This study aims to systematically review the performance of red blood cell distribution width to platelet ratio (RPR) in the diagnosis of significant or advanced fibrosis, and cirrhosis associated with hepatitis B virus (HBV).
MethodsThe relevant studies were comprehensively searched in English databases such as Web of Science, PubMed, EMBASE, Cochrane Library, as well as Chinese databases such as China National Knowledge Infrastructure, Wanfang Data from the inception to March 2021. Accuracy of RPR in diagnosing significant or advanced fibrosis and liver cirrhosis was assessed by area under the curve (AUC), pooled sensitivity and specificity, as well as positive and negative likelihood ratios. Stata 15.0 software was applied to analyze the data.
ResultsIn total, 13 literature met the requirements, including patients with significant fibrosis (n=1890), advanced fibrosis (n=645), and cirrhosis (n=499). The prevalence rates of significant fibrosis, advanced fibrosis and cirrhosis were 49.31% (range: 17.25–84.21%), 37.07% (range: 9.60–58.20%) and 2.18% (range: 2.78–44.19%), respectively. The AUCs for predicting significant fibrosis, advanced fibrosis, and cirrhosis by RPR were 0.73 (95%CI: 0.69–0.76), 0.80 (95%CI: 0.77–0.84) and 0.80 (95%CI: 0.76–0.83), respectively.
ConclusionRPR is of some diagnostic value to the prediction of HBV-related significant fibrosis, advanced fibrosis and cirrhosis. This conclusion is urgently needed to be verified by further multi-center studies of large sample size and rigorous design.
Este estudio tiene como objetivo revisar sistemáticamente la capacidad del cociente entre el ancho de distribución de los glóbulos rojos y el recuento plaquetario (RPR) para discriminar en pacientes con infección crónica por virus de la hepatitis B la existencia de fibrosis significativa, avanzada y cirrosis.
MétodosSe realizaron búsquedas exhaustivas de los estudios relevantes en bases de datos en inglés, como Web of Science, PubMed, EMBASE y Cochrane Library, así como en bases de datos chinas, como China National Knowledge Infrastructure y Wanfang Data, desde el inicio hasta marzo de 2021. La precisión de RPR en el diagnóstico de fibrosis avanzada y cirrosis hepática se evaluó mediante el área bajo la curva, la sensibilidad y la especificidad combinadas, así como las razones de probabilidad positiva y negativa. Se aplicó el software Stata 15.0 para analizar los datos.
ResultadosUn total de 13 publicaciones cumplieron con los requisitos, incluyendo pacientes con fibrosis significativa (n=1.890), fibrosis avanzada (n=645) y cirrosis (n=499). Las tasas de prevalencia de fibrosis significativa, fibrosis avanzada y cirrosis fueron del 49,31% (rango: 17,25-84,21), 37,07% (rango: 9,60-58,20) y 2,18% (rango: 2,78-44,19), respectivamente. El área bajo la curva para predecir fibrosis significativa, fibrosis avanzada y cirrosis por RPR fue 0,73 (IC 95%: 0,69-0,76), 0,80 (IC 95%: 0,77-0,84) y 0,80 (IC 95%: 0,76-0,83), respectivamente.
ConclusiónLa RPR tiene algún valor diagnóstico para la predicción de fibrosis significativa relacionada con el virus de la hepatitis B, fibrosis avanzada y cirrosis. Y esta conclusión debe ser verificada con urgencia mediante más estudios multicéntricos de gran tamaño de muestra y diseño riguroso.
Hepatitis B virus (HBV) infection, a crucial global health problem, had a prevalence rate of more than 3.5% worldwide in 2015.1 There are approximately 350 million chronic HBV infections in the world, of which about 240 million are chronic hepatitis B (CHB).1 The global mortality rate caused by viral hepatitis associated with HBV and hepatitis C virus (HCV) has risen rapidly, with ranking from 10th in 1990 to 7th in 2013.2 World Health Organization (WHO) has estimated that viral hepatitis is responsible for 1.34 million deaths in 20.3 Patients with decompensated cirrhosis without regular systematic treatment often experience unfavorable outcomes, with a 5-year survival rate of about 14–35%.4 Therefore, chronic HBV infection is still a vital factor affecting people's health at the present stage.
Abnormal deposition and increase of extracellular matrix resulting from chronic liver damage caused by different etiologies is the main pathological change of liver fibrosis.5 The physiological repair response after liver damage attributed to pathogenic factors is also an unavoidable stage in the development of chronic liver disease to liver cirrhosis.6 In recent years, a large number of clinical studies have confirmed that liver fibrosis may be reversed by effective treatments for liver protection, anti-virus and anti-fibrosis in the early stage of hepatic fibrosis.7,8 Therefore, the early diagnosis of liver fibrosis is critical, which plays a significant practical role in improving the quality of life of patients with chronic HBV infection.9
Liver biopsy is considered to be the gold standard for judging the degree of liver fibrosis. Nevertheless, as an invasive examination, it has potential risks, such as pain, bleeding, infection, etc.10 Moreover, the condition of liver fibrosis is dynamic, and repeated liver biopsy is not appropriate for judging disease development and treatment outcomes. In developed countries, non-invasive tests for liver fibrosis are commonplace, such as aspartate transaminase and alanine transaminase measurements.11,12 Simplifying fibrosis staging can improve efficiency in referrals from primary care to specialized care, especially in developing countries.
Serological models have been gradually applied to the diagnosis of liver fibrosis in recent years. Specifically, aspartate aminotransferase to platelet ratio index (APRI) and fibrosis-4 (FIB-4) have been widely studied as non-invasive diagnoses of liver fibrosis in CHB patients.13 In contrast to these unconventional laboratory indicators, whole blood cell count is a common item in laboratory tests. Studies have shown that routine blood parameters are related to the severity of many diseases and the risk of death.14–16 An increase of red blood cell distribution width is significantly correlated with fibrosis in CHB patients.17 Hence, the platelet count can be used as a serological index for the evaluation of liver fibrosis and liver cirrhosis.18 However, there is yet no clear conclusion about the relationship between the red blood cell distribution width to platelet ratio (RPR) and the degree of liver fibrosis in CHB patients. A meta-analysis by Cai et al.19 have shown that RPR has some value in predicting the severity of liver fibrosis in chronic liver disease patients, especially CHB. We have found a number of studies20–23 assessing RPR in CHB not included in the meta-analysis of Cai et al., so we present what we believe is a more complete picture of the available evidence on the use of RPR in CHB.
Therefore, in this study, we used the method of meta-analysis to explore the association between RPR and the severity of liver fibrosis in CHB patients, and comprehensively analyzed the diagnostic value of RPR in the severity of liver fibrosis in CHB patients.
MethodsWe conducted this meta-analysis in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA).24 Ethical approval was not needed because this is a meta-analysis.
