Colitis cystica profunda is a rare benign condition characterized by mucoid cysts in the colonic submucosal layer. The most frequent form is localized, typically located on the anterior rectal wall 6–7cm from the anal verge, although segmental and diffuse forms affecting only one part of the colon or the whole colon have been documented. The disease usually presents as a polypoid mass in the rectum associated with bloody or mucoid diarrhea and rectal tenesmus. This clinical presentation associated with the microscopic finding of mucus cysts in the submucosa has led to the mistaken diagnosis of adenocarcinoma and unnecessarily radical operative procedures. Attention to the completely normal cellular architecture provides the key to the correct diagnosis.
We here report two cases of colitis cystica profunda in one of which the initial misdiagnosis of rectosigmoid neoplasm caused unnecessary anterior resection of the rectum. In the other case, treatment with systemic and topical corticosteroids was necessary to control rectal bleeding. Both patients have remained asymptomatic for 7 and 10 years, respectively, after successful response to treatment with mesalamine and corticosteroid therapy.
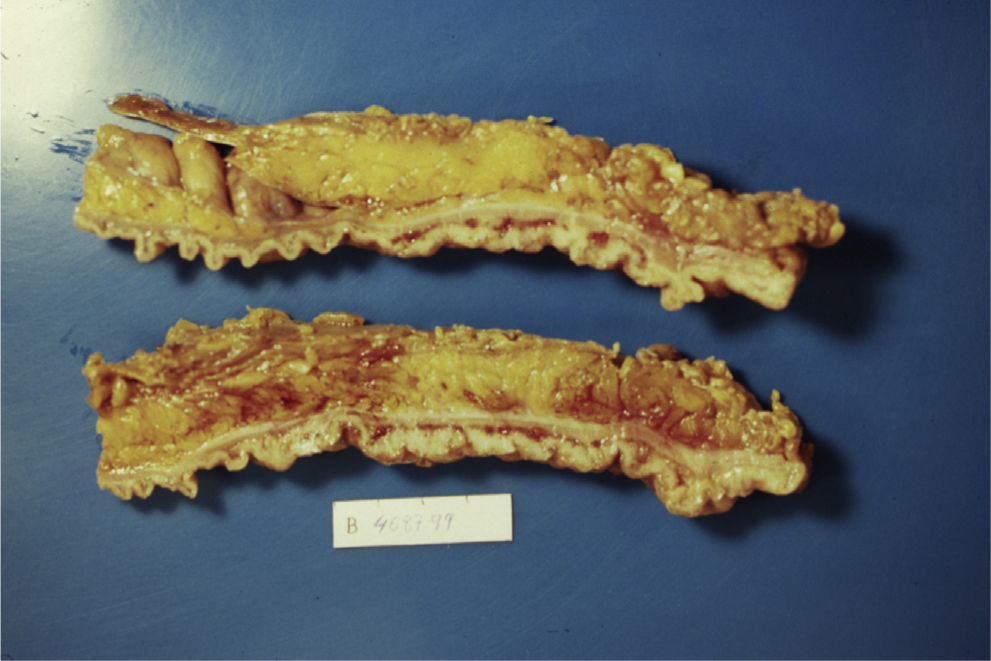
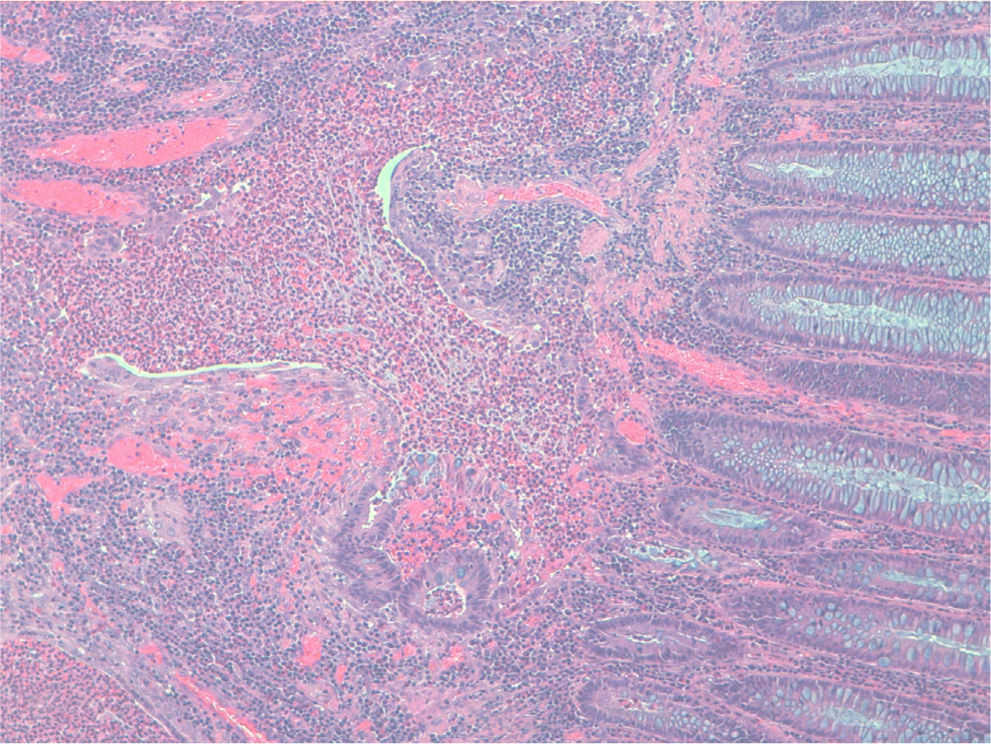
Case 1A 45-year-old man with family history of ulcerative colitis (one brother) was admitted in 1999 because of post-defecation rectal bleeding, rectal tenesmus and mucus discharge of one month's duration. He was a current smoker (30 pack-years) and reported daily alcohol consumption of 40g of ethanol. A firm rectal mass was palpated about 6cm from the anal verge. Barium enema showed a large infiltrating stenotic lesion with an extraluminal mass compatible with rectosigmoid neoplasm. A colonoscopy was performed revealing a 15-cm lobulated and ulcerated mass at 7cm from the anal verge suggestive of malignant neoplasm affecting the rectosigmoid area. Biopsies showed inflammatory changes without tumor infiltration. The abdominal computerized tomography (CT) showed rectosigmoid wall thickening of malignant appearance without lymph nodes or distant metastasis. A tentative diagnosis of rectal neoplasm was established and the patient underwent a low anterior resection and prophylactic appendectomy. Histopathological examination of the resected specimen (Figs. 1 and 2) showed numerous herniations of glands into the submucosa of different sizes and absence of neoplastic changes in columnar epithelium. Also, there were areas with ulcerative fissures and abscess-like fistulas, with abundant lymphoid tissue and fibrosis suggestive of Crohn's disease. Lymph nodes and the vermiform appendix did not show relevant changes. The postoperative course was uneventful but the patient began to have fecal incontinence (gas and stools) shortly after hospital discharge. A severe hypotonic internal anal sphincter confirmed by anorectal manometry and endoscopic ultrasonography of the anal canal was found. The episodes of fecal leakage decreased with biofeedback therapy.
