 40). Fifty-four percent were women and 38% were of foreign origin. Diagnosis in Spanish natives and immigrants varied over time, with immigrant patients having increased significantly in recent years (p<0.001). Immigrants were predominantly from Eastern Europe (46%), mainly Romania (87.5% of those from this area), followed by Africa (31%), mainly Morocco (43% of Africans), Asia (10%), Central America (9%) and the Middle East (4%).
In terms of epidemiology, 34 patients had a family history of HBV infection (24.6%), none were intravenous drug users or former users, 5.8% had previous transfusions, 5.1% had tattoos, and 1.45% had piercings.
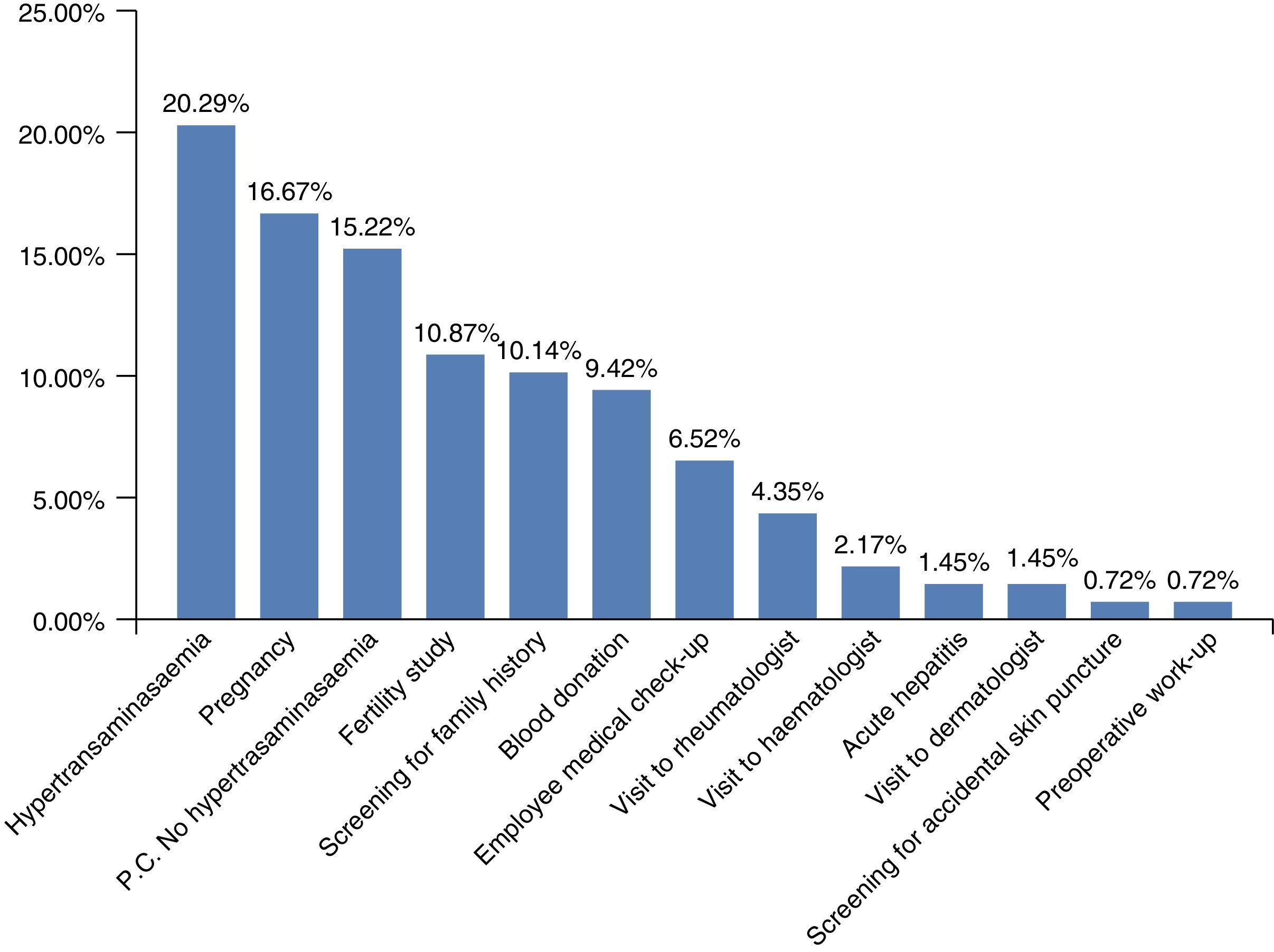
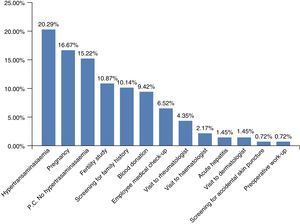
Chronic HBV infection had been diagnosed for various reasons (Fig. 2). The most common was hypertransaminasaemia (20.29%); however, grouping all the reasons for HBV screening together (pregnancy, fertility, family history, blood donors, or at the various clinics prior to immunosuppressant treatments) showed that hypertransaminasaemia was the reason for diagnosis in 55.07% of patients.
