Although adenomas and serrated polyps are the preneoplastic lesions of colorectal cancer, only few of them will eventually progress to cancer. This review provides a comprehensive overview of the present and future of post-polypectomy colonoscopy surveillance. Post-polypectomy surveillance guidelines have recently been updated and all share the aim towards more selective and less frequent surveillance. We have examined these current guidelines and compared the recommendations of each of them. To improve the diagnostic yield of post-polypectomy surveillance it is important to find predictors of metachronous polyps that better identify high-risk individuals of developing advanced neoplasia. For this reason, we have also conducted a literature review of the molecular biomarkers of metachronous advanced colorectal polyps. Finally, we have discussed future directions of post-polypectomy surveillance and identified possible strategies to improve the use of endoscopic resources with the COVID-19 pandemic.
Aunque los adenomas y los pólipos serrados son las lesiones preneoplásicas del cáncer colorrectal, solo algunas de ellas progresarán a cáncer. Esta revisión presenta una descripción del presente y el futuro de la vigilancia endoscópica tras la resección de pólipos. Las recomendaciones de vigilancia pospolipectomía se han actualizado recientemente y todas comparten el objetivo de una vigilancia más selectiva y menos frecuente. Hemos examinado estas directrices actuales y hemos comparado las recomendaciones de cada una de ellas. Para mejorar el rendimiento diagnóstico de la vigilancia posterior a la polipectomía es importante encontrar predictores de pólipos metacrónicos que identifiquen mejor a los individuos con alto riesgo de desarrollar neoplasias avanzadas. Por este motivo, también hemos realizado una revisión de la literatura de los biomarcadores moleculares de pólipos avanzados metacrónicos. Finalmente, hemos discutido las direcciones futuras de la vigilancia endoscópica tras la resección de pólipos e identificado posibles estrategias para mejorar el uso de recursos endoscópicos con la pandemia de COVID-19.
Sporadic colorectal cancer (CRC) is the type most commonly seen in clinical practice and it is defined as cancer arising from the colon or rectum without known contribution from germline causes or inflammatory bowel disease. Two-thirds of all CRCs fit this definition1 and usually occur in individuals at average risk for CRC (women and men over 50 years of age, with no personal or family history of this neoplasm). Most population-based CRC screening programmes use the faecal immunochemical test (FIT) as the preferable screening method for CRC2 and aim to reduce sporadic CRC mortality and, although to a lesser degree, CRC incidence.2,3
Most CRCs develop from a polyp but conversely, only a small number of polyps (5–10%) become cancers.4 Molecular and phenotypic characterization of CRCs has allowed us to understand that this cancer is a heterogeneous disease comprising different molecular entities. Commonly observed alterations show that CRCs develop via three distinct carcinogenesis pathways: chromosome instability (traditional adenoma–carcinoma pathway), microsatellite instability and, alterations in the serrated pathway accompanied with DNA methylation.5,6 These pathways can have some overlap. The sequential accumulations of genetic and epigenetic events that characterize sporadic CRCs are being used to find diagnostic, prognostic, and treatment biomarkers.7
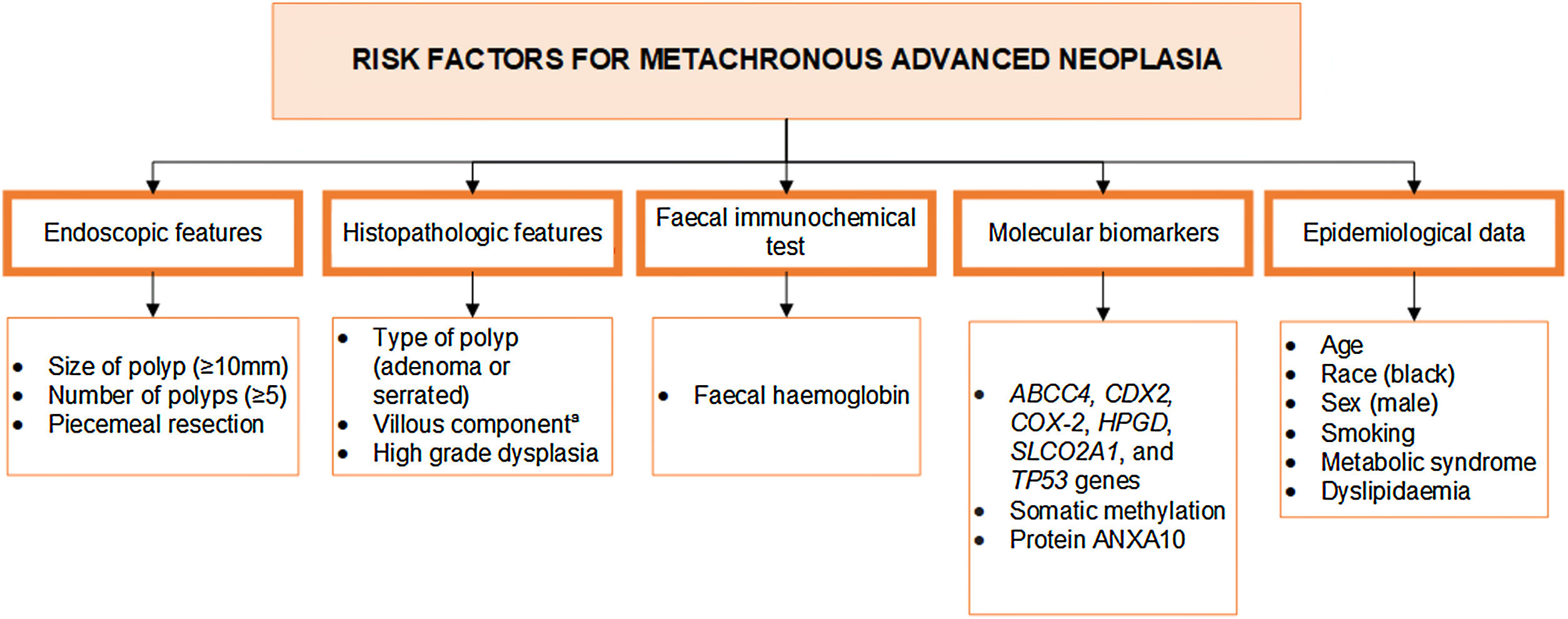
The implementation of CRC screening programs has increased the demand for surveillance colonoscopies which represent around one third of all colonoscopies performed.8,9 And this figure will inevitably rise because CRC screening participation is increasing with FIT implementation.10 The high prevalence of lesions (over 70% of the screening population)11 mandates a conservative surveillance management in order to allocate resources efficiently.12–14 Although there is a lot of effort in developing emerging CRC screening methods,7 post-polypectomy colonoscopy surveillance is recommended according to traditional endoscopic and/or pathological findings of polyps on the index colonoscopy. The yield of CRC and advanced polyps at each surveillance colonoscopy is low. It is estimated that in the high-risk group, 80% of colonoscopies will either be negative or detect only non-advanced neoplasia.15–18 As only a minority of polyps progress to cancer, detection and removal of these lesions may lead to overdiagnosis and overtreatment.19 Nevertheless, because it is unknown which are the polyps that would progress to CRC, the success of screening depends on a high detection and removal rate of all polyps.20,21 A more cost-effective method of surveillance following polyp removal is required. Fig. 1 summarizes all the risk factors associated with metachronous advanced colorectal polyps: endoscopic features, pathological features, FIT, molecular biomarkers, and epidemiological data.
