Intestinal ultrasound is considered to be a valid alternative for the evaluation of post-operative recurrence (POR) of Crohn’s disease. The aim of this study is to assess the correlation between ultrasound and endoscopic findings.
MethodsPatients with Crohn’s disease were retrospectively recruited who had undergone ileocecal resection, and for whom a colonoscopy and intestinal ultrasound had been performed for the detection of POR. Recurrence was assessed using the Rutgeerts score (RS). The ultrasound findings analysed were bowel wall thickness (BWT), parietal hyperaemia using power Doppler, loss of layer pattern and mesenteric fat hypertrophy.
ResultsA total of 31 patients were included, of which 15 (48.4%) had no POR (RS < 2b) and 16 (51.6%) had POR (RS ≥ 2b). A statistically significant association was identified between BWT and the presence of endoscopic recurrence (a mean of 2.75 mm vs. 5.68 mm, p > 0.001). There was also a statistically significant difference in hyperaemia between the 2 groups (p = 0.03). For wall thickness, an area under the ROC curve (AUC) of 92.9% was obtained, and with a cut-off point of 3.4 mm, a sensitivity of 100% and specificity of 86.6%. When comparing with the most frequent biomarkers (fecal calprotectin and serum CRP), a higher AUC was obtained for wall thickness (72.3% and 72.3% vs. 92.9%).
ConclusionsIn our experience, ultrasound has high diagnostic efficacy in the detection of POR and can be considered a valid non-invasive alternative to endoscopy.
La ecografía intestinal se considera una alternativa para la evaluación de la recurrencia posquirúrgica (RPQ) de la enfermedad de Crohn. El objetivo de este estudio es evaluar la correlación entre los hallazgos ecográficos y endoscópicos.
MétodosSe recogieron de forma retrospectiva los datos de pacientes con enfermedad de Crohn y resección ileocecal en los que se había realizado una colonoscopia y una ecografía intestinal para la detección de RPQ. La RPQ se evaluó empleando el índice de Rutgeerts (IR). Los hallazgos ecográficos analizados fueron el grosor de la pared intestinal, la hiperemia parietal por Doppler, la desestructuración del patrón de capas y la proliferación fibrograsa.
ResultadosSe incluyó a un total de 31 pacientes, 15 (48,4%) sin recurrencia (IR < 2b) y 16 (51,6%) con recurrencia (IR ≥ 2b). Se identificó una asociación significativa entre el grosor parietal y la presencia de recurrencia endoscópica (media 2,75 mm vs. 5,68 mm; p < 0,001). La hiperemia también se asoció de forma significativa con la recurrencia endoscópica (p = 0,03). Para el grosor parietal se obtuvo un área bajo la curva ROC (AUC) del 92,9% y, con punto de corte en 3,4 mm, la sensibilidad fue del 100% y la especificidad del 86,6%. Al comparar con los biomarcadores principales (calprotectina fecal y PCR sérica) se obtuvo un AUC superior para el grosor (72,3% y 72,3% vs. 92,9%).
ConclusionesEn nuestra experiencia, la ecografía tiene una alta rentabilidad diagnóstica para la detección de RPQ y puede considerarse en muchas ocasiones como una alternativa válida y no invasiva a la ileocolonoscopia.
Two thirds of patients with Crohn’s disease (CD) will require surgery at least once in the course of their illness.1 While postoperative recurrence (PR) rates vary by the type of study and the populations considered, one year after surgery, up to 80% of patients have endoscopic PR (ER) and 25% experience clinical symptoms.2
The onset of ER, left untreated, can result in complications that will ultimately lead to further surgery. This means that early recognition is essential so that treatment may be started or adjusted as soon as possible. Early colonoscopy after surgery, generally at six months after the procedure, has been advised to achieve this.3,4
However, there is no agreement on the frequency with which endoscopic examinations should be performed subsequent to this first evaluation. Given that colonoscopy is an invasive procedure that requires preparation and sedation, and also carries risks, non-invasive alternatives for detecting PR have been evaluated. Among the imaging techniques postulated for its management, bowel ultrasound, magnetic resonance enterography and computed tomography offer advantages over endoscopy. These enable examination of segments that, due to stenosis or technical difficulty, cannot be suitably assessed by endoscopy. They are also capable of detecting other transmural complications such as fistulas and abscesses.5,6
Among non-invasive endoscopic tests, capsule endoscopy could be useful for detecting PR, but it is up to 10 times more expensive than ultrasound and carries a risk of complications such as retention.