Twelve of the 138 patients (8.69%) presented signs or symptoms at diagnosis. These were clearly related with chronic HBV infection in only 1 patient (0.72%), as he presented OAD at diagnosis. Some patients presented symptoms that could be disease-related (3 patients with asthenia, 2 with arthralgia-arthritis and 1 with haematuria), while others presented more non-specific or unrelated symptoms (2 with dyspepsia, 1 with erythema nodosum, 1 with uveitis, and another with non-specific abdominal pain). On examination, only 1 patient presented stigmata of chronic liver disease at diagnosis (the same patient who had been diagnosed with OAD).
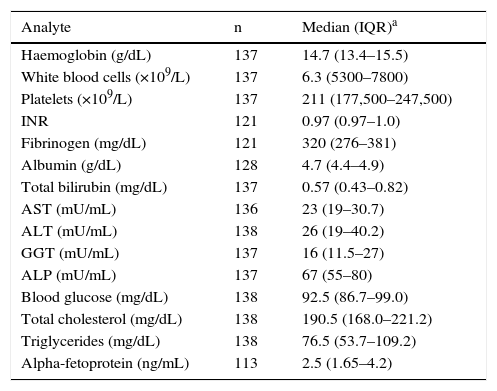
Table 1 shows the biochemistry results at the time of diagnosis. ALT at diagnosis was normal in 103 patients (74.6%). Of these, 95 (91.26%) presented HBV-DNA <20,000IU/mL and 67 (64.08%) <2000IU/mL at diagnosis. Patients with normal ALT at diagnosis vs abnormal ALT values had a statistically significant lower viral load (916 [IQR: 110–3830]IU/mL vs 6840 [IQR: 479–38,000]IU/mL; p=0.003). The mean quantitative viral load at diagnosis was 43,726±238,701IU/mL, with a median of 6230IU/mL. We defined 4 groups based on HBV-DNA at diagnosis: undetectable (14%); 0–2000IU/mL (42%); 2000–20,000IU/mL (27%), and >20,000IU/mL (17%); with patients with HBV-DNA <2000IU/mL accounting for 56%.
General laboratory results at diagnosis.
| Analyte | n | Median (IQR)a |
|---|---|---|
| Haemoglobin (g/dL) | 137 | 14.7 (13.4–15.5) |
| White blood cells (×109/L) | 137 | 6.3 (5300–7800) |
| Platelets (×109/L) | 137 | 211 (177,500–247,500) |
| INR | 121 | 0.97 (0.97–1.0) |
| Fibrinogen (mg/dL) | 121 | 320 (276–381) |
| Albumin (g/dL) | 128 | 4.7 (4.4–4.9) |
| Total bilirubin (mg/dL) | 137 | 0.57 (0.43–0.82) |
| AST (mU/mL) | 136 | 23 (19–30.7) |
| ALT (mU/mL) | 138 | 26 (19–40.2) |
| GGT (mU/mL) | 137 | 16 (11.5–27) |
| ALP (mU/mL) | 137 | 67 (55–80) |
| Blood glucose (mg/dL) | 138 | 92.5 (86.7–99.0) |
| Total cholesterol (mg/dL) | 138 | 190.5 (168.0–221.2) |
| Triglycerides (mg/dL) | 138 | 76.5 (53.7–109.2) |
| Alpha-fetoprotein (ng/mL) | 113 | 2.5 (1.65–4.2) |
Information on hepatic fibrosis was obtained during follow-up by histology in 36 cases and by FibroScan® in 28. No fibrosis was detected in 36 patients (55.4%), 25 presented different grades of inflammation-fibrosis (38.5%), and 4 presented cirrhosis (6.1%), one of whom was diagnosed by clinical-analytical and ultrasound findings at diagnosis, having commenced the study with OAD. The 4 patients with cirrhosis (3.1%) were men (p=0.024), with mean age greater than that of patients without cirrhosis (61.7±8.6 years vs 39.8±11.7 years; p=0.003), and classified as Child–Turcotte–Pugh stage A at diagnosis.