This review provides a comprehensive overview of the present and future of post-polypectomy colonoscopy surveillance. We first examine current guidelines of post-polypectomy surveillance and compare the recommendations of each of them. Next, we review all the evidence concerning molecular biomarkers of metachronous advanced colorectal polyps. Finally, we discussed future directions for research and practice, including the challenges related to post-polypectomy colonoscopy surveillance on overloaded endoscopic units after the lockdown period of the COVID-19 pandemic.
Comparison of the 2020s post-polypectomy surveillance guidelinesNowadays, post-polypectomy colonoscopy surveillance is scheduled according to the number, size, and/or pathological findings of polyps on the index colonoscopy. In 2020, three guidelines22–24 were published with updated recommendations for post-polypectomy surveillance to incorporate new data on long-term CRC incidence and mortality. Table 1 compares each guideline recommendation according to the baseline colonoscopy finding with the current European guideline for quality assurance in CRC screening and diagnosis (2012)25 and with the 2018 update of the Spanish (Spanish Association of Gastroenterology, the Spanish Society of Family and Community Medicine, the Spanish Society of Digestive Endoscopy, and the Colorectal Cancer Screening Group of the Spanish Society of Epidemiology) clinical practice guideline for diagnosis and prevention of colorectal cancer.26
Recommended post-polypectomy surveillance according to each guideline.a
| Baseline colonoscopy finding | European Society of Gastrointestinal Endoscopy (ESGE)22 | British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England23 | US Multi-Society Task Force24 | AEG, SEMFyC, SEED and SEE (Spain)26 | European guidelines for quality assurance in CRC screening and diagnosis25 | |
|---|---|---|---|---|---|---|
| Adenomas | 1–2 tubular adenomas <10mm | Return to screeningc | Return to screening | 7–10 yearsd | Return to screeningc | Return to screeningc |
| 3–4 tubular adenomas <10mm | Return to screeningc | Return to screening | 3–5 years | 3 years | 3 years | |
| 5–10 tubular adenomas <10mm | 3 years | 3 years | 3 years | 3 years | 1 year | |
| Adenoma≥10mm | 3 years | 3 years | 3 years | 3 years | 3 years (≥20mm→1 year) | |
| Adenoma with tubulovillous or villous histology | Return to screeningb | Return to screening | 3 years | 3 years | 3 years | |
| Adenoma with high grade dysplasia | 3 years | 3 years | 3 years | 3 years | 3 years | |
| >10 adenomas on single exam | Genetic evaluation | <60 years/FH or>20adenomas if ≥ 60 years | 1 year, consider genetic evaluation | Genetic evaluation | Genetic evaluation | |
| Piecemeal resection adenoma≥20mm | 3–6 months | 2–6 months | 6 months | 6 months | 2–3 months | |
| Serrated polyps | ≤20 HPs in rectum or sigmoid colon<10mm2 | No recommendation | No recommendation | 10 years | Return to screening (regardless of the number) | No recommendation |
| ≤20 HPs in proximal to sigmoid colon <10mm2 | No recommendation | No recommendation | 10 years | No recommendation | No recommendation | |
| 1–2 SSPs<10mm | Return to screeningc | Return to screening | 5–10 years | Return to screeningc | No recommendation | |
| 3–4 SSPs<10mm | Return to screeningc | Return to screening | 3–5 years | No recommendation | No recommendation | |
| 5–10 SSPs<10mm | No recommendation | 3 years | 3 years | No recommendation | No recommendation | |
| SSPs≥10mm | 3 years | 3 years | 3 years | 3 years | No recommendation | |
| SSP with dysplasia | 3 years | 3 years | 3 years | 3 years | No recommendation | |
| Hyperplastic polyp≥10mm | 3 years | ≥6mm→3 years | 3–5 yearse | No recommendation | No recommendation | |
| Traditional serrated adenoma | 3 years | Included in SSP definition | 3 years | No recommendation | No recommendation | |
| Piecemeal resection of SSP≥20mm | 3–6 months | 2–6 months | 6 months | 6 months | No recommendation | |
All recommendations assume examination complete to cecum with adequate bowel preparation; recommendations do not apply to individuals with a hereditary CRC syndrome, inflammatory bowel disease, personal history of CRC, or family history of CRC.