Bowel ultrasound has advantages over the other techniques, in its accessibility, immediacy, non-invasive character, the absence of radiation, its low cost and good tolerance by patients. It has also demonstrated good correlation with findings in surgery and endoscopy and with other imaging techniques.6,7
Various systematic reviews and position statements have proposed ultrasound as a valid technique in the initial diagnosis of Crohn’s disease, in the assessment of activity, in monitoring, the detection of complications (stenosis, fistulas and inflammatory masses) and in PR.8–11 Among the various ultrasound parameters used to assess activity, wall thickness and hyperaemia are those with the greatest diagnostic yield.12,13 In the context of PR, ultrasound has a sensitivity of 83.3% and a specificity of 97.7%.14
Based on these ideas, we aimed to determine the correlation between the various ultrasound parameters of activity and the Rutgeerts endoscopic score, and to evaluate ultrasound as a non-invasive method for monitoring PR.
Patients and methodsPatientsRetrospective data were collected on a total of 31 patients with Crohn’s disease and ileocaecal resection who had undergone a colonoscopy and a bowel ultrasound to assess PR between 2014 and 2018. Only patients with a lapse of less than six months between tests were selected, provided that no clinical or therapy change had occurred between them.
We collected all the demographic variables of the patients selected (age, gender and smoking habits) and those related to their Crohn’s disease (location, phenotype, treatment and number of surgeries) that could be important for the study objectives. Finally, the biological variables of activity most commonly used in clinical practice (C-reactive protein and calprotectin) were included. A cut-off point for faecal calprotectin of 50 μg/g was used to define disease remission.
IleocolonoscopyIleocolonoscopy was performed by expert endoscopists from the inflammatory bowel disease unit with extensive experience in the use of endoscopic indices in this disease. Olympus® 180/190 colonoscopes (Tokyo, Japan) were used. Colonoscopies were performed after a 48-h liquid diet. The preparation for bowel cleansing indicated by the physician was used on the day before the examination. In some cases, anastomotic dilatation was performed so that the neoterminal ileum could be suitably assessed.
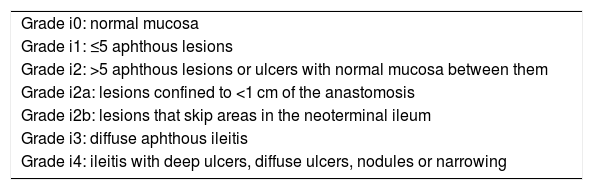
The Rutgeerts scale was used to assess ER. The Rutgeerts score (RS) establishes five grades of lesions (Table 1): grade i0, no lesions; grade i1, fewer than 5 aphthous lesions with normal mucosa between them; grade i2, more than 5 aphthous lesions or ulcers with normal mucosa between them, distinguishing whether they are confined to the anastomosis (i2a) or not (i2b); grade i3, diffuse ileitis; and grade i4, diffuse ileitis with larger ulcers, nodules, or stenosis. Cases with grade ≥i2b lesions were considered ER, and those with grades i0, i1, or i2a, lack of recurrence. Grades i3 and i4 were considered severe ER. The examination selected was not necessarily the first endoscopic evaluation after surgery, but those performed according to clinical practice in patient follow-up. Data was collected only for patients who had both techniques within a lapse of under 6 months. In no case included was data collected for more than one endoscopic study or ultrasound of the same patient.