Analytical follow-up was performed in 126 patients, with a mean follow-up time of 33.6±22.1 months, and an average of 6.5 laboratory tests per patient. Mean clinical follow-up time was 42.14±21.50 months. Median peak ALT was 32 (IQR: 23.5–65)mU/mL, and the median time to reach this peak was 10.4 (IQR: 4.4–25) months. The maximum median viral load value was 3620 (IQR: 531–13,900)IU/mL, and the minimum 216 (IQR: 30–1095)IU/mL Time to reach the HBV-DNA peak was a median of 11.2 (IQR: 3.5–23.6) months. Of the 108 patients who presented a detectable viral load at diagnosis, this remained detectable in 81 (75%), became undetectable in 18 (16.67%) and fluctuated between detectable and undetectable in 9 (8.33%). Of the 18 patients with an undetectable viral load, the viral load remained undetectable in 11 (61.11%%), became detectable in 6 (33.33%) and fluctuated in 1 (5.56%).
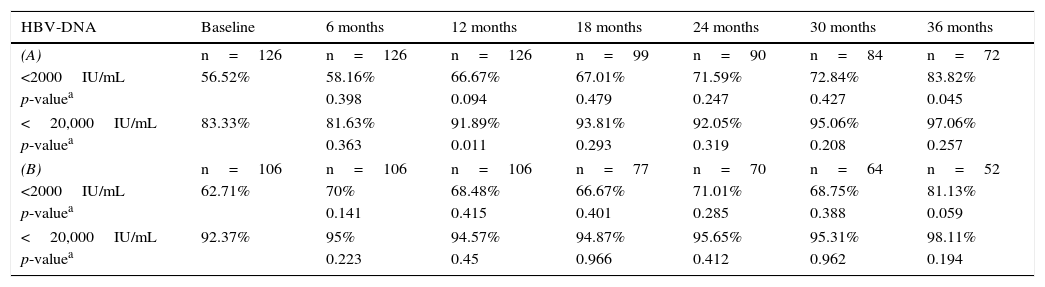
If we analyse the trend in the percentage of patients with HBV-DNA <2000IU/mL and <20,000IU/mL at each point in the study, we can see in Table 2A how the percentage of patients with lower HBV-DNA tends to increase over the study follow-up, although significant differences were only observed in 2 of the study periods. Excluding patients who started treatment from the study may cause bias, as these generally had a higher viral load, although as can be observed in Table 2B, this trend continues, but with no significant differences in any period.
Percentage of patients with HBV-DNA <2000IU/mL and <20,000IU/mL at each point in the study. (A) In the general series, and (B) excluding patients who were treated.
| HBV-DNA | Baseline | 6 months | 12 months | 18 months | 24 months | 30 months | 36 months |
|---|---|---|---|---|---|---|---|
| (A) | n=126 | n=126 | n=126 | n=99 | n=90 | n=84 | n=72 |
| <2000IU/mL | 56.52% | 58.16% | 66.67% | 67.01% | 71.59% | 72.84% | 83.82% |
| p-valuea | 0.398 | 0.094 | 0.479 | 0.247 | 0.427 | 0.045 | |