One year after surgery, the patient was referred to our unit of inflammatory bowel disease due to reappearance of rectal bleeding, diarrhea with mucus in stools and anal pain. The pelvic magnetic resonance imaging (MRI) was normal. A new colonoscopy showed hyperemia at the level of the anastomosis but superficial biopsies of the mucosa were unrevealing. Biopsies of the surgical specimen were re-examined and the diagnosis of colitis cystica profunda was established. The patient was treated with 2g/day of mesalamine and rectal symptoms resolved within 4 weeks. After one year, the patient remained asymptomatic and treatment with mesalamine was discontinued. During the next 5 years, he presented an episode of clinico-endoscopic recurrence, in 2007, which was successfully treated with mesalamine suppositories. From then until now the patient remained asymptomatic, with normal colonoscopic findings.
Case 2A previously healthy 16-year-old woman presented with rectal bleeding of one month's duration and chronic constipation. Rectal examination showed a prominent and irregular lesion in the anorectal zone. Laboratory tests, stool culture and abdominal and pelvic CT scan were normal. Pancolonoscopy with ileostomy revealed a mucous neoproliferative lesion of polypoid aspect, 2cm from the anal verge, with friable and ulcerated surface, which involved almost the entire circumference, suggestive of a malignant neoplasm. However, multiple biopsies revealed marked hyperplasia and gland dilatation in the submucosa, with cyst formations and mixed-type inflammatory changes, without evidence of malignant cells, compatible with cystic colitis. An endoscopic ultrasonography of the anal canal showed non-specific thickening of the submucosa without infiltration of the internal sphincter, and a videodefecography showed posterior rectal mucosal prolapse and non-obstructive anterior retrocele. The diagnosis of cystic colitis in the context of a mucosal rectal prolapse syndrome was made. Treatment with high-fiber diet and oral mesalamine was unsuccessful and the patient was admitted to the hospital and treated with full-doses systemic and topical corticosteroids and antibiotics with metronidazole and ciprofloxacin. Clinical response with disappearance of rectal bleeding and clear endoscopic improvement was achieved in 2 weeks. Treatment with high-fiber diet and topical corticosteroids was indicated after discharge from the hospital. The patient remained asymptomatic and topical corticosteroids were discontinued 6 months later. The patient has been in clinical remission over the course of the next 10 years, with progressive endoscopic improvement in the control sigmoidoscopies carried out every year until complete normal findings in 2010.