Rutgeerts endoscopic scale and its modifications.
| Grade i0: normal mucosa |
| Grade i1: ≤5 aphthous lesions |
| Grade i2: >5 aphthous lesions or ulcers with normal mucosa between them |
| Grade i2a: lesions confined to <1 cm of the anastomosis |
| Grade i2b: lesions that skip areas in the neoterminal ileum |
| Grade i3: diffuse aphthous ileitis |
| Grade i4: ileitis with deep ulcers, diffuse ulcers, nodules or narrowing |
Bowel ultrasound was performed by two gastroenterologists with expertise in this technique (JP and CS) using an Esaote® My Lab Desk 70 XVG system (Genoa, Italy). Two probes were used for the examination: a 3–5 MHz convex probe and a 7–10 MHz high-frequency linear probe. Neither oral nor intravenous contrast was employed, but only fasting for at least 5 h.
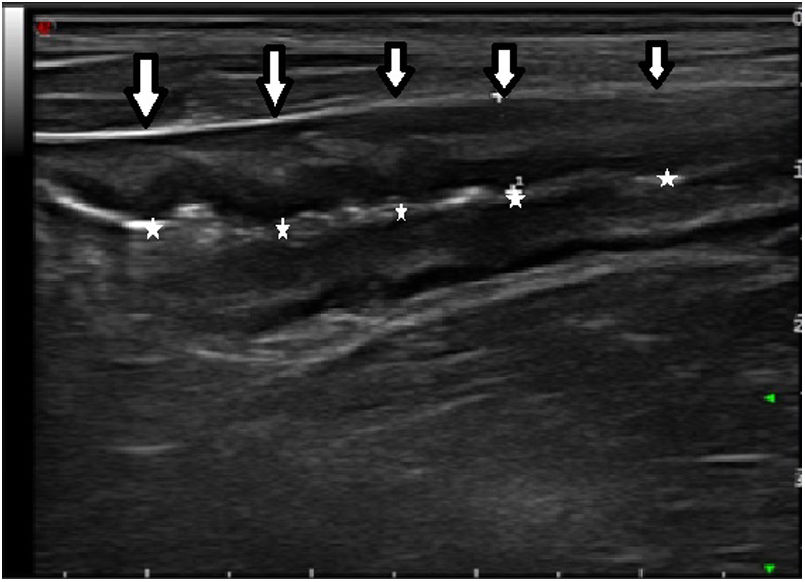
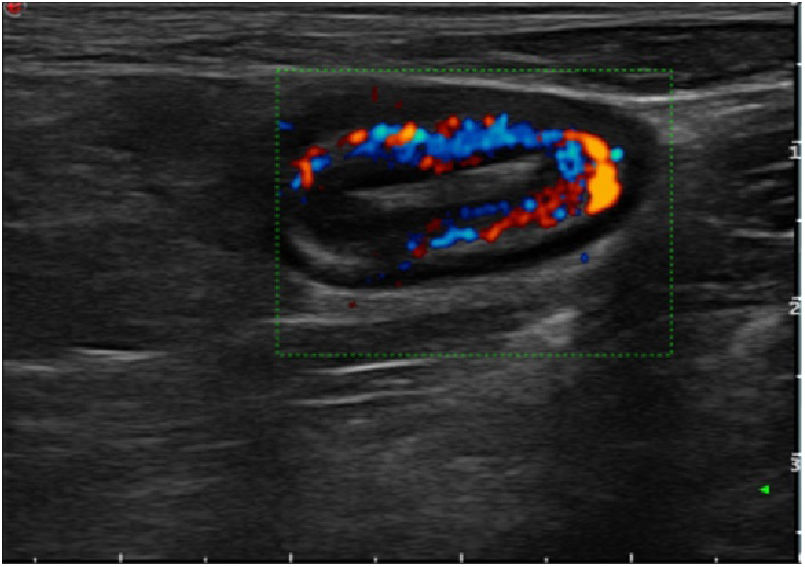
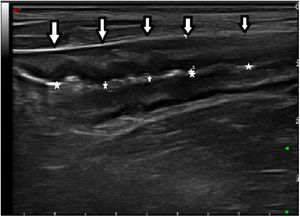
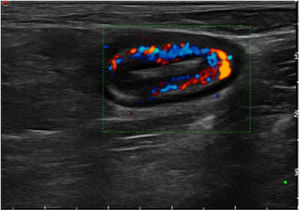
The ultrasound findings collected were maximum thickness of a longitudinal slice of the neoterminal ileum wall (expressed in millimetres), the hyperaemia of the bowel wall, the loss of layer pattern, fibrofatty proliferation and the presence of lymphadenopathy and transmural complications (such as stenosis, fistulas and abscesses). The modified Limberg scale was used to assess hyperaemia of the bowel wall.15 This scale grades vessel density from 0 to 3 (grade 0, absent; grade 1, 1–2 points per cm identified2; grade 2, 3–5 points per cm identified2; and grade 3, more than 5 points per cm2 and even vessels outside the wall identified). Grades 0 and 1 were considered absence of hyperaemia, and grades 2 and 3 were considered hyperaemia. Identification of decreased bowel lumen preceded by fixed dilatation of the prior loop with fluid and echogenic contents was considered diagnostic of anastomotic stenosis.7