| <20,000IU/mL | 83.33% | 81.63% | 91.89% | 93.81% | 92.05% | 95.06% | 97.06% |
| p-valuea | 0.363 | 0.011 | 0.293 | 0.319 | 0.208 | 0.257 | |
| (B) | n=106 | n=106 | n=106 | n=77 | n=70 | n=64 | n=52 |
| <2000IU/mL | 62.71% | 70% | 68.48% | 66.67% | 71.01% | 68.75% | 81.13% |
| p-valuea | 0.141 | 0.415 | 0.401 | 0.285 | 0.388 | 0.059 | |
| <20,000IU/mL | 92.37% | 95% | 94.57% | 94.87% | 95.65% | 95.31% | 98.11% |
| p-valuea | 0.223 | 0.45 | 0.966 | 0.412 | 0.962 | 0.194 | |
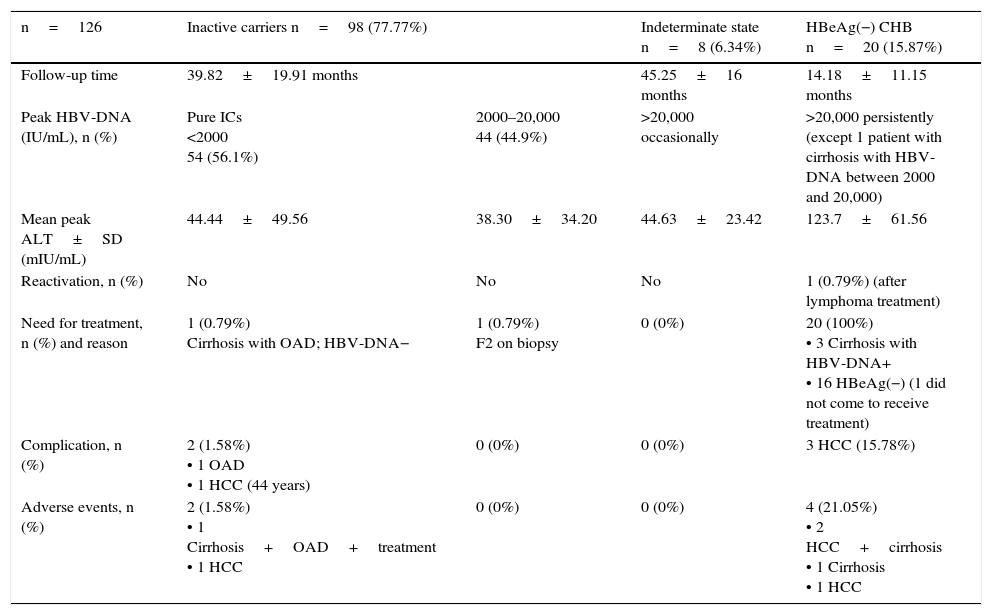
Just over three quarters of patients (77.77%) were in an IC state (56.1% with persistent HBV-DNA <2000IU/mL and 44.9% with occasional HBV-DNA between 2000 and 20,000IU/mL). Of the patients in this group, 27.5% presented elevated ALT <2ULN at diagnosis or occasionally during follow-up, associated with steatohepatitis and/or sporadic alcohol consumption, not related to the disease activity.
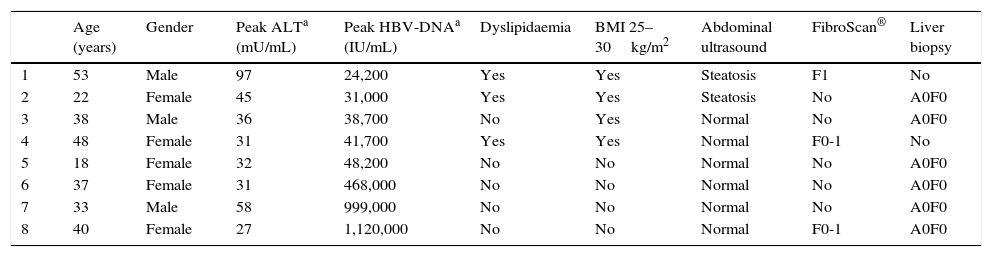
There were 8 patients (6.34%) in an indeterminate state (ALT normal or slightly fluctuating, with occasional HBV-DNA peak >20,000IU/mL). Of these, 5 presented persistently normal ALT, and 3 presented slight fluctuations in ALT, with peak ALT during the clinical course no higher than 97mU/mL (mean ALT of 64.25mU/mL), with no findings of liver disease in the ultrasound imaging; the highest ALT peak (97mU/mL) was observed in a patient with steatosis on the ultrasound and dyslipidaemia. These 8 patients underwent a liver fibrosis study, by FibroScan® and/or liver biopsy, all with normal results.
A total of 15.87% of patients were in an HBeAg(−) CHB state since diagnosis. No reactivations from IC to HBeAg(−) CHB were observed, although we received 1 patient who had been referred for treatment due to reactivation secondary to lymphoma treatment. Table 3 shows the evolution of the patients, as well as complications; Table 4 presents the characteristics of patients in an indeterminate state, and Table 5 compares ICs and patients in an indeterminate state.