Search strategy and study selectionEnglish databases such as PubMed, Web of Science, EMBASE, Cochrane Library, and Chinese databases such as China National Knowledge Infrastructure (CNKI) and Wanfang Data were searched to collect relevant literature on the diagnostic value of RPR for liver fibrosis in CHB patients up to March 2021. The search items were as follows: (“red blood cell distribution width” OR “RDW” OR “platelet” OR “PLT” OR “red blood cell distribution width to platelet ratio” OR “RPR”) AND (“hepatitis B virus” OR “HBV”) AND (“fibrosis” OR “cirrhosis”). There was no language limitation. Additionally, the references of identified studies and reviews were searched manually. Two researchers independently retrieved and finally cross-checked the literature.
Inclusion and exclusion criteriaInclusion criteria(1) Studies on the evaluation of the diagnostic value of RPR for fibrosis in patients with CHB. (2) Fibrosis was evaluated by the following scoring standard, such as METAVIR, Ishak, and Batts-Ludwig. In this study, significant fibrosis, advanced fibrosis, and cirrhosis were defined by METAVIR,25 Batts-Ludwig26 scoring system as stages F2-F4, F3-F4, and F4 or by Ishak27 scoring system as stages F3-F6, F4-F6, and F5-F6. (3) RPR was calculated as follows: RPR=RDW (%)/PLT(109/L). (4) The cut-off value was provided.
Exclusion criteria(1) Patients were co-infected with other hepatitis viruses or HIV. (2) Reviews, and non-original or unpublished articles. (3) The data were incomplete.
Data extractionThe variables collected were as follows: first author, year, country, number of cases and controls, scoring system for fibrosis, cut-off value, sensitivity, specificity. According to the number of patients and controls reported in the study, as well as sensitivity and specificity, the diagnostic characteristics of each study were calculated, such as false positive (FP), true positive (TP), true negative (TN), and false negative (FN).
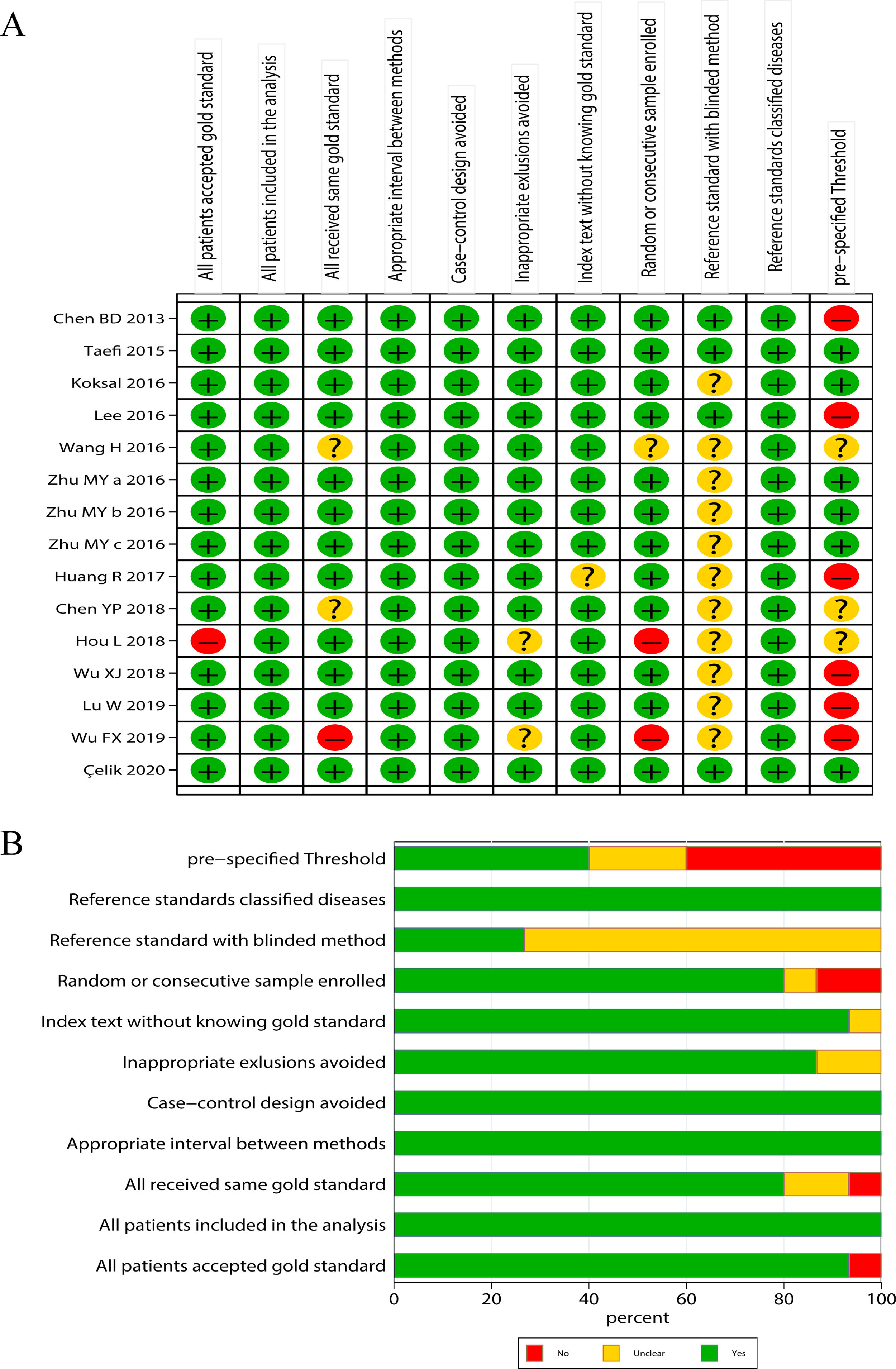
Literature quality evaluationThe qualities of the included studies were accessed by following the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2),28 a standard scale widely used for diagnostic accuracy in clinical trials. The scale includes 11 items, each evaluated as “yes”, “no” and “unclear”. “Yes” represents the literature meets this standard, indicating low risk; “no” represents the literature does not meet or mention this item, indicating high risk; “unclear” represents that the literature partially matched this item or item is partially matched, or it is unable to receive sufficient information from the study, indicating moderate risk.