Patients with >20 hyperplastic polyps with at least 5 being proximal to the rectum, as well as those with 5 serrated polyps proximal to the rectum >5mm, with at least two ≥10mm meet criteria for serrated polyposis syndrome and may require specialized management.
Patients with recommendations issued before 2020 for shorter than 7–10-year follow-up after diagnosis of 1–2 tubular adenomas may follow original recommendations. If feasible, physicians may re-evaluate patients previously recommended an interval shorter than 10 years and reasonably choose to provide an updated recommendation for 7–10-year follow-up, taking into account factors such as quality of baseline examination, polyp history, and patient preferences.
A 3 year follow up interval is favoured if concern about consistency in distinction between SSP and HP locally, bowel preparation, or complete excision.
AEG: Asociación Española de Gastroenterología; FH: family history of CRC or polyposis; HP: hyperplastic polyp, SEMFyC: Sociedad Española de Medicina Familiar y Comunitaria; SEE: Sociedad Española de Epidemiología; SEED: Sociedad Española de Endoscopia Digestiva; SSP: sessile serrated polyp; US: United States.
All guidelines focus on the importance of providing surveillance based on the best available evidence, and minimizing the frequency of colonoscopies to achieve the primary goals of post-polypectomy surveillance: reducing CRC incidence and mortality.
The Spanish Guideline for Diagnosis and Prevention of Colorectal Cancer raised in 2018 some of the key questions (serrated lesions, subsequent surveillance, age limit) that have been later developed in the 2020s guidelines. The recommendations proposed by the Multi-Society Task Force from the United States (US-MSTF) are more conservative and in some risk categories they recommend shorter surveillance intervals. Compared to the not yet updated European guidelines for quality assurance in CRC screening and diagnosis,27 the roles of some traditional risk factors (multiplicity or of villous histology) have been questioned and new evidence available with regard to serrated polyps has been added. Certainly, the incorporation of specific recommendations for serrated polyps has added a degree of complexity to guidelines. Moreover, a suggested limit age of 75–80 years (or earlier if life expectancy is thought to be limited by comorbidities) for performing surveillance colonoscopy has been added in Spanish, ESGE and UK guidelines. This is largely due to the risks associated with colonoscopy in individuals at advanced ages who may not benefit from treatment. In comparison, only the UK guidelines provide recommendations for management of young patients (<50 years).
The terms “low-risk”, “intermediate-risk” and “high-risk” when referring to patients or polyps have been abandoned. After high-quality colonoscopy, the new European guidelines (Spanish, ESGE and UK) simplify the findings of a colonoscopy into two categories: (1) “No need for surveillance” or “No high-risk finding” and; (2) “Need for surveillance” or “High-risk finding”. By contrast, the United States Multi-Society Task Force classifies into six risk categories and each of them with a respectively different recommendation.
Briefly, the Spanish guideline recommends surveillance in patients with EITHER: (1) ≥1 advanced adenoma (with villous component (>20%), high grade dysplasia or ≥10mm) or; (2) ≥3 tubular adenomatous lesions with low grade dysplasia and <10mm or; (3) ≥1 serrated polyp ≥ 10mm or with dysplasia. The ESGE guideline recommends surveillance following polypectomy for EITHER: (1) ≥1 advanced adenoma (≥10mm or with high grade dysplasia) or; (2) ≥5 adenomas or; (3) any advanced serrated polyp (≥10mm or with dysplasia). The British term of advanced colorectal polyp includes the same criteria as the ESGE definition (any adenoma≥10mm or with high grade dysplasia; or ≥1 serrated polyp≥10mm or with dysplasia) but they also differentiate the term premalignant polyp which includes both serrated polyps (excluding diminutive (1–5mm) rectal hyperplastic polyps) and adenomas. Thus, the British guideline recommends surveillance following polypectomy for EITHER: (1) ≥2 premalignant polyps including ≥1 advanced colorectal polyp or; ≥5 premalignant polyps. This way, the British guideline for the first time combines adenomas and serrated polyps in consideration for risk stratification. This approach has not been adopted in either the Spanish, US-MSTF or ESGE or in previous guidelines and it allows surveillance stratification of individuals with multiple polyps of different histology. Conversely, the other guidelines presents recommendations for adenomas and serrated polyps separately.
