To study the expression of defensin-5 (RD-5), soluble phospholipase A2 (sPLA2) and lysozyme in the intestine in a rat model of acute liver failure and its relationship with intestinal bacterial translocation (BT).
Patients and methodsSprague–Dawley (SD) rats were divided into two groups. The experimental group was divided into five subgroups according to the lapsing time after the model was established, which were designated accordingly as 8h, 16h, 24h, 48h, and 72h groups. Acute liver failure (ALF) model was induced by intraperitoneal injection of 10% d-galactosamine. The homogenates of mesenteric lymph nodes (MLNs), liver and spleen from each group were cultured in agar to determine the bacterial outgrowth. The mRNA expression of RD-5, sPLA2, lysozyme and the protein expression of sPLA2, lysozyme were determined.
ResultsNo bacteria grew in the organ cultures from the control group while experimental groups had positive cultures. Expression of the RD-5 and sPLA2 mRNA in the experimental groups gradually increased at early time points and peaked 16h after induction of ALF, then progressively decreased. The mRNA expression of lysozyme in the experimental group peaked at 8h after ALF induction, then progressively decreased. Similar results were obtained with Western blot and immunohistochemical staining.
DiscussionThe immune barrier function of the ileal mucosa in the rat model of acute liver failure was compromised as demonstrated by the decreased expression of RD-5, sPLA2 and lysozyme in Paneth cells along with increased intestinal bacterial translocation.
Estudiar la expresión de defensina-5 (RD-5), fosfolipasa A2 soluble (sPLA2) y lisozima en el intestino de un modelo de rata con insuficiencia hepática aguda y su relación con la traslocación bacteriana (TB) intestinal.
Pacientes y métodosSe dividieron ratas Sprague-Dawley® (SD) en 2 grupos. El grupo experimental se dividió en 5 subgrupos según el tiempo transcurrido desde que se estableció el modelo, y se designaron en consecuencia como grupos de 8, 16, 24, 48 y 72h. El modelo de insuficiencia hepática aguda (IHA) se indujo mediante inyección intraperitoneal de D-galactosamina al 10%. Se cultivaron homogeneizados de ganglios linfáticos mesentéricos (GLM), hígado y bazo de cada grupo en agar para determinar la proliferación bacteriana. Se determinaron la expresión de ARNm de RD-5, sPLA2 y lisozima, y la expresión de proteínas de sPLA2 y lisozima.
ResultadosEn los cultivos de órganos del grupo de control no creció ninguna bacteria, mientras que los grupos experimentales presentaron cultivos positivos. La expresión del ARNm de RD-5 y sPLA2 en los grupos experimentales aumentó gradualmente en los primeros momentos y alcanzó el máximo 16h después de la inducción de la IHA, para después disminuir de forma progresiva. La expresión de lisozima en el grupo experimental alcanzó el valor máximo 8h después de la inducción de la IHA y después disminuyó progresivamente. Se obtuvieron resultados similares con la inmunoelectrotransferencia y la tinción inmunohistoquímica.
DiscusiónLa función de barrera inmunológica de la mucosa ileal en el modelo de rata de insuficiencia hepática aguda se vio afectada, como lo demuestra la disminución de la expresión de RD-5, sPLA2 y lisozima en las células de Paneth junto con el aumento de la translocación bacteriana intestinal.
The mechanism of bacterial translocation due to acute liver failure and cirrhosis remains elusive. To date, several mechanisms have been proposed to explain the bacterial translocation occurring in these scenarios including bacterial overgrowth in the small intestine, change of the intestinal microecology, increased intestine permeability and decreased bile secretion in the intestine. However, these mechanisms are more related to the physical, chemical, and microbial barrier of the intestine with little connection with the immune barrier function of this organ.1,2
The majority of translocating bacterial are the normal commensal flora in the intestine, especially Escherichia coli and other bacilli that are frequently found translocating to the mesenteric lymph nodes. E. coli and other bacilli are also the most common bacteria that cause peritoneal infection in patients with severe acute liver failure and cirrhosis, which suggests that decreased host immunity is not sufficient in preventing bacterial translocation induced infection from occurring.3–5 Although sIg A has long been considered the key player in stopping bacterial translocation, the synthesis of sIg A is very slow and takes approximately 4–6 days, which is far behind the rapid reproduction of bacteria or viruses, and therefore, bacterial translocation cannot be effectively prevented. In contrast, intestinal epithelial cells are able to rapidly synthesize and secrete serials of antimicrobial peptides (AMPs) to stop the invasion of pathogens. Until now, it was unknown whether the innate immune functions of intestinal mucosa are altered in ALF. To answer this important question, in the current study we investigated the expression of defensin-5 (RD-5), soluble phospholipase A2 (sPLA2) and lysozyme secreted by intestinal Paneth cells and its relationship to bacterial translocation in ALF. Intraperitoneal injection of 10% d-galactosamine in rats may be considered as a model of acute liver failure.
