Colorectal (CRC) screening programs represent a large volume of procedures that need a follow-up endoscopy. A knowledge-based clinical decision support system (K-CDSS) is a technology which contains clinical rules and associations of compiled data that assist with clinical decision-making tasks. We develop a K-CDSS for management of patients included in CRC screening and surveillance of colorectal polyps.
MethodsWe collected information on 48 variables from hospital colonoscopy records. Using DILEMMA Solutions Platform © (https://www.dilemasolution.com) we designed a prototype K-CDSS (PoliCare CDSS), to provide tailored recommendations by combining patients data and current guidelines recommendations. The accuracy of rules was verified using four scenarios (normal colonoscopy, lesions different than polyps, non-advanced adenomas and advanced adenomas). We studied the degree of agreement between the clinical assessments made by expert doctors and nurses equipped with PoliCare CDSS. Two experts confirmed a correlation between guidelines and PoliCare recommendations.
Results56 consecutive endoscopy cases from colorectal screening program were included (62.8 years; range 53-71). Colonoscopy results were: absence of colon lesions (n=7, 12.5%), lesions in the colon that are not polyps (n=3, 5.4%) and resected colonic polyps (n=46, 82.1%; 100% R0 resection). Patients with resected polyps presented non-advanced adenoma (n=21, 45.6%) or advanced lesions (n=25, 54.4%). There were no differences in erroneous orders with PoliCare CDSS (Kappa value 1.0).
ConclusionsPoliCare CDSS can easily be integrated into the workflow for improving the overall efficiency and better adherence to evidence-based guidelines.
Los programas de cribado de cáncer colorrectal (CCR) generan un gran número de colonoscopias de seguimiento. Un sistema de soporte a la decisión clínica basado en el conocimiento (K-CDSS) es una tecnología que contiene reglas clínicas y asociaciones de datos que ayudan en la tarea de toma de decisiones clínicas. El objetivo fue desarrollar un K-CDSS para el manejo de los pacientes de cribado de CCR, y evaluar su eficacia.
MétodosRecolectamos información de 48 variables de registros de colonoscopia. Mediante el software DILEMMA (https://www.dilemasolution.com) diseñamos un prototipo de K-CDSS (PoliCare CDSS), para proporcionar recomendaciones personalizadas, combinando los datos de los pacientes y las recomendaciones de las guías actuales. La exactitud de las reglas se verificó mediante cuatro escenarios (colonoscopia normal, lesiones diferentes a pólipos, adenomas no avanzados y adenomas avanzados). Se estudió el grado de concordancia entre las valoraciones clínicas realizadas por médicos expertos y enfermeros equipados con PoliCare CDSS. Dos expertos confirmaron una correlación entre las pautas y las recomendaciones de PoliCare.
ResultadosSe incluyeron 56 casos consecutivos del programa de cribado (62,8 años; rango 53-71). Los resultados de la colonoscopia fueron: ausencia de lesiones de colon (n = 7, 12,5%), lesiones en el colon que no son pólipos (n = 3, 5,4%) y pólipos de colon resecados (n = 46, 82,1%; resección R0 del 100%). Los pacientes con pólipos resecados presentaron adenoma no avanzado (n = 21, 45,6%) o lesiones avanzadas (n = 25, 54,4%). No hubo diferencias en recomendaciones erróneas con PoliCare CDSS (valor Kappa 1.0).
ConclusionesPoliCare CDSS se puede integrar fácilmente en el flujo de trabajo de una unidad de endoscopia digestiva.