In European guidelines surveillance colonoscopy is not recommended in patients with “no high-risk criteria” or “No need for surveillance” but these patients should be strongly encouraged to participate in their national CRC screening programme. If organized screening is not available, a surveillance colonoscopy in 10 years is recommended.28,29 This is according to new evidence30–36 that found that long-term CRC risk in patients with non-advanced neoplasia was no higher than in the general population. The Spanish and the ESGE also suggest timing of second surveillance colonoscopy. If no polyps requiring surveillance are detected at the first surveillance colonoscopy, to perform a second surveillance colonoscopy after 5 years. After that, if no polyps requiring surveillance are detected, patients can be returned to screening.
Regarding the differences in surveillance for non-advanced polyps (<10mm in size without dysplasia) the US-MSTF and the Spanish guideline recommend surveillance for those with three to four polyps whereas the UK and ESGE guidelines recommend that individuals should return to routine screening. In the case of the Spanish guideline, as some studies published after have strengthened this recommendation.30,37,38 In the US-MSTF guideline this difference could be explained by the lack of a population-based CRC screening program using a faecal occult blood test unlike many European countries. Moreover, the ESGE emphasized the lower CRC incidence and mortality observed compared with the general population for the low-risk adenoma group and a similar colon cancer incidence compared with normal colonoscopy. They interpreted that the studies available showed that there was a lack of benefit of endoscopic surveillance for this group and should be returned to screening. By contrast, the US-MSTF emphasized the uncertainty regarding the role of surveillance in the outcomes that were observed and raised the possibility that an increased surveillance might have had a role in normalizing the difference in outcomes between the low-risk adenoma and no adenoma group. Another difference in adenomas is that villous histology has been moved into a non-surveillance group in ESGE and UK guidelines. Thus, for subjects with villous or tubulovillous adenomas Spanish and US-MSTF guidelines recommended three years whereas ESGE and UK guidelines recommended to return to screening if no other surveillance criteria were met. In fact, UK post-polypectomy guidelines have never included tubulovillous/villous histology as a high-risk criterion. They state that there is well documented lack of inter-observer agreement among histopathologists in the assessment of villous architecture39,40 and because it generates additional surveillance workload.30,41
Regarding serrated polyps, the US-MSTF guideline recommends different surveillance intervals for subjects with hyperplastic polyps, traditional serrated adenoma and sessile serrated lesions. This is contentious as interobserver reproducibility of serrated polyp subtypes is poor.42 The other guidelines have essentially merged all serrated polyp subtypes, with the exception of small rectal hyperplastic polyps, and post-polypectomy surveillance is based on polyp size and the presence of dysplasia. Another difference is that subjects with five to ten serrated polyps are recommended three years surveillance according to the 2020s guidelines. With five to ten serrated polyps ESGE does not give any recommendation and the US-MSTF and UK guideline recommend a 3-year surveillance colonoscopy. The Spanish guideline does not state any recommendation regarding the number of serrated non-hyperplastic lesions. Finally, for large hyperplastic polyps the US-MSTF allowed for a three-to-five-year follow-up whereas the ESGE and UK offered a three-year recommendation.
Loughrey et al.43 evaluated the impact of 2020s post-polypectomy surveillance guidelines in the Northern Ireland CRC screening programme. They reported a 76% reduction in numbers of individuals meeting surveillance criteria. This confirmed significant potential savings in colonoscopy related to implementation of these new surveillance guidelines. Furthermore, Cross et al.44 examined the appropriateness of these risk classification criteria and recommendations. They found that the 2020s British guideline accurately classifies post-polypectomy individuals and the respectively appropriateness of the surveillance recommendations (colonoscopy versus population-based non-invasive CRC screening). Compared with the general population, CRC incidence was 30% higher among high-risk subjects in the absence of surveillance and 25% lower among subjects with no high-risk criteria.
Molecular biomarkers of metachronous advanced neoplasiaCRC research over the past decade has improved our understanding of the aetiology and development of colorectal polyps. Much has been written about molecular changes during colorectal tumorigenesis and there are a lot of studies that analyse the molecular differences between normal mucosa, adenomatous polyp (less frequently serrated polyps) and cancer.7,45,46 However, few studies have investigated which molecular biomarkers are associated with risk of CRC neoplasia in the long term.
For the second objective, reviewing papers describing molecular biomarkers of metachronous advanced neoplasia, a literature search was conducted using PubMed database until February 2021. The search terms included were as follows: ((“colorectal adenoma” OR “adenomatous polyps” OR “advanced adenoma” OR “high risk adenoma” OR “high-risk adenoma” OR “intermediate risk adenoma” OR “intermediate-risk adenoma” OR “serrated polyps”) AND molecular biomarkers). Seventeen abstracts were individually reviewed, and reference lists were examined to include further appropriate publications. We excluded studies whose aim was to find a screening biomarker of neoplasia. Finally, we included eight studies47–54 that have investigated molecular predictors of metachronous neoplasia. The characteristics of the included studies are shown in Table 2. The study quality score of the seven cohort studies was analysed according to the Newcastle-Ottawa criteria55 (Supplementary Table 1). All of them had a retrospective study design. Seven have extracted DNA from formalin-fixed paraffin-embedded (FFPE) blocks of polypectomies and one50 performed immunostaining on 4μm sections of FFPE blocks.