Statistical analysisData were analyzed by Stata 15.0 statistical software. Heterogeneity was evaluated by using Cochran Q and I2 statistics. The threshold effect of RPR in predicting liver fibrosis in patients with CHB as assessed in accordance with the shape of the receiver operating characteristic (ROC) curve and the Spearman correlation coefficient between the sensitivity logarithm and 1-specificity logarithm. The sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) and diagnostic odds ratio (DOR) were calculated by the bivariate mixed-effect regression model. Publication bias was detected by using Deeks’ funnel plot test. Finally, sensitivity analysis was performed. The summary receiver operating characteristic (SROC) curve was constructed to describe the relationship between sensitivity and specificity. Meanwhile, AUC was calculated to measure RPR diagnostic performance in detecting CHB patients with significant fibrosis, advanced fibrosis and liver cirrhosis. When AUC≥0.70, it was considered to be a useful predictive index.29
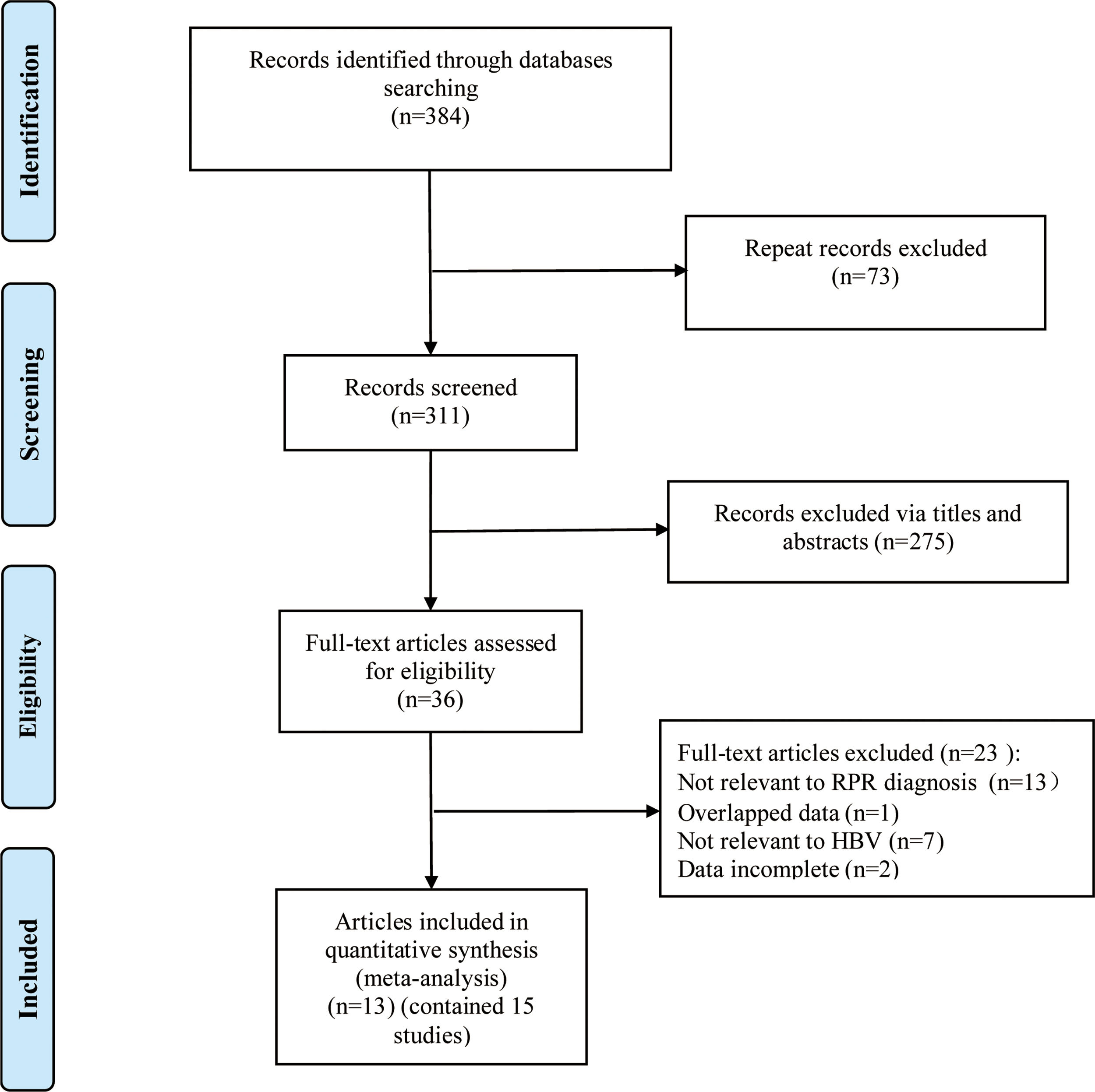
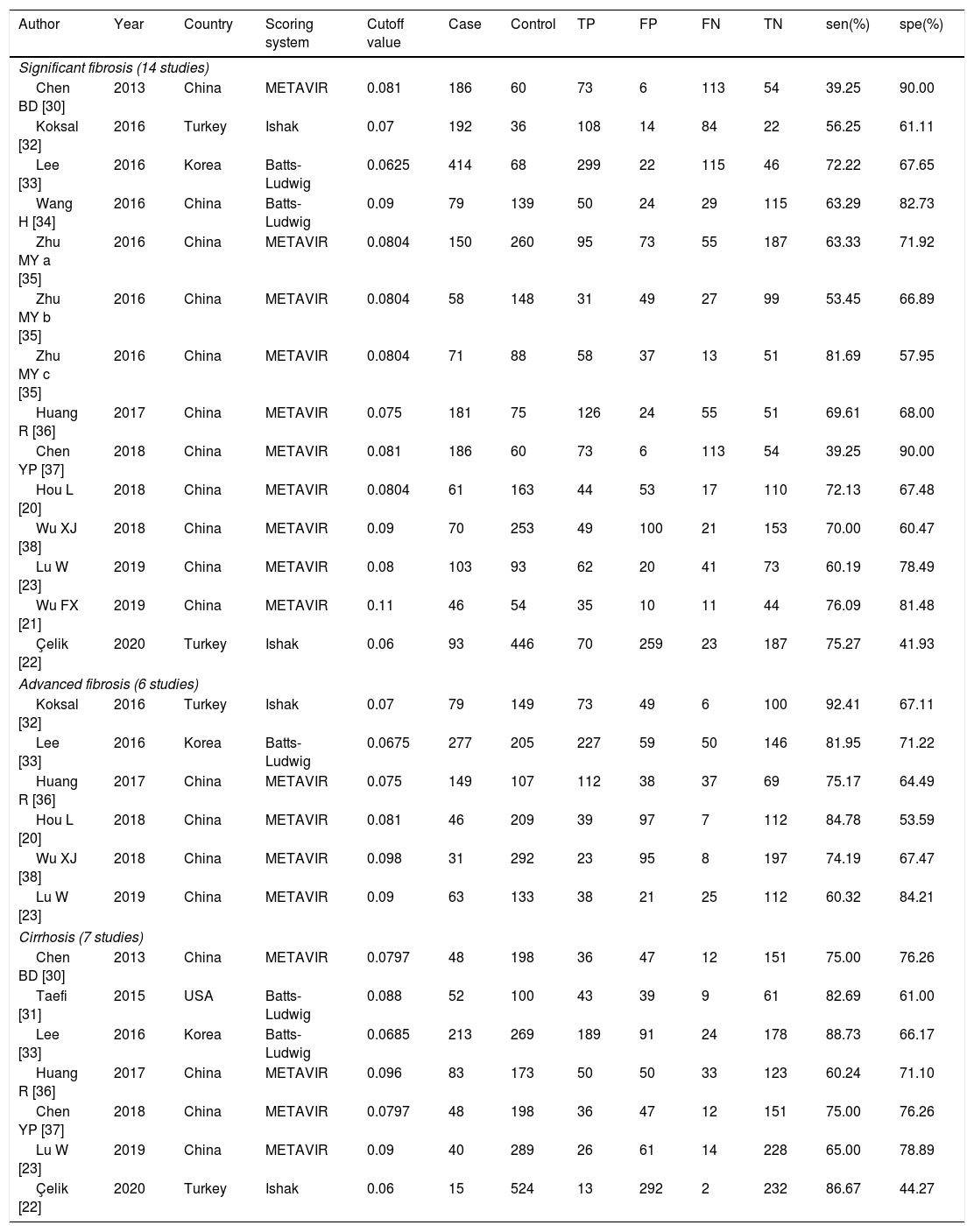
ResultsLiterature screening and characteristics of included studiesA total of 13 articles were included in this meta-analysis.20–23,30–38 The detailed flow diagram of literature screening is displayed in Fig. 1. Among the 13 included articles, 9 were from China,20,21,23,30,34–38 1 from South Korea,33 1 from the United States,31 and 2 from Turkey.22,32 Their histological scoring systems were different, including METAVIR score in 8 cases,20,21,23,30,35–38 Batts-Ludwig in 3 cases,31,33,34 and Ishak in 2 cases22,32. The prevalence rates of significant fibrosis, advanced fibrosis and cirrhosis were 49.31% (range: 17.25–84.21%), 37.07% (range: 9.60–58.20%) and 22.18% (range: 2.78–44.19%), respectively. The AUC for predicting significant fibrosis, advanced fibrosis and cirrhosis by RPR were 0.73 (95%CI: 0.69–0.76), 0.80 (95%CI: 0.77–0.84) and 0.80 (95%CI: 0.76–0.83), respectively. Results of quality assessment by QUADAS-2 are shown in Fig. 2(A, B). The basic characteristics of the included studies are shown in Table 1.