The current global outbreak of the COVID-19 related disease has drastically change our health care systems. Gastrointestinal endoscopy clinical practice has adapted its policies and transitioned to telehealth to perform diagnostic and therapeutic procedures.1,2 Artificial Intelligence (AI) has focused on clinical practice to develop clinical decision support systems (CDSSs) as an attractive method for minimizing medical errors and streamline healthcare processes.3 The use of CDSSs has been proposed as a potential remedy for improving the overall efficiency, better adherence to evidence-based guidelines, increase the availability of more accurate medical records, improve the use of preventive measures and quality of health care.4,5
Colorectal cancer (CRC) screening programs represent a large volume of procedures that need a follow-up endoscopy. Surveillance colonoscopy is required after polypectomy given an elevated risk of recurrent polyps and cancer. The long-term effectiveness of colonoscopy-based screening depends on appropriate surveillance intervals; however, there is substantial overuse and underuse.6 A knowledge-based CDDS (K-CDSS) is a technology which contains clinical rules and associations of compiled data that assist with clinical decision-making tasks. Existing CDSSs are limited,7,8 and the arrival of the latest generation of K-CDSS represents an opportunity to appropriate surveillance colonoscopy intervals. To overcome these limitations, we developed a stand-alone K-CDSS prototype (PoliCare CDSS), based on variables and clinical rules introduced by medical specialist for management of patients included in CRC screening and surveillance of colorectal polyps. The aim of the present study was to evaluate the effectiveness of PoliCare based on degree of adherence to current guidelines. As secondary objectives, we evaluated the effect of PoliCare on user satisfaction.
MethodsStudy setting and populationWe performed a retrospective cohort study of patients at a large referral center in Spain who underwent colonoscopy under CRC screening program. We included a cohort of consecutive patients between September 1, 2020, and October 30, 2020. This cohort excluded patients with a personal history of CRC, inflammatory bowel