Statistical studyA descriptive analysis was performed on the data obtained, both on the baseline characteristics of the patients and that relating to their inflammatory bowel disease, phenotype, location, type of surgical procedure, anastomosis and treatment. Mean and standard deviation were calculated for continuous variables, and percentages for the categorical variables. The univariate analysis used Student’s t test for quantitative values, comparing means, and the chi-squared test for categorical variables and comparison of proportions. ROC curves were prepared to study the sensitivity and specificity of the test. A multivariate analysis was performed by means of logistic regression, using significant endoscopic recurrence categorically as a dependent variable. A p value <0.05 was considered statistically significant. The statistical analysis was performed with the Stata software program. The study was approved by the local Research Ethics Committee.
ResultsIn our sample, 29% (9 patients) were smokers, 45% (14) were former smokers and 25% (8) were non-smokers. The majority of the patients, 87% (27), had been treated with a single surgical resection, and the remaining 13% (four) had undergone two surgical procedures. Table 2 summarises the baseline characteristics of the patients.
Baseline characteristics of the study population.
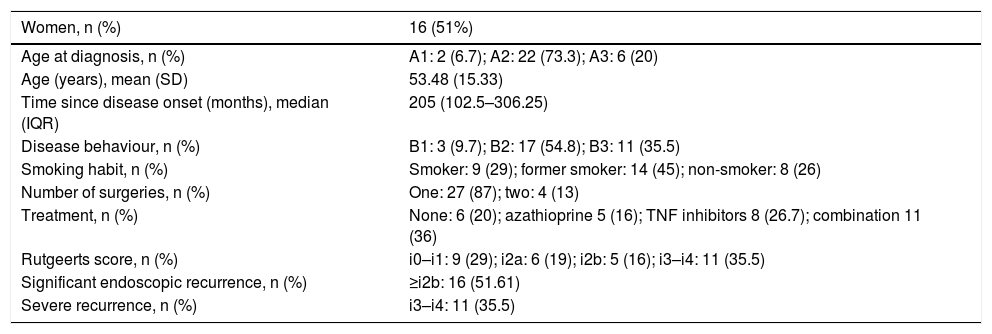
| Women, n (%) | 16 (51%) |
|---|---|
| Age at diagnosis, n (%) | A1: 2 (6.7); A2: 22 (73.3); A3: 6 (20) |
| Age (years), mean (SD) | 53.48 (15.33) |
| Time since disease onset (months), median (IQR) | 205 (102.5–306.25) |
| Disease behaviour, n (%) | B1: 3 (9.7); B2: 17 (54.8); B3: 11 (35.5) |
| Smoking habit, n (%) | Smoker: 9 (29); former smoker: 14 (45); non-smoker: 8 (26) |
| Number of surgeries, n (%) | One: 27 (87); two: 4 (13) |
| Treatment, n (%) | None: 6 (20); azathioprine 5 (16); TNF inhibitors 8 (26.7); combination 11 (36) |
| Rutgeerts score, n (%) | i0–i1: 9 (29); i2a: 6 (19); i2b: 5 (16); i3–i4: 11 (35.5) |
| Significant endoscopic recurrence, n (%) | ≥i2b: 16 (51.61) |
| Severe recurrence, n (%) | i3–i4: 11 (35.5) |
Regarding the treatment patients received to manage their disease, up to 20% (6 patients) were not being treated, 16.7% (5) were being treated with azathioprine, 26.7% (8) with tumour necrosis factor (TNF) inhibitors and 36.6% (11) were on combination treatment with TNF inhibitors and azathioprine.
