Effective vaccines against the SARS-CoV-2 are already available and offer a promising action to control the COVID-19 pandemic. IBD patients on biological agents accept the vaccine as well as an additional dose if recommended.
BackgroundVaccination against COVID-19 prevents its severe forms and associated mortality and offers a promising action to control this pandemic. In September 2021, an additional dose of vaccine was approved in patients with immunosuppression including IBD patients on biologic agents. We evaluated the vaccination rate and additional dose willingness in this group of at risk patients.
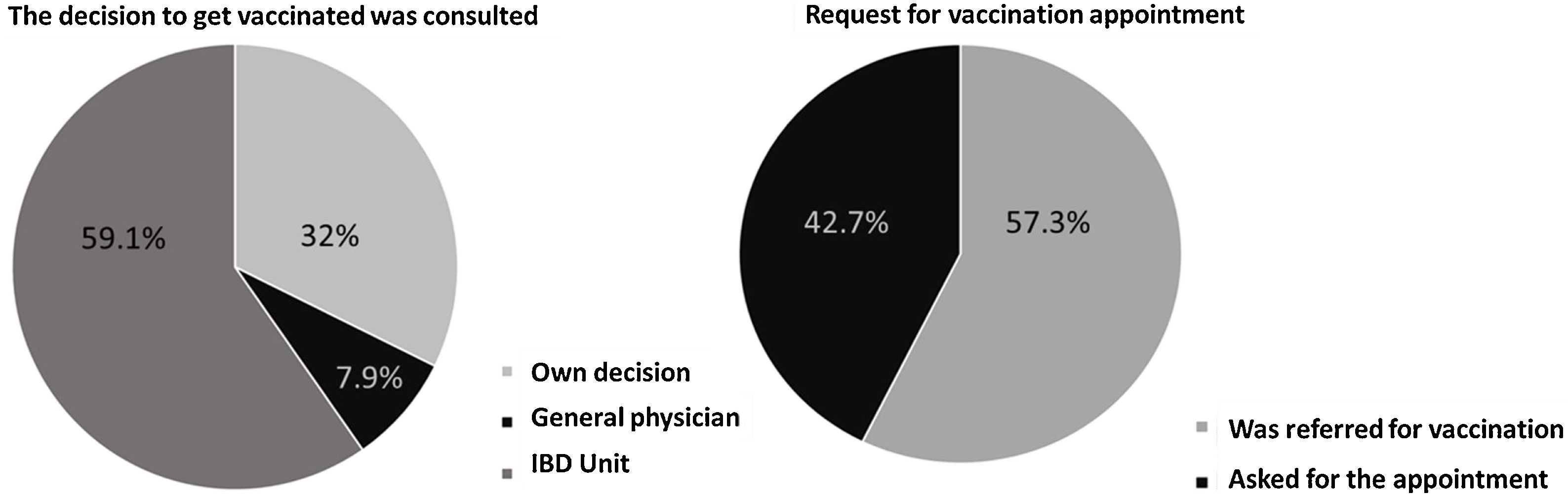
MethodsA single-center, cross-sectional study was performed among IBD patients on biologic agents and eligible for an additional dose of the COVID-19 vaccine. IBD clinical characteristics and type of vaccine and date of administration were checked in medical records. Acceptance was evaluated after telephone or face-to-face surveys in IBD patients.