disease (IBD), hereditary polyposis syndromes, prior colectomy, Boston Bowel Preparation Scale (BBPS)<5 or an endoscopic report of poor preparation quality (inadequate visualization of polyps<5mm), incomplete colonoscopy (defined as failure to intubate the cecum), and polyp retrieval failure. Ethics approval for the conduct of this study was obtained from our Ethical Committee (Registered number: 2020-640-1).
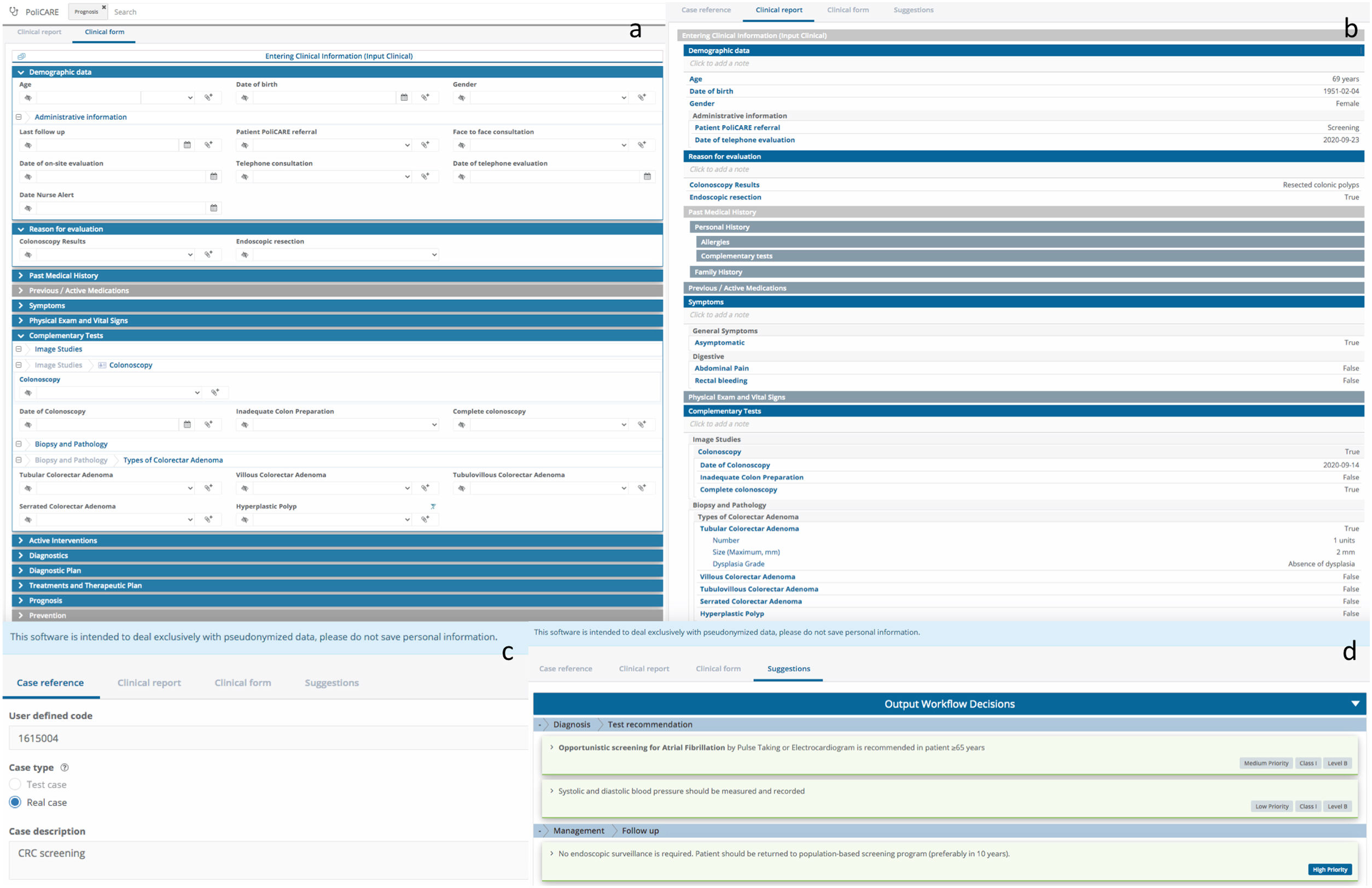
Development of the K-CDSSUsing DILEMMA Solutions Platform © (https://www.dilemasolution.com) we developed the prototype K-CDSS (PoliCare CDSS), to provide tailored recommendations by combining patients data and current guidelines recommendations from the Spanish (AEG) and European (ESGE) Scientific Societies.6,9 We selected DILEMMA because it offered medical specialists to model knowledge directly and has functionalities of natural language processing (NLP) to facilitate data management. To generate patient-specific guidance and reminders we collected information on 48 variables from hospital colonoscopy records, including: (1) cecal intubation, (2) quality of bowel preparation (BBPS score≥6), (3) total number of polyps removed, (4) size of the largest polyp, (5) pathology data (histology, type of dysplasia), and (6) completeness of polyp removal. Endoscopic data were captured from Orion Clinic, our healthcare system's electronic colonoscopy reporting system. The accuracy of rules was verified using four scenarios (normal colonoscopy, lesions different than polyps, non-advanced adenomas and advanced adenomas). Advanced adenomas were defined as ≥3 adenomas, adenomas measuring≥1cm, sessile serrated adenoma/polyps, and any polyp containing high-grade dysplasia or villous histology (Fig. 1).
AssessmentsPoliCare CDSS was designed to be easy to use and to be completed quickly by endoscopists or nurses with minimal workflow disruption. Provided recommendations were compared with the AEG/ESGE guidelines and classified as guideline-adherent or nonadherent. To validate PoliCare we studied the degree of agreement between the clinical assessments made by expert doctors and nurses equipped with PoliCare CDSS. Two experts confirmed a correlation between guidelines and PoliCare recommendations.