The median time elapsed between endoscopy and ultrasound was 2 months (x = 1.74 months; SD = 1.29).
Endoscopy and ultrasound findingsIn total, 48.4% (15 patients) did not have ER (grade i0: 7; grade i1: 2; grade i2a: 6), 16.1% (5 patients) had grade i2b, and the remaining 35.5% had severe ER (grade i3-i4). This means that ER was identified in half the patients (51.6%; 16 patients).
Analysis of the relationship between wall thickness and the RS found statistically significant differences in thickness between patients without ER and patients with ER (mean 2.75 versus 5.68 mm ≥i2b; p < 0.001).
In addition, mean thickness was analysed by RS grade, revealing that thickness steadily increased with the RS, with a mean of 2.17 mm (SD 0.45) in grade i0; 4.15 mm (SD: 3.04) in grade i1; 2.96 mm (SD: 1.55) in grade i2; 3.8 mm (SD: 0.32) in grade i2b; 7.2 mm (SD: 0) in grade i3 and 6.47 mm (SD: 1.26) in grade i4. These observed differences were statistically significant in the ANOVA test (p < 0.004). Significant differences were also seen between the mean thickness of RS subgroups i0–i1 and that of subgroups i2–i4 (2.61 vs. 4.94 mm; p = 0.003).
A ROC curve determined the sensitivity and specificity of the thickness for detecting ER, with an area under the curve of 92.9%. With a cut-off point of 3.4 mm for wall thickness, a sensitivity of 100%, a specificity of 86.6%, a positive predictive value (PPV) of 88.8% and a negative predictive value (NPV) of 100% were obtained. Of the two patients with false positives, one had grade i2a endoscopic lesions and the other, grade i1. It should be noted that when wall thickness exceeded 6 mm, a diagnostic specificity of 95% was seen for detecting severe ER.
Regarding hyperaemia of the bowel wall evaluated by Doppler, 92.8% (13/14) of patients without hyperaemia did not have ER. Among patients with ER, 93% were found to have hyperaemia. Notably, none of the patients without ER had hyperaemia. In contrast, 100% of the patients with severe ER had it. These differences were statistically significant (p = 0.03). With these results, the colour Doppler showed a sensitivity of 93.3%, a specificity of 86.6%, a PPV of 87.5% and an NPV of 92.8%.
Comparing the layer pattern to the endoscopic findings revealed that 100% (14/14) of patients without ER had the layer pattern preserved. In addition, 100% (9/9) of patients with wall destructuring had ER, in most cases as severe recurrence (grade i4, in six out of nine cases) (p < 0.001).
Finally, the association between involvement of mesenteric fat and ER was analysed by ultrasound. In our series, it was spared in all cases without ER (15 patients), while it was involved in 54% of the cases (6/11 patients) with grade i3–i4 (p = 0.014).