The basic characteristics of the studies included.
| Author | Year | Country | Scoring system | Cutoff value | Case | Control | TP | FP | FN | TN | sen(%) | spe(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Significant fibrosis (14 studies) | ||||||||||||
| Chen BD [30] | 2013 | China | METAVIR | 0.081 | 186 | 60 | 73 | 6 | 113 | 54 | 39.25 | 90.00 |
| Koksal [32] | 2016 | Turkey | Ishak | 0.07 | 192 | 36 | 108 | 14 | 84 | 22 | 56.25 | 61.11 |
| Lee [33] | 2016 | Korea | Batts-Ludwig | 0.0625 | 414 | 68 | 299 | 22 | 115 | 46 | 72.22 | 67.65 |
| Wang H [34] | 2016 | China | Batts-Ludwig | 0.09 | 79 | 139 | 50 | 24 | 29 | 115 | 63.29 | 82.73 |
| Zhu MY a [35] | 2016 | China | METAVIR | 0.0804 | 150 | 260 | 95 | 73 | 55 | 187 | 63.33 | 71.92 |
| Zhu MY b [35] | 2016 | China | METAVIR | 0.0804 | 58 | 148 | 31 | 49 | 27 | 99 | 53.45 | 66.89 |
| Zhu MY c [35] | 2016 | China | METAVIR | 0.0804 | 71 | 88 | 58 | 37 | 13 | 51 | 81.69 | 57.95 |
| Huang R [36] | 2017 | China | METAVIR | 0.075 | 181 | 75 | 126 | 24 | 55 | 51 | 69.61 | 68.00 |
| Chen YP [37] | 2018 | China | METAVIR | 0.081 | 186 | 60 | 73 | 6 | 113 | 54 | 39.25 | 90.00 |
| Hou L [20] | 2018 | China | METAVIR | 0.0804 | 61 | 163 | 44 | 53 | 17 | 110 | 72.13 | 67.48 |
| Wu XJ [38] | 2018 | China | METAVIR | 0.09 | 70 | 253 | 49 | 100 | 21 | 153 | 70.00 | 60.47 |
| Lu W [23] | 2019 | China | METAVIR | 0.08 | 103 | 93 | 62 | 20 | 41 | 73 | 60.19 | 78.49 |
| Wu FX [21] | 2019 | China | METAVIR | 0.11 | 46 | 54 | 35 | 10 | 11 | 44 | 76.09 | 81.48 |
| Çelik [22] | 2020 | Turkey | Ishak | 0.06 | 93 | 446 | 70 | 259 | 23 | 187 | 75.27 | 41.93 |
| Advanced fibrosis (6 studies) | ||||||||||||
| Koksal [32] | 2016 | Turkey | Ishak | 0.07 | 79 | 149 | 73 | 49 | 6 | 100 | 92.41 | 67.11 |
| Lee [33] | 2016 | Korea | Batts-Ludwig | 0.0675 | 277 | 205 | 227 | 59 | 50 | 146 | 81.95 | 71.22 |