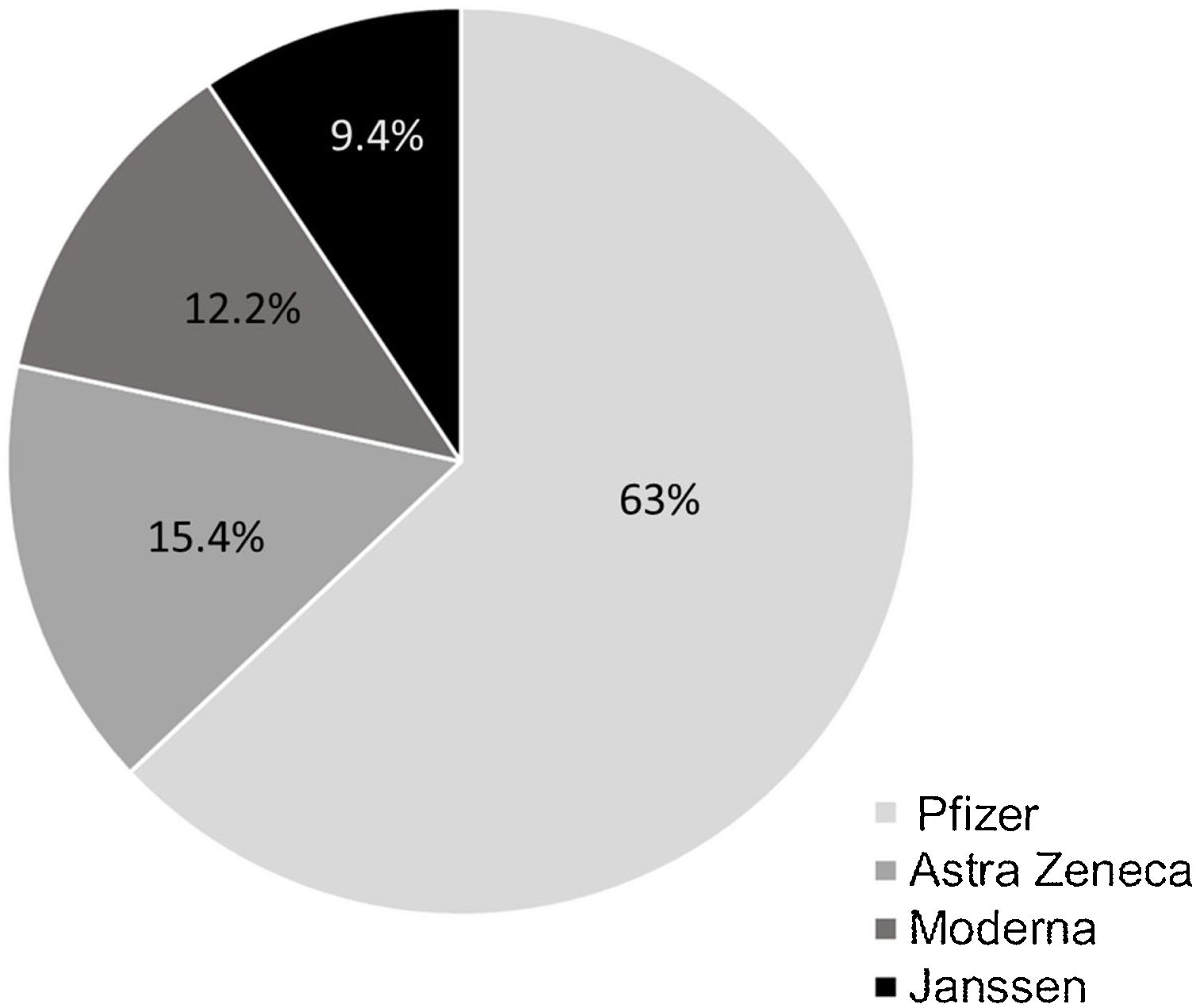
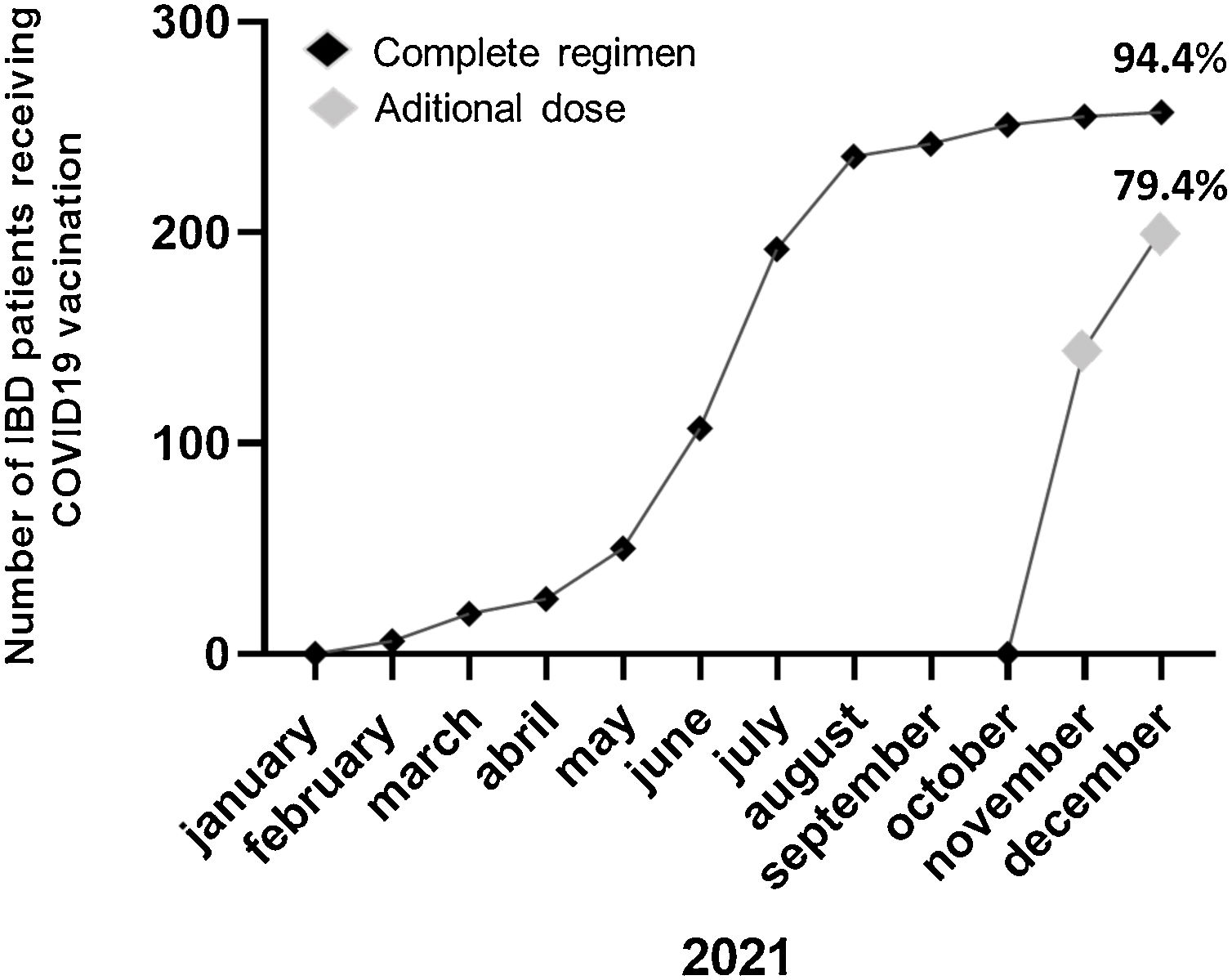
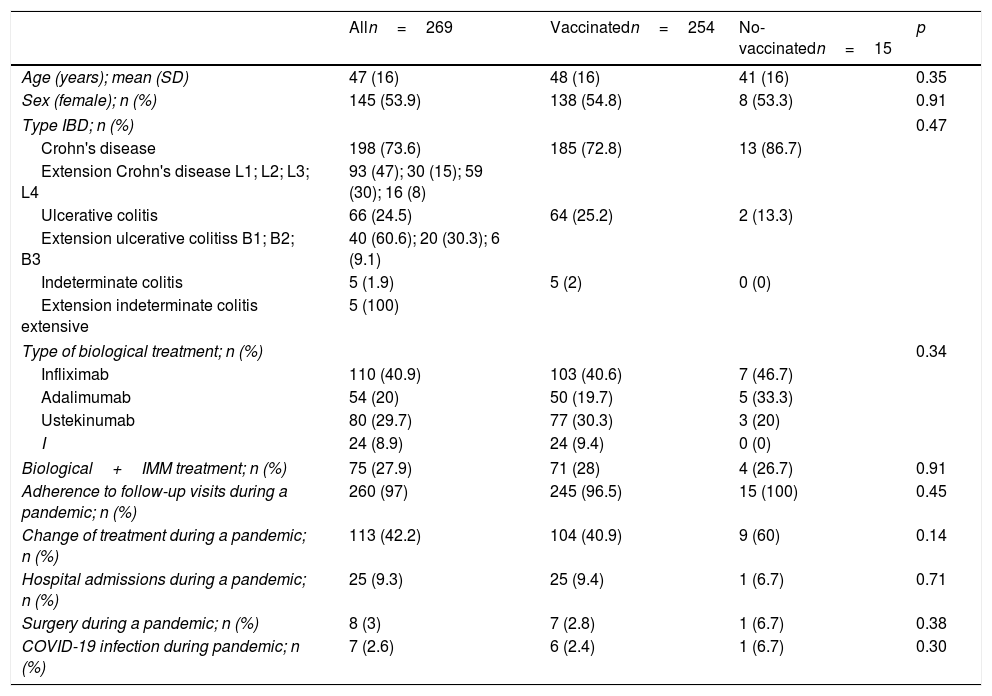
ResultsOut of a total of 344 patients, 269 patients (46.1% male; mean age 47±16 years; Crohn's disease 73.6%) were included. Only 15 (5.6%) patients refused the COVID-19 vaccine mainly (40%) for conviction (COVID-19 pandemic denial). 33.3% would re-consider after discussing with their doctor and/or receiving information on the adverse effects of the vaccine. Previous to the additional dose, the COVID-19 vaccination was present in 94.4% of patients (n=254). Adverse effects occurred in 53.9% of the cases, mainly pain in the arm (40%). Up to 94.1% of the patients agreed to an additional dose and 79.4% had already received the additional dose at the final time of the assessment.
ConclusionsIBD patients on biological agents accept the vaccine as well as an additional dose if recommended. Physicians in charge of IBD units should provide information and confidence in the use of the vaccine in these IBD patients.