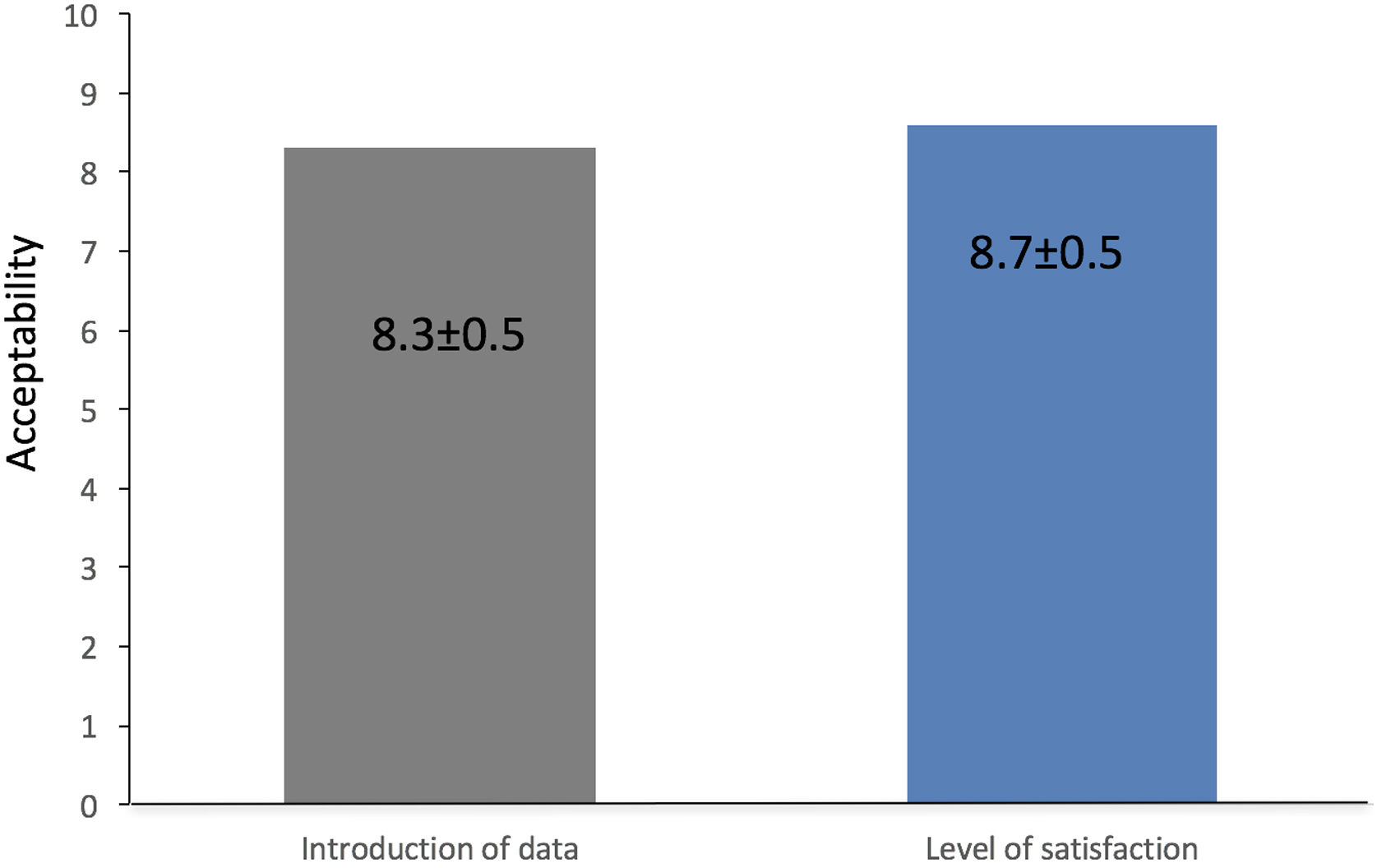
Secondary objectives, to evaluate the effect of PoliCare on user satisfaction, three nurses were assessed with a specific questionnaire at time of the evaluation. Users were asked about their satisfaction with the K-CDSS. Again, the endoscopist was blinded to the answers. The items read as follows: (1) “How easy was the introduction of data?”; (2) “Which is your level of satisfaction with PoliCare?”; (3) “Did you have any difficulty?”. User responses to the questionnaire were categorical (yes or no; question 3) or numerical scale answers (0–10), from very difficult or very bad to very easy or very good (items 1 and 2).
Statistical analysisResults are expressed as mean±SD, median and range, or proportions as indicated. Our primary outcome was presence of guideline-adherent surveillance recommendations, defined as a dichotomous outcome (guideline-adherent vs nonadherent). We used a generalized linear mixed model to compare guideline adherence of PoliCare. Cohen's Kappa coefficient was used to estimate interrater reliability.
Questionnaire responses were evaluated by the X2 test. All analyses were performed with SPSS software version 14.0 (SPSS Inc., Chicago, Ill).