| Huang R [36] | 2017 | China | METAVIR | 0.075 | 149 | 107 | 112 | 38 | 37 | 69 | 75.17 | 64.49 |
| Hou L [20] | 2018 | China | METAVIR | 0.081 | 46 | 209 | 39 | 97 | 7 | 112 | 84.78 | 53.59 |
| Wu XJ [38] | 2018 | China | METAVIR | 0.098 | 31 | 292 | 23 | 95 | 8 | 197 | 74.19 | 67.47 |
| Lu W [23] | 2019 | China | METAVIR | 0.09 | 63 | 133 | 38 | 21 | 25 | 112 | 60.32 | 84.21 |
| Cirrhosis (7 studies) | ||||||||||||
| Chen BD [30] | 2013 | China | METAVIR | 0.0797 | 48 | 198 | 36 | 47 | 12 | 151 | 75.00 | 76.26 |
| Taefi [31] | 2015 | USA | Batts-Ludwig | 0.088 | 52 | 100 | 43 | 39 | 9 | 61 | 82.69 | 61.00 |
| Lee [33] | 2016 | Korea | Batts-Ludwig | 0.0685 | 213 | 269 | 189 | 91 | 24 | 178 | 88.73 | 66.17 |
| Huang R [36] | 2017 | China | METAVIR | 0.096 | 83 | 173 | 50 | 50 | 33 | 123 | 60.24 | 71.10 |
| Chen YP [37] | 2018 | China | METAVIR | 0.0797 | 48 | 198 | 36 | 47 | 12 | 151 | 75.00 | 76.26 |
| Lu W [23] | 2019 | China | METAVIR | 0.09 | 40 | 289 | 26 | 61 | 14 | 228 | 65.00 | 78.89 |
| Çelik [22] | 2020 | Turkey | Ishak | 0.06 | 15 | 524 | 13 | 292 | 2 | 232 | 86.67 | 44.27 |
TP: true positive; FP: false positive; TN: true negative; FN: false negative; sen: sensitivity; spe: specificity
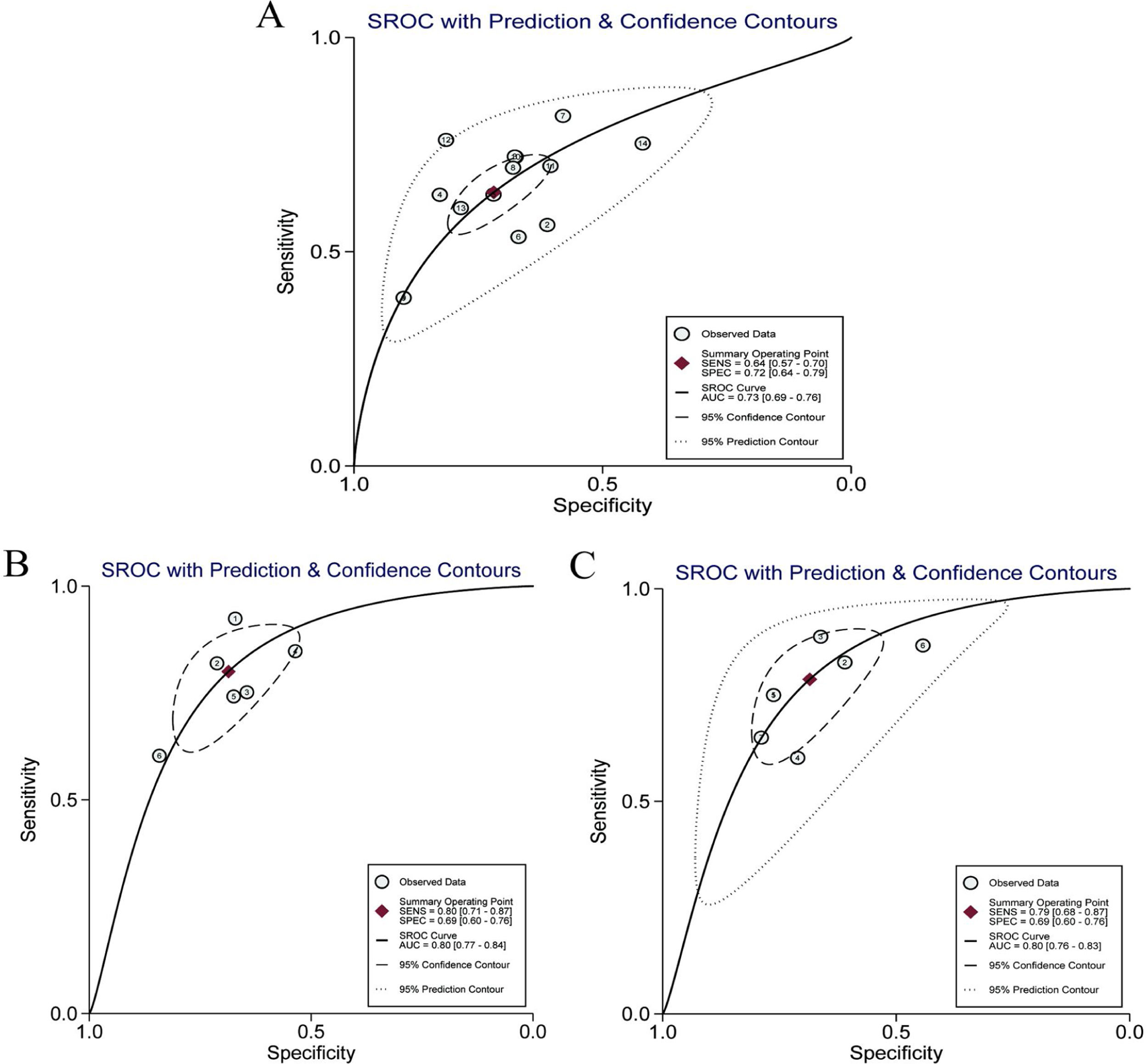
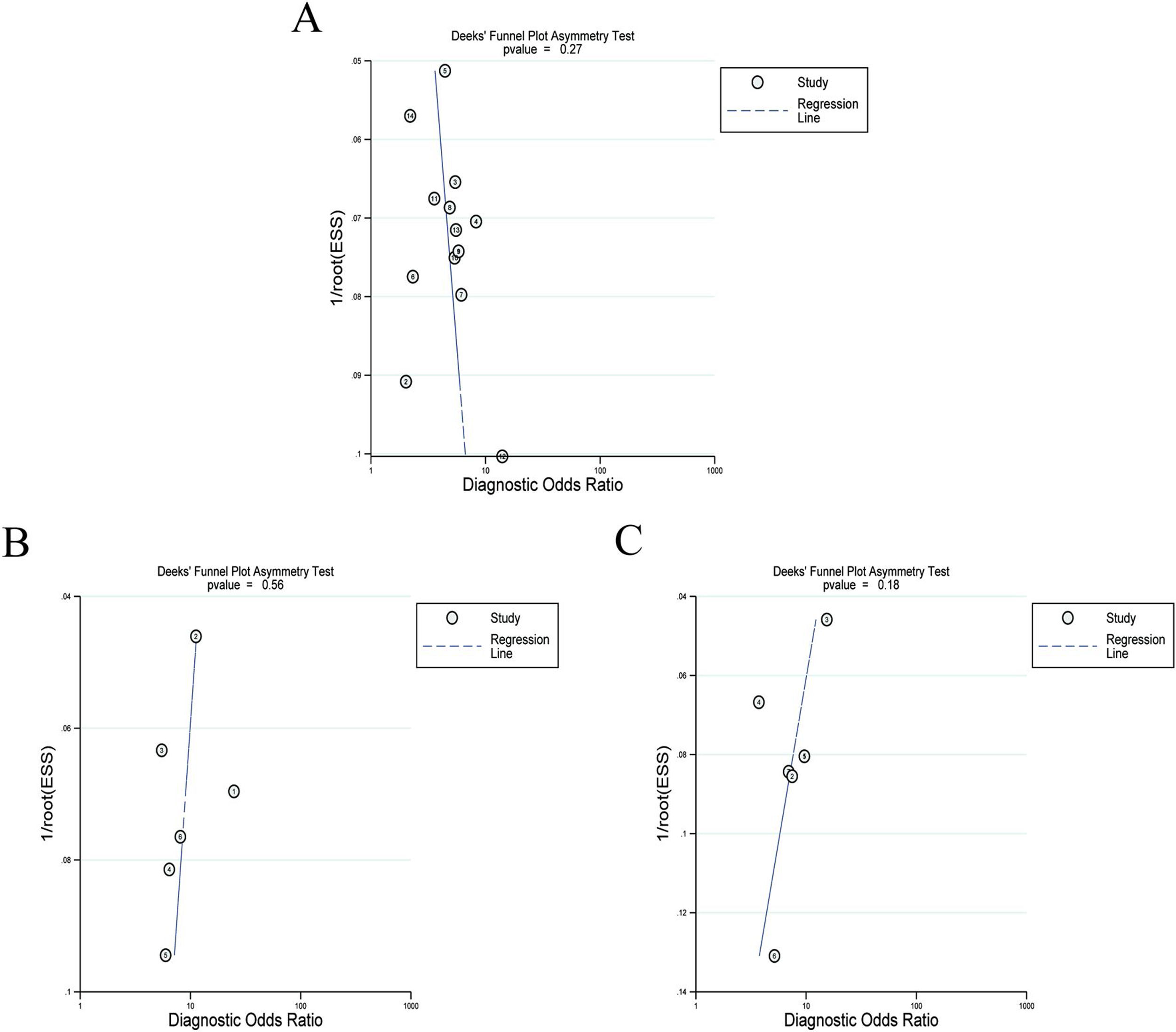
Twelve articles evaluated RPR in detecting significant fibrosis, including 14 trials and 1890 patients with HBV-related hepatic fibrosis. The results showed that the AUC value was 0.73 (Fig. 3A). By using the bivariate mixed-effect model, the pooled DOR was 5.00 (95%CI: 4.00–6.00) (Table 2). The ROC curve did not present a typical “shoulder-arm shape”, indicating no significant threshold effect (Spearman correlation coefficient: 0.529, P>0.05). The sensitivity, specificity, PLR, NLR and DOR are shown in Table 2. Deeks’ funnel plot revealed that RPR had no significant publication bias in detecting significant fibrosis (P>0.05) (Fig. 4A).