Evolution of patients with 3 or more ALT tests in follow-up.
| n=126 | Inactive carriers n=98 (77.77%) | Indeterminate state n=8 (6.34%) | HBeAg(−) CHB n=20 (15.87%) | |
|---|---|---|---|---|
| Follow-up time | 39.82±19.91 months | 45.25±16 months | 14.18±11.15 months | |
| Peak HBV-DNA (IU/mL), n (%) | Pure ICs <2000 54 (56.1%) | 2000–20,000 44 (44.9%) | >20,000 occasionally | >20,000 persistently (except 1 patient with cirrhosis with HBV-DNA between 2000 and 20,000) |
| Mean peak ALT±SD (mIU/mL) | 44.44±49.56 | 38.30±34.20 | 44.63±23.42 | 123.7±61.56 |
| Reactivation, n (%) | No | No | No | 1 (0.79%) (after lymphoma treatment) |
| Need for treatment, n (%) and reason | 1 (0.79%) Cirrhosis with OAD; HBV-DNA− | 1 (0.79%) F2 on biopsy | 0 (0%) | 20 (100%) • 3 Cirrhosis with HBV-DNA+ • 16 HBeAg(−) (1 did not come to receive treatment) |
| Complication, n (%) | 2 (1.58%) • 1 OAD • 1 HCC (44 years) | 0 (0%) | 0 (0%) | 3 HCC (15.78%) |
| Adverse events, n (%) | 2 (1.58%) • 1 Cirrhosis+OAD+treatment • 1 HCC | 0 (0%) | 0 (0%) | 4 (21.05%) • 2 HCC+cirrhosis • 1 Cirrhosis • 1 HCC |
HCC, hepatocellular carcinoma; OAD, oedematous ascitic decompensation; IC, inactive carrier.
Patients in indeterminate state in follow-up.
| Age (years) | Gender | Peak ALTa (mU/mL) | Peak HBV-DNAa (IU/mL) | Dyslipidaemia | BMI 25–30kg/m2 | Abdominal ultrasound | FibroScan® | Liver biopsy | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | Male | 97 | 24,200 | Yes | Yes | Steatosis | F1 | No |
| 2 | 22 | Female | 45 | 31,000 | Yes | Yes | Steatosis | No | A0F0 |
| 3 | 38 | Male | 36 | 38,700 | No | Yes | Normal | No | A0F0 |
| 4 | 48 | Female | 31 | 41,700 | Yes | Yes | Normal | F0-1 | No |
| 5 | 18 | Female | 32 | 48,200 | No | No | Normal | No | A0F0 |
| 6 | 37 | Female | 31 | 468,000 | No | No | Normal | No | A0F0 |
| 7 | 33 | Male | 58 | 999,000 | No | No | Normal | No | A0F0 |
| 8 | 40 | Female | 27 | 1,120,000 | No | No | Normal | F0-1 | A0F0 |
ALT, alanine transaminase; BMI, body mass index.
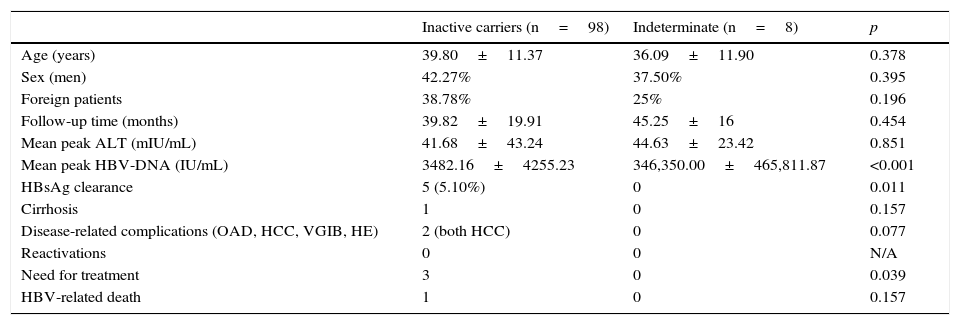
Comparison of inactive carriers vs patients in indeterminate state.
| Inactive carriers (n=98) | Indeterminate (n=8) | p | |
|---|---|---|---|
| Age (years) | 39.80±11.37 | 36.09±11.90 | 0.378 |
| Sex (men) | 42.27% | 37.50% | 0.395 |
| Foreign patients | 38.78% | 25% | 0.196 |
| Follow-up time (months) | 39.82±19.91 | 45.25±16 | 0.454 |
| Mean peak ALT (mIU/mL) | 41.68±43.24 | 44.63±23.42 | 0.851 |
| Mean peak HBV-DNA (IU/mL) | 3482.16±4255.23 | 346,350.00±465,811.87 | <0.001 |
| HBsAg clearance | 5 (5.10%) | 0 | 0.011 |
| Cirrhosis | 1 | 0 | 0.157 |
| Disease-related complications (OAD, HCC, VGIB, HE) | 2 (both HCC) | 0 | 0.077 |
| Reactivations | 0 | 0 | N/A |
| Need for treatment | 3 | 0 | 0.039 |
| HBV-related death | 1 | 0 | 0.157 |
ALT, alanine transaminase; HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma; HE, hepatic encephalopathy; NA, not analysable; OAD, oedematous ascitic decompensation; VGIB, variceal gastrointestinal bleeding.