La vacunación frente al COVID-19 constituye una acción prometedora para controlar esta pandemia. En septiembre de 2021, se aprobó una dosis adicional de vacuna en pacientes con inmunosupresión, incluidos los pacientes con enfermedad inflamatoria intestinal (EII) que reciben agentes biológicos. En este estudio se evaluó la tasa de vacunación y la disposición de recibir la dosis adicional de vacuna en este grupo de pacientes de riesgo.
MétodosSe realizó un estudio transversal unicéntrico con pacientes afectos de EII con tratamiento biológico y elegibles para una dosis adicional de la vacuna COVID-19. Se evaluó la aceptación y los efectos adversos de la vacuna mediante encuesta telefónica o presencial y se recopiló en las historias clínicas las características de la EII, el tipo de vacuna recibida y la fecha de administración.
ResultadosDe un total de 344 pacientes, 269 (46,1% varones; edad media 47±16 años; enfermedad de Crohn n=198) fueron incluidos. Solo 15 (5,6%) pacientes rechazaron la vacuna frente al COVID-19, el 40% por convicción (negación de la pandemia COVID-19). Antes de la dosis adicional, la vacuna COVID-19 se había administrado en el 94,4% de los pacientes (n=254). En el 53,9% de los casos presentaron efectos adversos, principalmente dolor en el brazo (40%). Hasta el 94,1% de los pacientes refería la aceptación de una dosis adicional de la vacuna y el 79,1% ya había recibido esta dosis adicional en el momento de la evaluación final.
ConclusionesLos pacientes con EII que reciben agentes biológicos aceptan la vacuna frente al COVID-19, así como una dosis adicional si se les recomienda. Los médicos responsables de las unidades de EII deben proporcionar información y confianza en el uso de la vacuna en estos pacientes.