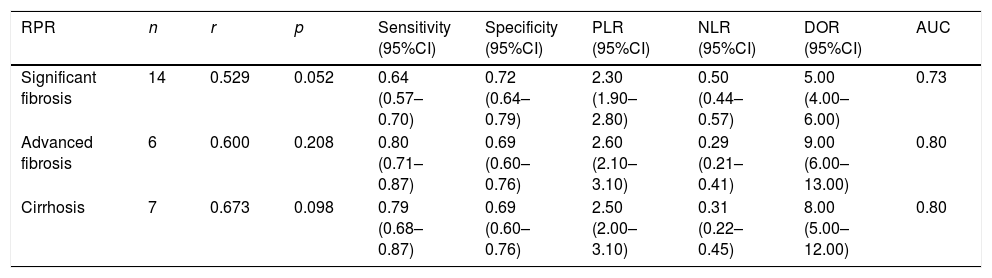
The pooled estimates of RPR index for the prediction of hepatitis B-related fibrosis.
| RPR | n | r | p | Sensitivity (95%CI) | Specificity (95%CI) | PLR (95%CI) | NLR (95%CI) | DOR (95%CI) | AUC |
|---|---|---|---|---|---|---|---|---|---|
| Significant fibrosis | 14 | 0.529 | 0.052 | 0.64 (0.57–0.70) | 0.72 (0.64–0.79) | 2.30 (1.90–2.80) | 0.50 (0.44–0.57) | 5.00 (4.00–6.00) | 0.73 |
| Advanced fibrosis | 6 | 0.600 | 0.208 | 0.80 (0.71–0.87) | 0.69 (0.60–0.76) | 2.60 (2.10–3.10) | 0.29 (0.21–0.41) | 9.00 (6.00–13.00) | 0.80 |
| Cirrhosis | 7 | 0.673 | 0.098 | 0.79 (0.68–0.87) | 0.69 (0.60–0.76) | 2.50 (2.00–3.10) | 0.31 (0.22–0.45) | 8.00 (5.00–12.00) | 0.80 |
RPR: red cell volume distribution width-to-platelet ratio; PLR: positive likelihood ratio; NLR: negative likelihood ratio; DOR: diagnostic odds ratio; AUC: area under the curve.
Six articles evaluated RPR in the detection of advanced fibrosis, including 645 patients with HBV-related hepatic fibrosis. The results showed that the AUC value was 0.80 (Fig. 3B). By utilizing the bivariate mixed-effect model, the pooled DOR was 9.00 (95%CI: 6.00–13.00) (Table 2). No typical “shoulder-arm shape” in the ROC curve was found, indicating no significant threshold effect (Spearman correlation coefficient: 0.600, P>0.05). Deeks’ funnel plot showed that RPR had no significant publication bias in predicting advanced fibrosis (P>0.05) (Fig. 4B).
Diagnostic accuracy of RPR for predicting cirrhosisSeven articles assessed RPR in the detection of cirrhosis, including 499 patients with HBV-related hepatic fibrosis. The pooled analysis results showed that the AUC value was 0.80 (Fig. 3C). By using the bivariate mixed-effect model, the pooled DOR was 8.00 (95%CI: 5.00–12.00) (Table 2). The ROC curve did not show a typical “shoulder-arm shape”, indicating no significant threshold effect (Spearman correlation coefficient: 0.673, P>0.05). Deeks’ funnel plot revealed that RPR had no significant publication bias in predicting cirrhosis (P>0.05) (Fig. 4C).
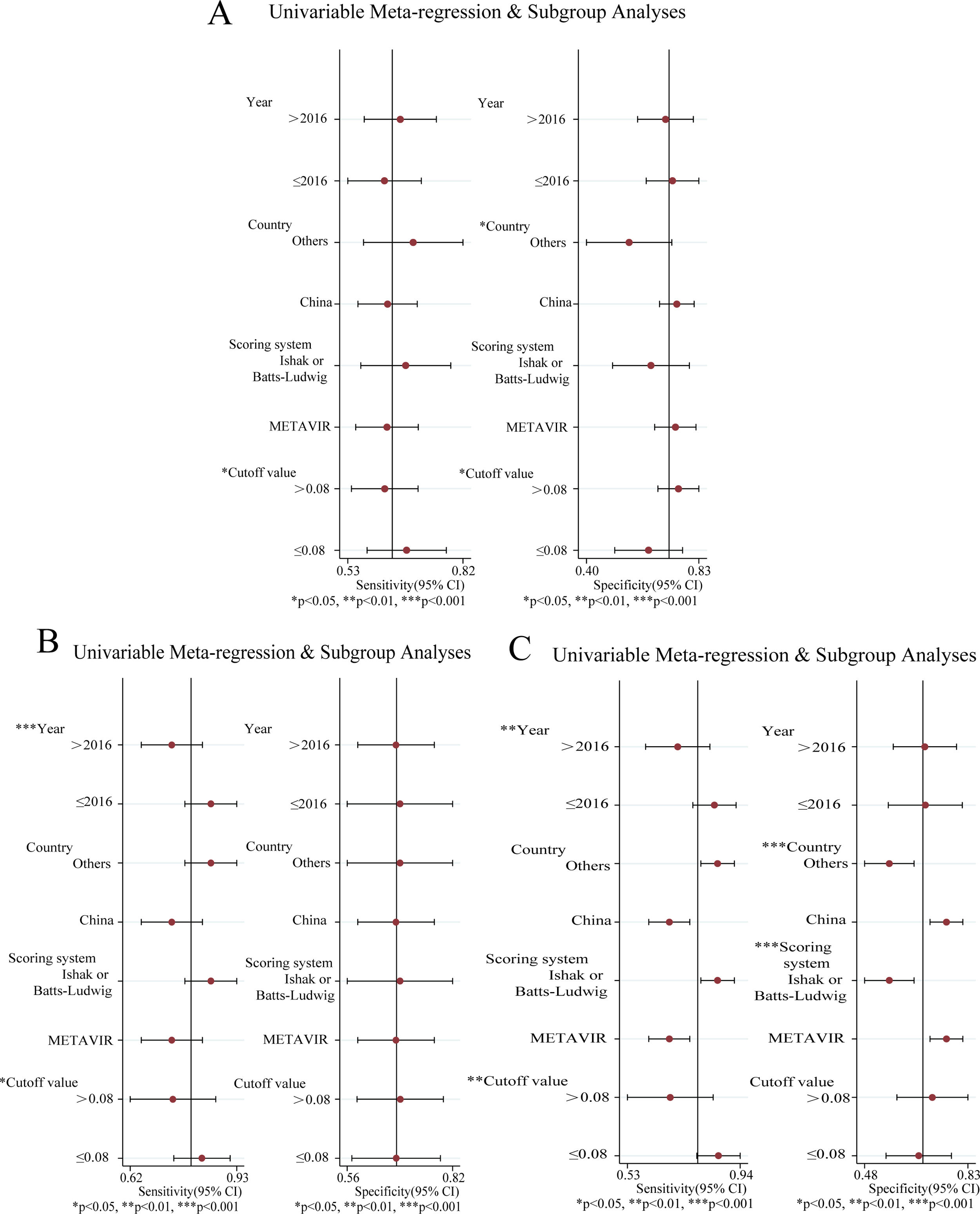
Analysis of heterogeneityConsiderable heterogeneity across the studies was identified (I2>50%) in the meta-analysis of RPR for predicting significant fibrosis, advanced fibrosis and cirrhosis. Therefore, meta-regression and subgroup analysis were adopted to explore potential sources of heterogeneity: publication year, country, scoring system, cut-off value (Fig. 5A–C). The