Twenty patients received pharmacological treatment, according to the indications of the current clinical guidelines,16,17 accounting for 15.5% of the series. Treatment indications were: 4 for liver cirrhosis (20% of those treated) – 3 on detection of viral load and 1 who started the study with OAD –; 1 on presentation of an HBV-DNA peak >2000IU/mL and F2 on liver biopsy; and 16 patients for presenting an HBeAg(−) CHB phase (80% of those treated), one of whom did not start treatment as he abandoned follow-up. Of the 16 patients in an HBeAg(−) CHB state, 13 had presented abnormal ALT levels since diagnosis, and 3 during the course of the study.
Five patients lost HBsAg (4%). Median time to HBsAg clearance was 26.47 (IQR: 8.64–43.94) months. Mean annual rate of HBsAg loss was 0.94%, and the cumulative rate at 3 years was 2.83%. Mean age of patients with HBsAg loss was 54.7±7.1years, while the mean age of those with no HBsAg loss was 38.7±10.9years (p=0.004). None of the patients with HBsAg loss presented complications over their clinical course; 3 of the 5 patients with HBsAg loss presented seroconversion to Anti-HBs (60% of those who cleared HBsAg). Median time from HBsAg loss to seroconversion was 10.5 (IQR: 0–22.7) months. Eighty percent of patients who cleared HBsAg presented ALT levels <40mU/mL and HBV-DNA <2000IU/mL (80% HBV-DNA undetectable) at diagnosis. Table 6 shows the comparative study of the ALT and HBV-DNA (baseline and peak) between patients with and without HBsAg loss.
Comparison of ALT and HBV-DNA between patients with and without HBsAg loss.
| n=126 | HBsAg clearance n=5 (3.97%) | No HBsAg clearance n=121 (96.03%) | p |
|---|---|---|---|
| ALT at diagnosis (mU/mL) | 25.80±17.81 | 31.97±28.72 | 0.421 |
| Peak ALT (mU/mL) | 34.00±22.53 | 43.74±44.94 | 0.566 |
| HBV-DNA at diagnosis (IU/mL) | 21.60±48.30 | 5214.70±16,074 | 0.002 |
| Peak HBV-DNA (IU/mL) | 1089.60±1869.56 | 31,100.22±154,440.70 | 0.053 |
ALT, alanine transaminase; HBsAg, hepatitis B surface antigen.
Five patients developed complications (3.6%). One had OAD and 4 HCC. Mean time to the complication was 3.5 years; 4 patients developed HCC (2.9%), 2 of whom presented cirrhosis and 2 did not (50% of those who developed HCC). All were Spanish and over 40 years of age (mean age 61.75±11.9), and 3 of the 4 were men. In two of these patients, cirrhosis was not identified by symptoms, laboratory tests, ultrasound or liver biopsy (both A1F0). The annual rate of development of HCC in patients with cirrhosis was 17.85%. Patients with complications were significantly older than those with no complications: 62.5±10.3 years vs 39.63±11.5 years (p=0.001).
During follow-up, 5 patients were admitted for causes related to the HBV infection (3.87%), 80% in relation to the HCC diagnosis, treatment or surgery, and 20% for OAD.
Two patients died (1.4%), one due to pulmonary squamous cell carcinoma and another to cholangiocarcinoma; the latter is the only death that might be related to the HBV infection (0.72%). The patient was a Romanian man who had been diagnosed at age 42 as a result of a study for dissociated cholestasis. At diagnosis he had ALT <40mU/mL and undetectable HBV-DNA; the HBV-DNA remained undetectable during follow-up, with no HBsAg loss. Gastroscopy did not show portal hypertension, abdominal ultrasound found no signs of chronic liver disease, and the patient presented no clinical, analytical or supplementary tests suggestive of cirrhosis.
DiscussionFew recent studies have focussed on chronic HBV infection in Spain, and even fewer focused only on HBeAg(−) patients, although these make up the largest group in our clinics. Patients with HBeAg(−) CHB in older studies were between 40 and 55 years of age,18,19 older than patients with HBeAg(+) CHB.20 In a more recent French series, the mean age of HBeAg(−) patients was 46.4±14.4 years, although a sub-analysis of naive patients in the same study found that the mean age was 40.9±14.8 years,7 which is consistent with our findings, and with other studies similar to ours.9
Older series noted a predominance of men (male/female ratio of 4.6/1.7),20 and this continues to be the case in recent series (75–88%).21,22 Although in recent studies with well-selected, treatment-naive patients the male/female ratio remains predominantly male (2.3/1.5),7,13,23 the difference is smaller, and some even show a slight predominance of women.24 Our findings show a predominance of women (54%). This could be due to the strict selection criteria used to avoid bias in the natural course of the disease, such as excluding patients with alcohol consumption—which is usually more common in men—or to the high percentage of patients diagnosed during antenatal screening (16.6%).