Studies that investigated biomarkers for metachronous neoplasia.
| Author and year | Age population | N | Characteristic analysed | Adjusting | Follow-up (median) | Conclusions |
|---|---|---|---|---|---|---|
| Habermann et al., 201152 | 56–85 years | n=18 adenomasn=23 adenomas of pTis/CRC patientsn=6 pTis/CRCs | Gene copy number alterations | Not adjusted for multiple comparisons | 78 months | EGFR, MYC, NCOA3, and RAB20 amplifications correlate with adenoma recurrence |
| Pereira et al., 201651 | 50–75 years | n=480 controlsn=195 adenomas | 43 SNP in prostaglandin E2 pathway | Age, sex, smoking, false-positive report probability | 76 months | rs689466 in COX-2 (OR=3.2; 95% CI: 1.5–6.9), rs6439448 in SLCO2A1 (OR=0.4; 95% CI: 0.2–0.7) and rs1751051 in ABCC4 genes (OR=2.8; 95% CI: 1.6–4.8) were associated with adenoma recurrence |
| Macaron et al., 201650 | Mean age 59 years | n=179 with serrated polyps | ANXA10 expression | Age, sex, smoking, or family history of CRC. | 44 months | High ANXA10 increased risk of serrated polyps (HR=2.7; 95%: 1.01, 7.0) |
| Fiedler et al., 201947 | 50–88 years | n=59 adenomas with or without recurrencen=10 recurrent adenomasn=3 normal | DNA methylation | False discovery rates | 21 months | No differences in DNA methylation |
| Fiedler et al., 201948 | Median age 67 years | n=15 adenomas with recurrencen=15 adenomas without recurrencen=14 matched pair samples | Gene copy number alterations | Not adjusted for multiple comparisons | 20 months | Gains of CDX2 were seen in primary adenomas with recurrenceNo differences in the clonal composition |
| Hua et al., 202049 | 20–75 years | n=553 individuals with serrated polyps (n=420 hyperplastics) | DNA methylationBRAF-mutations | Age, sex, smoking, body mass index, number of baseline polyps | 72 months | Loss of MLH1 was a predictor of serrated polyps’ recurrenceNo differences in BRAF-mutations |
| Murcia et al., 202153 | Mean age 65 years | n=281 patients with high-riska lesions | DNA methylationKRAS- and BRAF-mutations | Age, sex, smoking, previous CRC, KRAS- and BRAF-mutations, number and histology of baseline polyps | 36 months | CIMP status was a predictor of recurrence (OR=4.50, p=0.002)No differences in KRAS- and BRAF-mutations |
| Carot et al., 202154 | 50–69 years | n=537 patients with high-riskb lesions | KRAS, NRAS, BRAF, APC, TP53, FBXW7, CTNNB1, SMAD4, Ki-67, MLH-1, CYTOKERATIN 7, CYTOQUERATIN 20, and CDX2 genes | Age, sex, smoking, metabolic syndrome, previous CRC, gene mutations, number and histology of baseline polyps, among others. | 36 months | Loss of MLH1 was a predictor of recurrence of high-risk lesions (OR 4.59; 95% CI: 1.4–14.6)No differences in other genes |
Under the hypothesis that about 80% of CRC show aneuploidy, Habermann et al.52 investigated if gene copy number alterations predicted metachronous adenoma. They compared 18 adenomas of patients without subsequent CRC and 23 adenomas of patients with subsequent CRC. By measuring the ploidy status of the samples and specific genes copy number, they found that TP53 deletion was more frequently observed in adenomas from patients with subsequent CRC. Also, they concluded that genomic instability in adenomas reflected by EGFR, MYC, NCOA3 and RAB20 amplification were indicative of metachronous neoplasia. Also investigating copy number alterations, Fiedler et al.48 compared fifteen primary adenomas with recurrence, fifteen adenomas without recurrence, and fourteen matched pair samples (primary adenoma and the corresponding recurrent adenoma) by using array-comparative genomic hybridisation (aCGH) and single-cell multiplex-interphase fluorescence in situ hybridisation (miFISH). No differences between groups were observed in the clonal composition of adenomas neither in the average number of copy number alterations. However, gains in CDX2 gene were exclusively seen in primary adenomas with recurrence compared to primary adenomas without recurrence. The same group analysed aberrant methylation of DNA in colorectal polyps47 comparing 59 adenomas with and without recurrence using the Illumina Human Methylation 450K BeadChip array. As a result, no significantly differentially methylated CpG positions were identified comparing primary adenomas with and without recurrence. However, the functional analysis showed that differentially methylated CpG positions of recurrent adenomas were predominantly associated with the immune system and inflammation. Two recent investigations have also analysed the relationship between somatic hypermethylation in colonic polyps and the risk of developing metachronous advanced colorectal lesions. Murcia et al.53 found that patients with CIMP+polyps exhibited shorter time to metachronous advanced lesions development even with adjustment for polyp size and number in 281 consecutive patients. CIMP status improved the metachronous advanced lesions risk estimation compared to the classical risk factors alone, especially the sensitivity. Carot et al.54 examined genes most commonly involved in colorectal carcinogenesis (KRAS, NRAS, BRAF, APC, TP53, FBXW7, CTNNB1, SMAD4, Ki-67, MLH-1, CYTOKERATIN 7, CYTOQUERATIN 20, and CDX2) and clinical covariates and the risk of metachronous adenomas or serrated polyps in 537 patients. Only the loss of nuclear expression of the MLH1 gene increased the risk of proximal advanced adenomas and multiplicity and HRLs. A susceptibility study by Pereira et al.51 analysed polymorphisms (43 