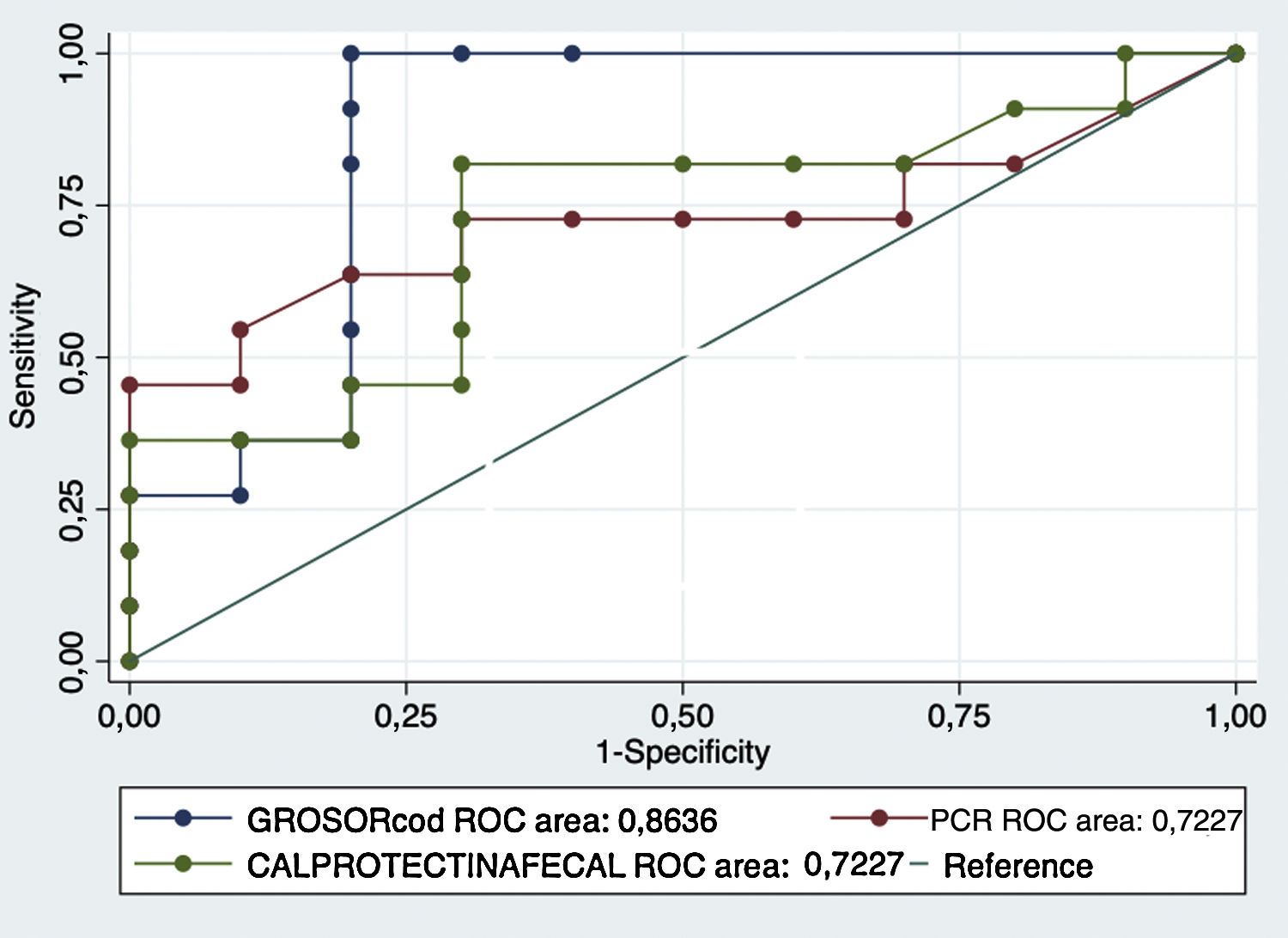
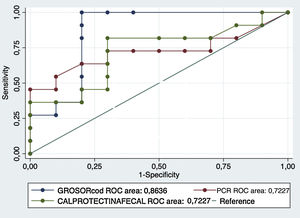
Analysis of calprotectin as a biomarker of recurrence found that 57.1% (12 patients) had high levels (>50 μg/g) while 42% had normal levels. In our series, the mean level in patients without PR was 74.7 μg/g versus 232.7 μg/g in patients with PR (p = 0.047). Analysis of the ROC curve yielded an area under the curve of 72.2%; with a cut-off point of 68 μg/g, a sensitivity of 81% and a specificity of 70% were obtained (PPV: 75%; NPV: 77%; AUC: 72.2%) (Figs. 1 and 2).