results of significant fibrosis showed that there was no statistical significance in meta-regression (Fig. 5A). In the subgroup analysis, the specificity and sensitivity increased significantly when the country was China and the fibrosis scoring system was METAVIR (P<0.05), and the sensitivity increased markedly when the cut-off value was less than 0.08 (P<0.05). This demonstrated that country, scoring system and cut-off value could be the sources of heterogeneity.
The results of advanced fibrosis showed that the published year, country and scoring system were statistically significant in meta-regression (Fig. 5B). In the subgroup analysis, the sensitivity decreased significantly when the published year was later than 2016 and the cut-off value was larger than 0.08 (P<0.05). This indicated that the year of publication, country, scoring system and cut-off value could be the sources of heterogeneity.
The results showed that the country and the scoring system had statistical significance in meta-regression (Fig. 5C). In the subgroup analysis, the sensitivity was significantly increased when the published year was earlier than 2016 and the cut-off value was less than 0.08. The specificity was markedly increased when the country was China and the scoring system was METAVIR, (P<0.05). This demonstrated that the year of publication, country, scoring system and cutoff value could be the sources of heterogeneity.
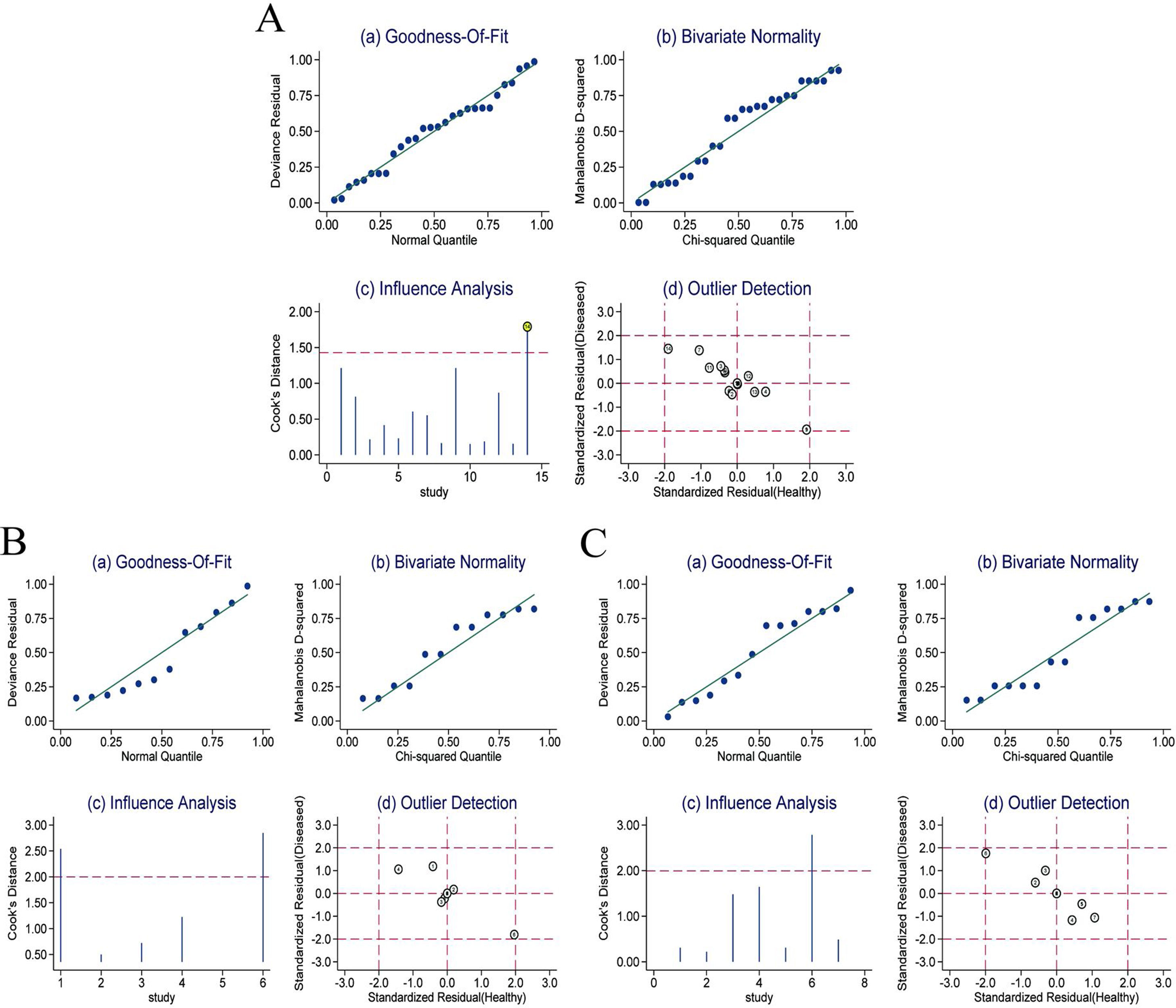
Sensitivity analysisThe sensitivity analysis of RPR in detecting significant fibrosis, advanced fibrosis and cirrhosis in CHB patients (Fig. 6A–C) showed that the goodness-of-fit and binary normality fitting were satisfactory (Fig. 6Aa, Ab; Ba, Bb; Ca, Cb). The sensitivity analysis identified a bias study in which a large weight may affect the robustness of the meta-analysis (Fig. 6Ac). After removing this study, no significant changes of the overall analysis was identified in sensitivity (0.67 vs. 0.63), specificity (0.71 vs. 0.74), PLR (2.30 vs. 2.40), NLR (0.46 vs. 0.50), DOR (5.00 vs. 5.00) and AUC (0.74 vs. 0.74). No outliers (Fig. 6Ad) were found. This suggested the meta-analysis of the diagnostic value of RPR in predicting significant fibrosis in CHB patients was robust. The sensitivity analysis and outlier test of RPR for predicting advanced fibrosis in CHB patients (Fig. 6Bc, Bd) and cirrhosis (Fig. 6Cc, Cd) did not find any deviation study that affected the robustness of the meta-analysis.