tagSNPs) in Prostaglandin E2 pathway under the hypothesis that deregulation of prostaglandin levels is a key step in early stages of cancer development. They included 480 unscreened individuals and 195 patients with personal history of adenomas and found polymorphisms associated with adenoma development in COX-2, HPGD, SLCO2A1, and ABCC4 genes. Finally, two studies were focused on serrated polyps. First, Macaron et al.50 studied the role of ANXA10 expression as a marker of development of subsequent polyps at follow-up colonoscopy. They stained 179 serrated polyps for protein ANXA10 expression and found that patients with high levels of expression were at an increased risk of metachronous serrated neoplasms. Secondly, Hua et al.49 analysed the prevalence of BRAF mutation, CpG island methylator phenotype, and MLH1 methylation in 553 subjects with serrated polyps (420 hyperplastic polyps and 133 sessile serrated polyps) and 795 subsequent colonoscopies. They reported that BRAF-mutation and a CpG island methylator phenotype-high were not associated with subsequent advanced neoplasia. Among sessile serrated polyps, MLH1 methylation was a predictor of metachronous advanced neoplasia.
Another approach to find molecular biomarkers of metachronous advanced neoplasia is the use of high-magnification chromoendoscopy to identify aberrant crypt foci. Rectal aberrant crypt foci are preneoplastic lesions and play a role in CRC carcinogenesis. However, a recent large-scale study56 demonstrated that there was no consistent morphological characteristic complicating the use of aberrant crypt foci as biomarkers for CRC risk.
Future directions for surveillance colonoscopyThe current recommendations for post-polypectomy surveillance are based solely on the characteristics of the polyps and do not take into account patient-related factors. Robertson et al.57 reviewed participants’ characteristics (age, race, sex, smoking, obesity and others) that increase the risk of developing polyps and cancer. These same characteristics could also be used to personalize surveillance but there is little evidence that lifestyle factors increase the risk of metachronous neoplasia.54,58–61 Additionally, multiple models have been developed to stratify the risk of metachronous neoplasia and guide surveillance with little additional value compared to current criteria.62–70 Tailored screening and surveillance are challenging to implement given the difficulty in obtaining accurate information of the population. Moreover, complicating screening and surveillance recommendations could decrease overall participation in screening and adherence of surveillance guidelines. Further studies are needed to understand whether potential risk factors might influence CRC risk after an index colonoscopy.
There are few studies71–76 that have investigated whether performing a FIT before a scheduled colonoscopy (for symptoms or a personal or familiar history of CRC) would result in detection of advanced neoplasia. Lane et al.72 performed colonoscopy either following positive FIT or, in those testing FIT negative, which allowed to calculate the performance of annual FIT in surveillance. Sensitivity of repeated FIT was 86% for CRC and 63% for advanced adenomas. Interval examinations using the FIT detected neoplasia sooner (median of 25 months) than scheduled surveillances. Digby et al.77 reported that detectable faecal haemoglobin had negative predictive values of 99% for CRC and 97% for CRC plus higher-risk adenoma. All these studies suggest that the FIT could be a useful tool for surveillance following polyp removal. However, only the study of Cross et al.78 has examined the performance of the FIT in patients undergoing post-polypectomy surveillance. They evaluated the use of annual FIT in intermediate risk patients (with three to four adenomas<10mm or with one to two adenomas ≥10mm) undergoing 3 yearly surveillance colonoscopies in a CRC screening programme. With a positivity threshold of 40μg Hb/g faeces replacing 3-yearly colonoscopies with annual FIT would result an 87% reduction in colonoscopies. However, such an approach could miss up to 40% of CRCs and 70% of advanced adenomas. At the lowest threshold of 10μg Hb/g faeces, this strategy could miss up to 30% of CRCs and 40% of advanced adenomas. Regardless of the cost savings, such an outcome is likely to be deemed unacceptable. Symonds et al.79 suggests using the quantitative FIT value to personalise the frequency of colonoscopy rather than omit colonoscopy altogether. Completing an FIT prior to surveillance colonoscopy could provide the underlying risk of advanced neoplasia, and could tailor the scheduling of colonoscopy.77 In fact, the FIT has been proved to be useful to triage patients with lower abdominal symptoms for CRC80–83 and this strategy is being used to mitigate the impact of delays during the COVID-19 pandemic.84 Indeed, some endoscopic units have started to determine levels of faecal haemoglobin to prioritize colonoscopies for symptomatic patients85 as the amount of faecal haemoglobin is related to severity of disease. In fact, a faecal haemoglobin threshold of 150μg Hb/g faeces has been proposed as a rule in criteria for urgent evaluation.83,86,87 We would have to wait to new studies such as the Polyprev88 trial which is designed to evaluate the potential of annual FIT for CRC surveillance of patients with advanced colonic lesions instead of surveillance colonoscopy.