Immigration has significantly affected the epidemiology of this infection. The last 2–3 decades have seen an increase in immigration from regions with high or intermediate prevalence of chronic HBV infection to industrialised countries,7,25 including Spain.8,9 In our series, the immigrant population accounts for 38% on average, with the ratio of immigrants to Spanish nationals increasing in the last few years of the study, a finding comparable to that described in a recent study conducted in Greece over a similar period.26 Hence, screening is important in this population group, especially in pregnant women. Thus, in our study, 16% of patients were diagnosed during pregnancy, but there was a significant difference between Spanish and immigrant women (22.2% vs 44.8%; p=0.04).
Twenty-four percent of patients in our series had a family history of HBV infection; other studies have reported percentages ranging from 13 to 63%.23,27 A recent Brazilian study13 described possible vertical or intrafamilial transmission in 43% of patients. None of our patients had been infected by vertical transmission, no doubt because this is rarely seen in countries with developed healthcare systems, where antenatal screening programmes (16% of patients in our series were identified for this reason, accounting for 38% of women), vaccination, and primary prophylaxis in childbirth and the post-partum period are well-established. Vertical transmission is highly unlikely in this setting. Ten percent of patients in our series were diagnosed by screening prompted by family history, possibly because this testing is not done in the large foreign population. Saliva has been suggested as a possible mechanism of HBV transmission,28 and could explain the percentage of patients with no obvious risk factors for acquiring the infection.
At present, based on the accumulated evidence,29 serological screening for hepatitis B has been established in patients scheduled for immunosuppressant treatments or chemotherapy. Thus, up to 8% of patients in our series (Fig. 2) were diagnosed with chronic HBV infection as a result of screening performed in rheumatology, haematology and dermatology clinics. Nevertheless, we must continue to raise awareness of the need for screening, since reactivations continue to occur in this patient group. An example of this is the reactivation to HBeAg(−) CHB presented by 1 patient treated for lymphoma in our series.
Most patients with HBeAg(−) chronic HBV infection in our series were asymptomatic when they came to the clinic, and of the patients with symptoms (fewer than 9%), these were clearly related to the underlying liver disease in only one patient, who already had OAD. The other symptoms were non-specific. During follow-up, only one patient death was HBV-related, and 5 patients required admission for infection-related causes (3.87%); 80% were admitted in relation to HCC diagnosis, treatment and surgery. Thus, the quality of life of patients with HBeAg(−) chronic HBV infection can be considered to be good or acceptable in most cases.
Since chronic HBV infection is usually a silent disease, the absence of symptoms is not sufficient to distinguish patients with significant progressive liver disease from ICs.30 Therefore, a patient with HBsAg(+), HBeAg(−), Anti-HBe(+), normal ALT and undetectable HBV-DNA in a single test should not be classified as an IC without appropriate follow-up. In our study, we observed how the median peak ALT value was not obtained until 10.4 months, and the peak HBV-DNA until 11.2 months. Studies have reported that the risk of normal ALT at diagnosis changing during follow-up is greater during the first year (15–20%),31,32 and decreases after 3 years of follow-up,32–34 which is consistent with our findings. In the early years of follow-up, therefore, ALT monitoring is indicated in order to establish the disease state in these patients.30
In line with other studies,35,36 HBV-DNA levels in HBeAg(−) patients decreased in the first year of follow-up due to the transformation of most of these types of patients to ICs, where the viral load becomes low or undetectable. Thus, in Table 2, we can see how the percentage of patients with low viral load gradually increases over time, suggesting a decrease in the viral load in ICs.