The study investigates the expression of RD-5, sPLA2 and lysozyme in the intestine in a rat model of acute liver failure and its relationship with intestinal bacterial translocation.
MethodsEthics statementThe Sprague–Dawley (SD) rats were obtained from Nanchang Royo Biotech Co., Ltd. (Nanchang, China). All animal experiments were carried out in accordance with the guidelines of the First Affiliated Hospital of Nanchang University institutional committee for the care and use of laboratory animals and were approved by the First Affiliated Hospital of Nanchang University laboratory animal management ethics committee. The study was approved by the Institutional Review Board of First Affiliated Hospital of Nanchang University.
ModelSprague–Dawley rats were divided into two groups. The experimental group was divided into five subgroups according to the lapsing time after the model was established, which were designated accordingly as 8h, 16h, 24h, 48h, and 72h groups. Acute liver failure (ALF) model was induced by intraperitoneal injection of 10% d-galactosamine. The homogenates of mesenteric lymph nodes (MLNs), liver and spleen from each group were cultured in agar to determine the bacterial outgrowth. The mRNA expression of RD-5, sPLA2, lysozyme and the protein expression of sPLA2, lysozyme were determined. Rats were sacrificed by neck dislocation after the experiments.
Establishment of the AFL animal model and sample collectionFood and water were provided ad libitum. One week after purchase, the male Sprague–Dawley rats of 180–220g weight were randomly divided into the control and experimental groups. Eight rats in the control group were i.p. injected with 0.9% saline (1.2ml/100g). The rats in the normal control group were sacrificed after 72h. Forty rats in the experimental group were further divided into five subgroups according to the different time points when the data were collected after the model was established, which were designated as 8h, 16h, 24h, 48h, and 72h subgroups with 8 rats each. All of the rats in the experimental group were i.p. injected with a full dose of 10% d-galactosamine (1.2ml/100g). At the end of the experiments, the animals were sacrificed by neck dislocation after the experiments. The samples were then collected under sterile conditions. A total of 2ml of whole blood from the abdominal aorta was centrifuged at 1500rpm for 15min for serum isolation, and the isolated serum was stored at −8°C. The isolated mesenteric lymph nodes, liver and spleen were washed with PBS (0.1ml/0.1g) and then homogenized. A total of 100μl of the homogenate was inoculated into MacConkey and whole blood agar plates and incubated at 37°C. The plates were checked 24–48h later for bacterial outgrowth. After the liver and terminal ileum were washed with PBS, some part of each tissue was fixed with 10% neutral buffered formalin, embedded with paraffin and sectioned, and the other part of each tissue was snap frozen in liquid nitrogen and later stored in a −80°C freezer.
Histopathological examination of the liver and ileumThe haematoxylin and eosin (H.E) stained sections were examined for pathological changes.
Serological testsThe serum levels of ALT, AST, TBil, DBil and Alb were determined using a 7600 automatic biochemistry analyser.