ResultsPatient characteristicsThe cohort was derived from 56 patients who underwent colonoscopy from CRC screening program. Median age was 63 years (range, 53-71). Colonoscopy results were: absence of colon lesions (n=7), lesions in the colon that are not polyps (n=3) and resected colonic polyps (n=46; 100% R0 resection). Patients with resected polyps presented non-advanced adenoma (n=21) or advanced lesions (n=25). The total adenoma detection rate (ADR) was 82.1%, and advanced ADR (AADR) was 44.6% (Table 1).
Effectiveness of PoliCare CDSSAmong the 56 patients, the extent of agreement in guideline-adherent surveillance recommendations was complete. Surveillance recommendations were: (i) return to screening (n=28, 50%), (ii) surveillance colonoscopy after 3 years (n=25, 44.6%), (iii) referred for clinical management (n=3, 5.4%), (iv) referred for genetic counselling (n=0, 0%), and (v) early repeat colonoscopy (n=0, 0%). There were no differences in erroneous orders with Policare CDSS. Number of observed agreements 56 (100% of the observations) (Kappa value=1.00). Otherwise, the degree of agreement between the clinical assessments made by expert doctors and nurses equipped with this PoliCare CDSS was also complete (Kappa=1.00).
AcceptabilityThe mean punctuation of the items “How easy was the introduction of data?” was 8.33±0.47, and the level of satisfaction was 8.66±0.47. Users that have reported any difficulty was none (Fig. 2).
DiscussionThis study showed that PoliCare CDSS can easily be integrated into the workflow. Also, nurse-reported acceptability responses confirmed that Policare can be carry out by endoscopy nursery to manage patients included in CRC screening and surveillance of colorectal polyps.
Medical decisions are highly dependent on the experience and knowledge of the healthcare professionals, that progressively have less time to collect information, which generate variability. CDSS are capable of processing a significant number of patient data from electronica medical records, and structured clinical rules to generate personalized recommendations to improve health outcomes.10,11 The development of K-CDSS requires a proper user-oriented design, the validation of the clinical rules by the attending physicians, and updating of the knowledge to ensure the quality of the data entered into the system.12–14 K-CDSS are different from mobile applications for health care. K-CDSS are based on clinical rules introduced and reviewed by healthcare professionals, and are accessible from any device through a link. This knowledge-based systems manage medical knowledge holistically. Knowledge can be updated and incorporated according to knowledge demands.15 Healthcare apps are built for mobile devices with the intent of helping users effectively manage their medical conditions (telemedicine, lab results review, prescription & appointment management and more), or for health case professionals to improve the overall health of the patients.
The present study aims to evaluate the effectiveness of the developed PoliCare CDSS, to generate appropriate colonoscopic surveillance intervals. This point is crucial because current data confirm that 15% of surveillance recommendations were not adherent with guidelines, and most non adherent recommendations represented potential overuse.7,16 Patients included in CRC screening program presented a high prevalence of colorectal polyps, 54% of them as advanced adenomas, being a quality indicator in colonoscopy.17 Our results confirm a complete agreement in guideline-adherent surveillance recommendations. Otherwise, the degree of agreement between the clinical assessments made by expert doctors and nurses equipped with PoliCare CDSS was also complete. This favourable data permits the integration of this tool into the workflow of the endoscopy unit, and can be carry out by endoscopy nursery. This can be justified given the high prevalence of patients requiring endoscopic surveillance. Automated identification of patient problems can enhance clinical performance by offsetting repetitive or monitoring activities, thus freeing physicians to focus on more demanding and sophisticated tasks. Nursery-reported acceptability responses confirm a great level of satisfaction and the absence of any difficulty. We know our study has the limitation that is a single-center study and that more studies are necessary to validate this K-CDSS. Our next step will be to integrate and incorporate information from different databases and to use natural language processing technology through PoliCare CDSS to process and analyze large amounts of natural language date to make decisions based on the information because the tool can be use stand alone.
In summary, the use of K-CDSS can easily be integrated into the workflow and can be carry out by endoscopy nursery to manage patients included in CRC screening and surveillance of colorectal polyps.
Conflict of interestVLZ, MBB and VPP declare no conflict of interest. CPG has developed dilemasolution.com