Comparing the areas under the ROC curve (AUC) for the main biomarkers (faecal calprotectin and serum CRP) to thickness yielded higher values for thickness and therefore higher sensitivity and specificity values. The results are shown in Fig. 3.
A multivariate analysis performed to determine the specific weight of the calprotectin, wall thickness, hyperaemia and layer pattern found an association with wall thickness (OR: 4.6) and calprotectin (OR: 3.2) (Table 3).
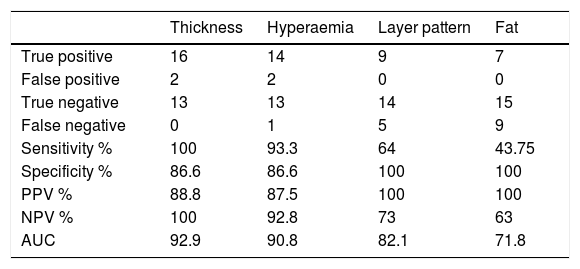
Values for sensitivity, specificity, positive predictive value, negative predictive value and area under the curve of the ultrasound parameters.
| Thickness | Hyperaemia | Layer pattern | Fat | |
|---|---|---|---|---|
| True positive | 16 | 14 | 9 | 7 |
| False positive | 2 | 2 | 0 | 0 |
| True negative | 13 | 13 | 14 | 15 |
| False negative | 0 | 1 | 5 | 9 |
| Sensitivity % | 100 | 93.3 | 64 | 43.75 |
| Specificity % | 86.6 | 86.6 | 100 | 100 |
| PPV % | 88.8 | 87.5 | 100 | 100 |
| NPV % | 100 | 92.8 | 73 | 63 |
| AUC | 92.9 | 90.8 | 82.1 | 71.8 |
Up to two thirds of patients with Crohn’s disease and ileocaecal resection present disease recurrence.1 At present, various treatment options are available for its prevention, particularly, immunosuppressive drugs and biological therapies. The indication for, or optimisation of, these treatments are largely based on endoscopic examination of the neoterminal ileum; however, this is an invasive procedure that is not free from complications and is uncomfortable for the patient. Consequently, in recent years, ultrasound has been proposed as an alternative imaging method to ileocolonoscopy for the assessment of postoperative recurrence.7,8,10,16
According to our results, ultrasound parameters show a good correlation with the presence and severity of endoscopic recurrence. In our series, there were statistically significant differences with regard to neoterminal ileum wall thickness in patients with ER versus patients without ER. In addition, thickness steadily increased as the RS increased, also reaching statistical significance, with the exception of subgroup i1. This anomaly can be explained by the fact that this subgroup was under-represented, with just two patients. These findings were consistent with prior studies, in which bowel wall thickness proved a good indicator of recurrence.6,14,17
Doppler ultrasound also showed a good correlation and supplied additional information supporting that obtained by thickness. In our series, all cases with a thickness <3.4 mm were free of hyperaemia, except for one patient. This patient in particular was a false positive, with a wall thickness of 6.2 mm but Doppler activity (1/3) presenting an endoscopic grade of i1. In a prospective study by Paredes et al.,18 none of the patients with thickness <3 mm showed hyperaemia in the bowel wall on Doppler.
Layer pattern disruption, unlike thickness, was seen to be less sensitive but more specific (64% and 100%, respectively) and was associated with severe forms of endoscopic recurrence (grade i3–i4). According to Rigazio et al.,19 layer pattern distortion is a good marker of disease activity and is even associated with increased risk of surgery. Our results support the findings in the literature and also showed it to be a good marker of recurrence.