DiscussionThis meta-analysis was conducted to explore the role of RPR in predicting different degrees of fibrosis in CHB patients, showing moderate accuracy. As a non-invasive indicator, RPR had several advantages, such as convenient detection, easy quantification of parameters and simple calculation.22,23 Therefore, in recent years, RPR has attracted wide attention in predicting liver fibrosis.21,38 Early detection and evaluation of liver fibrosis are essential for the judgment of disease progression and treatment.
The AUC of diagnostic value of RPR for significant fibrosis, advanced fibrosis and liver cirrhosis in CHB patients were 0.73, 0.80, 0.80, respectively, which were all over 0.7, and the DOR were 5, 9 and 8, respectively, which were statistically significant. That is, the AUC and DOR were markedly increased from significant fibrosis to advanced fibrosis. The pooled sensitivity and specificity of RPR diagnostic accuracy ranged from 0.6 to 0.9, and the funnel plot was basically symmetrical, suggesting no significant publication bias in this meta-analysis.
The results of meta-regression and subgroup analysis showed that the variables including publication year, country, scoring system and cut-off value had different effects on heterogeneity and were likely to be the sources of heterogeneity. In the prediction of different degrees of liver fibrosis by RPR, the scoring system, in particular, had statistical significance in the meta-regression equation of both advanced fibrosis and liver cirrhosis. Cut-off value, another crucial factor, had a significant effect on sensitivity or specificity. Specifically, with 0.08 as a cut-off value, the sensitivity of the group with a value less than 0.08 was higher than that of the group with the value more than 0.08, with the differences being statistically significant. The specificity was correspondingly lower. This finding was suitable for the groups with significant hepatic fibrosis, advanced fibrosis, and cirrhosis. In other words, the group with a lower cut-off value had higher sensitivity. Therefore, exploring the appropriate cut-off value has critical clinical significance for predicting different degrees of fibrosis in CHB patients and improving the accuracy of diagnosis.
A sensitivity analysis was performed to investigate the robustness of RPR in diagnosing the severity of fibrosis in CHB patients. The results showed that the diagnostic performance of RPR did not change significantly after excluding a heavily weighted study. In the prediction of advanced fibrosis and liver cirrhosis in CHB patients with CHB, there were no studies that had remarkable impacts on the diagnostic performance. It verified that the conclusions of our study were robust.
Currently, there are numerous non-invasive indicators to diagnose liver fibrosis in CHB patients, such as APRI and FIB-4, among others. Xiao et al.39 have demonstrated that the AUC of APRI in predicting significant fibrosis, severe fibrosis, and cirrhosis in CHB patients were 0.72, 0.76, and 0.72, respectively, while the AUCs of FIB-4 are 0.76, 0.80, and 0.78, respectively. It can be seen that in predicting the severity of liver fibrosis in CHB patients, RPR is not inferior to APRI and FIB-4. Moreover, red blood cell distribution width and platelet count are routine detection indexes in routine blood, which are easy to operate and quantify. The predictive value of RPR in liver fibrosis is of great clinical significance. The meta-analysis by Cai et al.19 have showen that RPR had a moderate and good diagnostic value for significant fibrosis (AUC=0.73) and cirrhosis (AUC=0.84) in CHB patients, which were consistent with the conclusions of our study. Cai et al.19 showed that RPR had a moderate diagnostic value for severe fibrosis in CHB patients (AUC=0.73), while our study revealed that RPR had a good diagnostic value for severe fibrosis (AUC=0.80). Based on the inclusion of more studies, our study further verified the predictive value of RPR on the severity of liver fibrosis in CHB patients.
Although liver biopsy is still the “gold standard” for determining the staging of liver fibrosis, its extensive clinical application is limited due to its defects such as invasibility, sampling error, observer variation and related complications. RPR, a diagnostic model based on serum indicators, is one of the non-invasive alternative methods for evaluating liver fibrosis. Accurate assessment of the stage of CHB fibrosis is particularly significant for clinical treatment decisions, to prevent the progression of HBV-related diseases effectively.40 Therefore, the conclusions of this meta-analysis have crucial clinical significance for the establishment of the RPR diagnostic model and can reduce the need for liver biopsy in CHB patients.
This study still has some limitations. Firstly, the included studies only covered limited areas. Most studies were conducted in China, one in South Korea, one in the United States, and two in Turkey. However, there were few studies in Europe and the United States, and no studies in African countries. Thus this may limit the generalization of the conclusions to the caucasian population. Secondly, the number of the included studies, as well as the sample size, was relatively small, which was likely to affect the robustness of the conclusions. Thirdly, the studies included in this meta-analysis are only published literature, while the high-quality unpublished studies were not included, which will probably lead to some publication bias. Fourthly, different studies have applied several different fibrosis scoring systems, which would affect the determination of the severity of fibrosis and have some impact on heterogeneity.
In conclusion, RPR has moderate diagnostic accuracy in predicting significant fibrosis, advanced fibrosis and liver cirrhosis in CHB patients. Although the diagnostic accuracy of RPR is not high, it is worthy of being widely used in the clinics because of its great advantages, such as simplicity, clinical contexts where it would be of most use, etc. As this study still has limitations, it is urgently needed to conduct further multi-center studies of larger sample size, tobetter clarify the clinical significance of RPR in the diagnosis of liver fibrosis in CHB patients.
AuthorshipCMF, WZG, DZ, LJ: Critical revision of the manuscript; CMF, WZG, WJ, LJ: Substantial contribution to the conception and design of the work, manuscript drafting; CMF, DZ, SSL, WJ: Acquisition, analysis, and interpretation of the data; CMF, WZG, DZ, SSL, WJ, LJ: Revising the manuscript critically, final approval of the version to be published. All authors have read and approved the final manuscript.
Ethical approvalEthical approval was not needed because this is a meta-analysis.
Consent for publicationNot applicable.
Availability of data and materialThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
FundingOur study is supported by the Science and Technology Program Foundation of Shenzhen (Grant No: JCYJ20190812172005670), The National Natural Science Foundation of China (Grant No: 81860113), and The Medical Science and Technology Research foundation of Guangdong Province (Grant No: B2020057).
Conflict of interestsAll the authors declare that they have no conflict of interest.