DiscussionThe aim of surveillance colonoscopy is to reduce the risk of CRC. Yet, we lack high quality evidence that post-polypectomy surveillance decreases long-term CRC incidence and mortality. Currently, guidelines identify which baseline findings are highly predictive of subsequent advanced neoplasia or CRCs, and do not take into account performance quality of endoscopists. In fact, it is possible that a high-quality baseline colonoscopy, with detection and complete removal of polyps, protects against subsequent cancer, and that surveillance may have very little added effect. The results of a 10-year follow-up study after screening colonoscopy proposed that surveillance should be informed by performance quality of endoscopists and characteristics of removed adenomas.89
The reasons for the recent changes in post-polypectomy surveillance guidelines are mainly related to the high prevalence of polyps with the widespread of CRC screening programs and the implementation of quality assurance and high-definition colonoscopy. This improvement simultaneously leads to an overestimation of the risk of CRC detection. Consequently, the risk of CRC incidence and mortality is ranked as a more relevant outcome than the risk of metachronous advanced neoplasia for estimating the benefit of post-polypectomy surveillance. The guidelines were also necessary to incorporate new evidence into the clinical recommendations of serrated polyps, multiplicity or villous histology. They all share the aim towards more selective and less frequent surveillance but there are small discrepancies despite having reviewed almost the same body of evidence. Although it will take time to evaluate which are the best recommendations, we already have data to state that some of the differences have virtually no repercussions. For example, although the guidelines vary in their approach on the tubulovillous/villous histology risk factor, a study that pooled data from eight prospective studies observed that size may be more important than villous features for predicting metachronous advanced adenomas.15 However, this difference may be not be important, because many polyps with villous elements are more than 1cm in size. Moreover, all guidelines have included recommendations for serrated polyps but only the US-MSTF base surveillance intervals on serrated polyp subtype. A recent study90 has evaluated the implications of the different criteria used to determine surveillance of serrated polyps according to the serrated polyp subtype. They found that most subjects were recommended identical colonoscopy surveillance intervals whether following the ESGE or US-MSTF guidelines suggesting that surveillance recommendations do not need to consider the serrated polyp subtype.
Identifying patients who are at high risk for subsequent advanced neoplasia can help to determine who could require shorter surveillance intervals. However, we believe that the population that would benefit from surveillance could be selected even more. The optimization of post-polypectomy surveillance intervals in patients with different categories of CRC risk is challenging. This is reflected by the continuous variations in the recommendations over time. Since the National Polyp Study,16 each update has led to increase the interval length. Fortunately, the European Polyp Surveillance (EPoS) trials,91 which were designed to determine the optimal surveillance strategy and to address the possibility of extending the surveillance interval, are now underway. Until we have the results from these trials, a microsimulation study13 has evaluated the additional benefit of colonoscopy surveillance in the Dutch population screening program. Adding surveillance to FIT-screening reduced mortality by an additional 2% (52% with surveillance and 50% without surveillance) but increased lifetime colonoscopy demand by 62%. They conclude that adding surveillance to FIT-screening was not cost-effective. Another study,92 which analysed failures in the screening process, found that surveillance accounted for only 1% of CRC deaths. Finally, in the PLCO colonoscopy cohort the surveillance colonoscopy was estimated to prevent 30% of CRC cases during 10 years of follow-up.93
Several approaches have been developed to improve post-polypectomy surveillance. In reviewing the literature, very little was found about molecular biomarkers of metachronous advanced neoplasia to improve risk stratification for post-polypectomy colonoscopy surveillance. We believe there is insufficient data to assess clinical utility of the biomarkers reviewed before they are incorporated into practice. Studies to date involved small cohorts and only one has been validated using bootstrap resampling. Given the low cost associated with performing an immunohistochemical staining, the most cost saving biomarker analysed might be ANXA10. However, its use would be limited to predict metachronous serrated polyps which are less frequent polyps than adenomas. Nowadays, we are a long way from using biomarkers to predict metachronous neoplasia therefore all the post-polypectomy recommendations are based on the histologic and endoscopic features of the polyps detected.