The involvement of mesenteric fat showed a specificity of 100%, as this was spared in all patients without recurrence. However, it had low diagnostic sensitivity (40%) for detecting postoperative recurrence; it was also a more common finding in cases of severe recurrence.
Our study was novel in that it evaluated wall thickness determined by ultrasound as a tool in the follow-up of these patients — that is to say, after the first evaluation following surgery. Using the ROC curve, an area under the curve of 92.9% was obtained for a thickness of 3.4 mm (sensitivity 100%, specificity 88%, PPV 86% and NPV 100%). These results were similar to those of a recent meta-analysis by Rispo et al.,14 which included five studies and a total of 219 patients, with the following values: sensitivity 94%, specificity 84%, PPV 100% and NPV 25.3%. We found differences between the predictive values obtained in our study and that meta-analysis, likely due to different pre-test probabilities. The studies included in the meta-analysis considered recurrence to be the presence of any endoscopic lesion — that is, subgroup i1 or higher — and therefore had higher percentages of patients with recurrence (53%–78%). Our series, however, considered ER to correspond to grade i2b or higher lesions, given the greater clinical and treatment-related interest of these, since it is as from this subgroup that optimisation of treatment of recurrence is indicated.4 This choice gave us a prevalence of PR of 51.6%, significantly lower than in other series. This meta-analysis also found that, when wall thickness exceeded 5.5 mm, specificity was 97.7%, which was similar to that found in our sample, with a specificity of 95% for severe ER when thickness exceeded 6.0 mm.
The faecal calprotectin level was also significantly related to finding recurrence, as reported in other studies.20,21 Faecal calprotectin as a biomarker of PR showed high sensitivity (81%) and a high NPV (77%), though lower than that observed by other studies, which obtained a higher NPV (91%–94%) to rule out PR.22,23 These differences were due to the cut-off point used to diagnose recurrence being higher, around 150–250 μg/g, and the fact that our series only had the calprotectin levels for 21 patients, pointing to a need for a larger sample size.
Comparison of the area under the ROC curve of the biomarkers (faecal calprotectin and serum CRP) to wall thickness revealed that thickness showed a higher diagnostic yield, with better sensitivity, specificity and positive and negative predictive values than the biomarkers. Hence, in our sample, we would not have achieved a better yield with their combination.
In view of our results, we propose ultrasound as a non-invasive diagnostic test that could be considered as an alternative to ileocolonoscopy, with a high diagnostic yield for monitoring PR, as it enabled suitable follow-up and treatment decision-making, and decreased the indication for ileocolonoscopy in our patients. This management would have allowed us to rule out patients without PR (grade i2a or lower) with an NPV of 100%, thus avoiding endoscopy in up to 48% of our patients. It is also interesting to note that patients with a wall thickness exceeding 6.0 mm had severe forms of recurrence with a specificity of 95%, offering the option to consider changes in treatment with no need for endoscopic confirmation.
As limitations, it should be noted that this was a retrospective study with a limited number of patients (n = 31); therefore, prospective studies with a larger sample size are needed to validate our results. In addition, the points in time when endoscopy and ultrasound were performed did not match, although on average they were no more than 2 months apart. Time since disease onset was not evaluated in each subject and likely differed among them. As a strength, the study was based on real-world clinical practice data.
ConclusionIn our experience, there is a good correlation between the ultrasound parameters of activity, primarily bowel wall thickness, and endoscopic recurrence. We propose bowel ultrasound as an imaging test with a high diagnostic yield that in many cases would avoid performing endoscopies or imaging tests that are less accessible such as magnetic resonance imaging in our patients.
FundingNone.
Conflicts of interestNone.
Please cite this article as: Yebra Carmona J, Poza Cordón J, Suárez Ferrer C, Martín Arranz E, Lucas Ramos J, Andaluz García I, et al. Correlación entre la endoscopia y la ecografía intestinal para la evaluación de la recurrencia posquirúrgica de la enfermedad de Crohn. Gastroenterol Hepatol. 2022;45:40–46.