HBsAg clearance is a rare event in the clinical course of patients with chronic HBV infection; thus, it has an estimated incidence of approximately 0.1–0.8% in patients who acquire the infection in childhood, and 0.4–2% in those who acquire it in adolescence or adulthood,37–40 as could be the case in our population. More recent studies with 25–30-year follow-up estimate an annual HBsAg clearance rate of between 1%41 and 1.8%,42 similar to our findings (0.94%). Of these patients, approximately half develop Anti-HBs, sometimes several months later.43 Eighty percent of patients who cleared HBsAg presented ALT <40mU/mL and viral load <2000IU/mL at diagnosis in all cases, being undetectable in 80% of them. This is consistent with the literature,44 which suggests that patients who clear HBsAg present low or persistently undetectable HBV-DNA years before seroconversion. Although these patients did not present complications over the course of this study, it should be taken into account that patients aged over 50 who clear HBsAg, as in our case, have an increased risk of developing HCC.43 Therefore, even though at present the accumulated evidence is insufficient to recommend including them in an HCC screening programme, it would be prudent to schedule them for annual or bi-annual follow-up. In fact, some studies suggest that the risk of HCC is not reduced in patients who clear HBsAg compared to those who do not,45,46 and that clearing HBsAg does not necessarily imply good prognosis.
The annual rate of progression to cirrhosis in patients with chronic HBV infection is between 1% and 7%,41,47 and higher (closer to 7%) in HBeAg(−) patients. The percentage of cirrhotic patients in our series was 6%, with an annual rate of 1.03% (data not shown, as the study was not conducted specifically with this aim); these low rates have been reported in other studies.47 This low percentage could be explained by the large number of patients in the IC state. Age is a factor that is clearly related to the development of cirrhosis48,49; thus, in our study, the mean age of patients with cirrhosis was clearly greater than those without. Most studies indicate that the possibility of significant histological damage should be considered in patients over the age of 40.50 Some studies in developing countries suggest younger ages (around 30 years old51), taking into account that these are countries where the infection is mainly transmitted in pregnancy, childbirth and infancy. Thus, the disease has a longer course, which could explain the greater histological damage at an early stage. Another factor clearly related with the development of cirrhosis is being male.49 This was corroborated in our study, where all patients with cirrhosis were men.
There is a clear association between HCC and liver disease due to HBV,52 but up to 30–50% of HBV-associated HCC cases occur in the absence of cirrhosis; in fact, only 50% of our patients who developed HCC presented cirrhosis. In our study, 75% of patients who presented HCC were men, which is consistent with other studies.53
The mortality described in these types of patients varies from a low rate (1%),41 to other studies where higher mortality rates are found (15%).54 We only had 2 deaths; both patients were men over 40 years old at diagnosis. One of the 2 deaths was probably related to HBV infection (0.72% of the series), as the patient developed cholangiocarcinoma.55
In our study, as in others in the literature, the largest patient group with HBeAg(−) CHB were the ICs. The latest European guidelines refer to IC in patients with persistently normal ALT and HBV-DNA <2000IU/mL (pure ICs).17 However, as explained, this infection is dynamic, and some patients with normal ALT present HBV-DNA peaks between 2000 and 20,000IU/mL over the course of the disease (almost 45% of the ICs in our series). This patient group did not present greater histological damage or more complications during the study follow-up than the pure ICs (Table 3). Furthermore, 6% of patients presented an indeterminate state, with HBV-DNA peaks sometimes higher than 20,000IU/mL, but with no significant histological damage or greater incidence of complications compared to ICs (Tables 3–5). Thus, we propose the term semi-inactive carrier for this group of indeterminate state patients. This group requires closer follow-up until their prognosis can be determined, but it is unclear whether their clinical course is poorer than that of ICs.
Twenty patients (15.5%) were treated during follow-up, 80% in the HBeAg(−) CHB state. Of these, 81.25% had abnormal ALT at diagnosis, so in the vast majority of cases, patients who required treatment had abnormal ALT levels since the start of follow-up. A recent Spanish study obtained very similar findings to ours in this respect.9
In summary, patients with HBeAg(−) chronic HBV infection are generally asymptomatic at diagnosis and during the course of the disease. The occurrence of cirrhosis is rare. It must be remembered that up to half of cases of HCC, which is the main complication, develop in patients without cirrhosis. The percentage of patients who require treatment must also be taken into account.
Conflicts of interestThe authors declare that they have no conflicts of interests.
We would like to thank Dr. Marcelino Sánchez Casado, ICU staff physician in Hospital Virgen de la Salud de Toledo, for his assistance in both the methodology and statistics.
Please cite this article as: Guardiola Arévalo A, Gómez Rodríguez R, Romero Gutiérrez M, Gómez Moreno AZ, García Vela A, Sánchez Simón R, et al. Características y evolución de la infección crónica por virus de la hepatitis B antígeno e negativo. Gastroenterol Hepatol. 2017;40:59–69.