The strategy that is more likely to be implemented but added to colonoscopy would be the FIT. Colonoscopy is an expensive procedure and negative colonoscopies provide no therapeutic benefit other than reassurance while contributing to risk and cost. The FIT is very cheap and non-invasive but it seems that it could not substitute a colonoscopy. However, given that there is significant debate over the recommended interval between colonoscopies within surveillance programmes, the implementation of the FIT could improve the outcomes of surveillance. Additional studies to confirm the role of the FIT in predicting metachronous neoplasia and evaluating the FIT performance in the recently defined “high-risk” group are needed. Moreover, further studies should investigate whether molecular stool testing in addition to the FIT could improve sensitivity.94,95
The different lockdown periods resulted in the cancellation of elective gastrointestinal endoscopies as only emergent endoscopies were performed (75% to 100% endoscopy volume reduction96). Now, endoscopic units face the immense challenge of rescheduling the lockdown endoscopy patient list into the already overloaded endoscopy agenda. Gastroenterology societies85,96,97 recommended prioritizing endoscopy procedures of symptomatic patients or those at higher risk of gastrointestinal cancer based upon previous examination findings (such as a positive FIT). Conversely, post-polypectomy surveillance patients were recommended to be postponed.96
The COVID-19 lockdown and the consequently fall in endoscopy activity has been associated with a reduction in CRC incidences during and after the lockdown.98–100 CRC population screening programs have been paused or markedly curtailed in most countries85 and it is proved that screening delays beyond 5 months increase advanced CRC cases.101,102 Disruption of the CRC screening programmes will have a marked impact on colorectal cancer incidence and deaths between 2020 and 2050 attributable to missed screening.103 However, this unprecedented period may provide an opportunity to better allocate resources. We should think about what proportion of endoscopy capacity is allocated to screening, to diagnosis and to surveillance, and how to maximize its yield with limited capacity. Given the need for colonoscopy in screening and diagnosis, it makes sense to decrease surveillance colonoscopies. The diagnostic yield of a surveillance post-polypectomy colonoscopy is small and a greater impact would be achieved by performing FIT-positive diagnostic confirmatory colonoscopies.13 Indeed, we should not forget that what has been shown to improve CRC survival is screening3,104 as opposed to post-polypectomy surveillance.
To mitigate the impact of the CRC screening delay due to COVID-19, we should use as soon as possible the new post-polypectomy surveillance guidelines as their implementation would imply a huge reduction of colonoscopies. According to their recommendations, a lower number of individuals meets surveillance criteria (81% under 2010s criteria needed surveillance compared with only 19% under 2020s criteria43). Furthermore, introducing a suggested limit age for performing surveillance colonoscopy will further reduce demand on overburdened colonoscopy capacity. To reduce the inadequate demand for colonoscopies, dissemination and implementation of post-polypectomy surveillance guidelines should be increased, because it is already known that the average adherence to recommended surveillance colonoscopy intervals is less than 50%.105 Moreover, an update of the European guidelines for quality assurance in CRC screening and diagnosis25 and a subsequent rapid implementation is also needed.
In conclusion, the implementation of the new post-polypectomy surveillance guidelines reduces the number of colonoscopies of the endoscopy units so they could reallocate resources and prioritize follow-up colonoscopies after a positive FIT. However, further research is needed in order to better select high-risk subjects of metachronous advanced neoplasia (for instance, identifying biomarkers) and make the best use of available resources and avoid unnecessary colonoscopies.
Authors’ contributionsGemma Ibáñez-Sanz and Montse Garcia conceived the idea for the article and performed the literature search; Gemma Ibáñez-Sanz, Rebeca Sanz-Pamplona and Montse Garcia drafted and critically revised the work. All the authors agreed to submit the article for publication.
FundingThis study was funded by Instituto de Salud Carlos III through project PI16/00588 (co-funded by the European Regional Development Fund (ERDF), a way to build Europe) and Secretaria d’Universitats i Recerca del Departament d’Empresa i Coneixement de la Generalitat de Catalunya, grant number 2017 SGR 1283. We thank CERCA Programme/Generalitat de Catalunya for institutional support.
Conflict-of-interestThe authors declare no conflicts of interest.
The members of the characterization and molecular subtyping of interval cancers in colorectal cancer screening research group (MSIC-SC) are as follows, in alphabetical order:
- ∘
Biobanc HUB-ICO-IDIBELL, L’Hospitalet de Llobregat, Barcelona, Spain: Susana López.
- ∘
Biomarkers and Susceptibility Unit, Oncology Data Analytics Programme, Catalan Institute of Oncology (ICO), L’Hospitalet de Llobregat, Barcelona, Spain: Rebeca Sanz-Pamplona.
- ∘
Cancer Screening Unit, Prevention and Cancer Control Programme, Catalan Institute of Oncology, L’Hospitalet de Llobregat, Barcelona, Spain: Gemma Binefa, Montse Garcia, Núria Milà, Carmen Vidal.
- ∘
Catalonian Health Service (CatSalut), Barcelona, Spain: Elvira Torné.
- ∘
Department of Gastroenterology, Bellvitge University Hospital, Hospitalet, Spain: Gemma Ibáñez-Sanz, Francisco Rodríguez-Moranta.
- ∘
Department of Pathology, Bellvitge University Hospital (HUB-IDIBELL), L’Hospitalet de Llobregat, Barcelona, Spain: Xavier Sanjuan, Mar Varela.
- ∘
Department of Pathology, Parc de Salut Mar, Barcelona, Spain: Mar Iglesias.
- ∘
Department of Pathology, Hospital Clínic, Barcelona, Spain: Míriam Cuatrecasas.
- ∘
Endoscopic Unit, Gastroenterology Department, Institut de Malalties Digestives i Metabòliques, Hospital Clínic, Barcelona, Spain: Maria Pellisé.
- ∘
Oncology Data Science Group, Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain: Fiorella Ruiz-Pace.
- ∘
Prevention and Cancer Registry Unit, Service of Epidemiology and Evaluation, Parc de Salut Mar, Barcelona, Spain: Francesc Macià.
- ∘
School of Nursing, University of Barcelona, Fundamental Care and Medical-Surgical Nursing Department, L’Hospitalet de Llobregat, Barcelona, Spain: Llúcia Benito.
We thank CERCA Program, Generalitat de Catalunya for institutional support.