Colitis cystica profunda has been gradually gaining recognition through sporadically reported cases, some of them accompanied by review of the literature.1,2 The etiology and pathogenesis of this condition remains unclear, however, an unusual mucosal repair, with herniation of the gland epithelium into the submucosa in response to inflammatory, infectious, traumatic or ischemic processes has been suggested.1 This hypothesis is supported by the frequent association of colitis cystica profunda with rectal prolapsed,3 solitary rectal ulcer, adenomatous polyp, post-radiation colitis, diverticulitis, colostomy stoma, surgical anastomosis, infectious colitis and inflammatory bowel disease.4
Colitis cystica profunda has been associated also with adenocarcinoma of the colon in a number of cases.5 Burt6 described a patient in who colitis cystica profunda and mucinous adenocarcinoma were found in the same lesion of the sigmoid, followed a year later by resection of the transverse colon for a lesion with features of colitis cystica profunda only. Bhuta7 reported a case of colitis cystica profunda with coexisting carcinoma in situ. Franzin and Novelli8 documented a number of surgical specimens resected for gastric cancer that also contained submucosal cysts lined by benign epithelium in relation with gastritis cystica profunda, a pathology with similar properties to colitis cystica profunda. The areas of gastritis cystica profunda were found adjacent to invasive cancer but also at sites where the overlying mucosa showed an almost normal appearance. Qizilbash9 described histologic changes suggestive of both gastritis cystica profunda and carcinoma at the site of anastomosis 35 years after gastrojejunostomy for benign peptic ulcer. More recent reports have described dysplastic changes and early gastric cancers within the submucosal glands of select cases of gastritis cystica profunda suggesting an adenocarcinomatous precursor lesion. In addition to these cases showing direct association between submucosal cysts and cancer, colon adenocarcinoma has been associated with lesions in which colitis cystica profunda also has been found concurrently, such as ulcerative colitis and adenomatous polyps. Further investigation may help to elucidate a still undefined relationship between colitis cystica profunda and malignant disease.
The most frequent symptoms are hematochezia, diarrhea and mucus in feces, less commonly patients present with rectal tenesmus and abdominal pain. Intestinal obstruction because of large lesions is rare. Neither barium enema nor colonoscopy may not be useful to distinguish benign from malignant polypoid lesions. An endoscopic ultrasonography can show hypoechoic cysts in the submucosal layer without infiltration of the deep layers or local lymph nodes.10 CT and MRI may help in the diagnosis.
The definitive diagnosis is based on histopathological findings of endoscopic biopsies (which should be large and deep enough to include the submucosal layer) or resected surgical specimens. Small endoscopic biopsies are frequently unrevealing. Microscopically, the disease is characterized by submucosal mucin-filled cysts of variable size covered by an epithelium without cellular atypia that extend below the muscularis mucosa and may intrude into the muscularis propria of the colonic wall. The surrounding connective tissue may show chronic inflammation and fibrosis. Occasionally, superficial ulcers, fissures or abscesses may be present mimicking an inflammatory bowel disease, as occurred in our first case. Also, in many cases, the diagnosis is reached after surgery.
Currently, it is recommended to initiate medical treatment with high-fiber diet to correct constipation and glucocorticoid enemas. In most cases, symptomatic treatment may suffice. Treatment with oral mesalamine was indicated in both of our patients because of similarities between colitis cystica profunda and inflammatory bowel disease, which allowed the relapsing episode to resolve in one case, whereas the other patient was successfully treated with corticosteroids. Surgical treatment is necessary in obstructive symptoms due to large lesions, chronic bleeding or in severe cases of rectal prolapse.
In summary, knowledge of colitis cystica profunda and its correct diagnosis are indispensable to differentiate this benign entity from malignancy and prevent unnecessary radical surgical procedures. Our experience with the first patient avoided to perform surgery in the second patient who showed a complete response to medical treatment. Both patients are asymptomatic after 7 and 10 years of follow-up, respectively.
Sources of support: None to be declared.
Conflicts of interestThe authors do not have any conflict of interest to disclose.
The authors thank Marta Pulido, MD, for help in translating and editing.