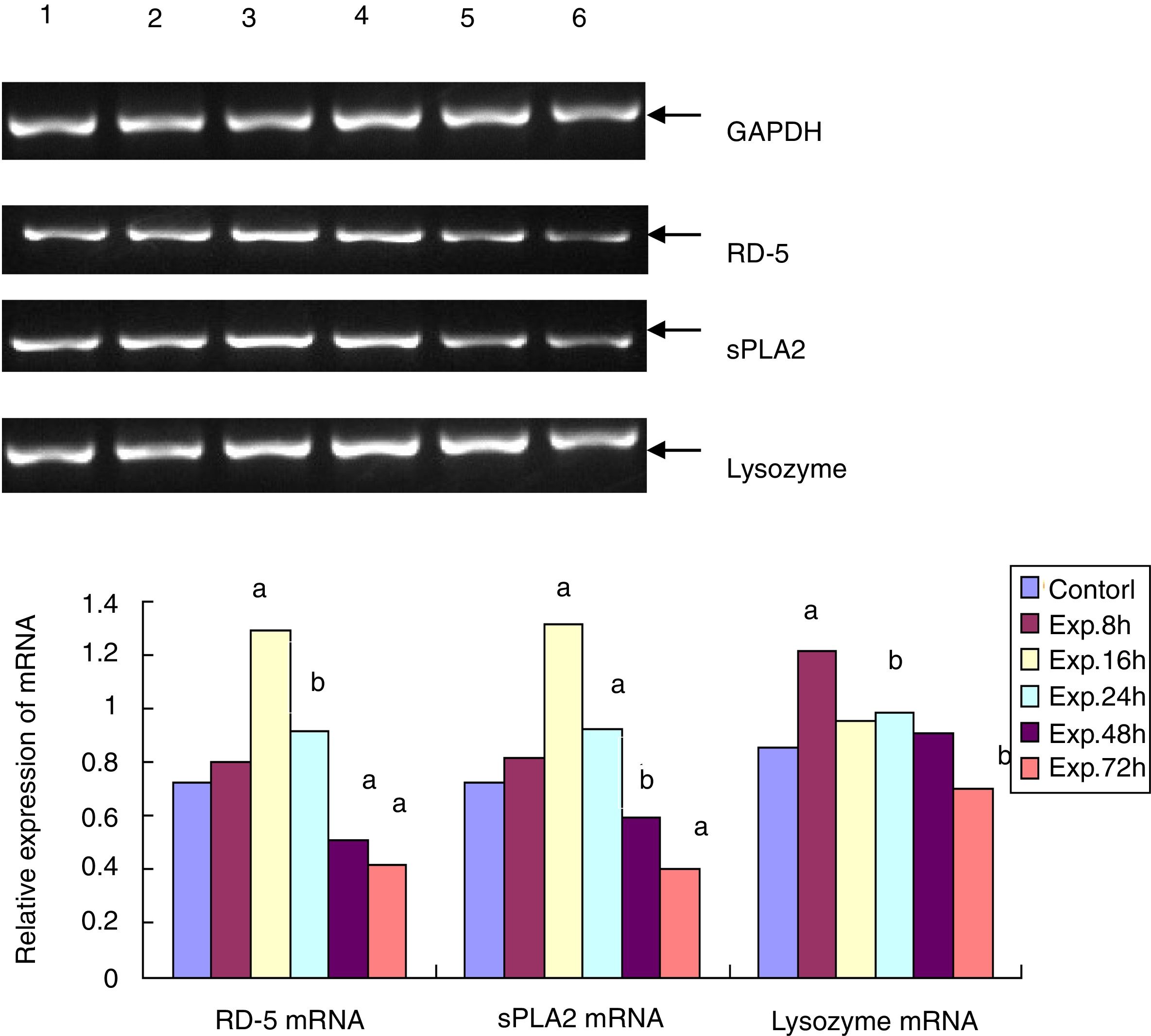
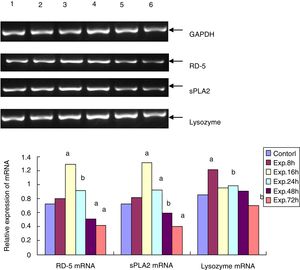
The RT-PCR for RD-5, sPLA2 and lysozyme mRNA levels in the terminal ileumThe total RNA was extracted from frozen ileum according to the manufacturer's instructions. After the concentration was determined using a spectrophotometer, the total RNA was transcribed into cDNA. With the sequence information in Genbank, the primers for RD-5, sPLA2 and lysozyme were designed with Primer Premier 5.0 and synthesized by Shanghai Bioengineering Company in China. The primers for RD-5 are 5′-agcactccgaccaagaagaat-3′ and 5′-ggaagaggaaactcaaggacat-3′, and the cycling condition was 94°C 5min, 94°C 30s, 60°C 30s, and 72°C 20s for 33 cycles; the primers for sPLA2 are 5′-cagattggtgctgtgtgactc-3′ and 5′-cctcggtaggagaacttgtagg-3′, and the cycling condition was 94°C 5min, 94°C 30s, 57°C 30s, and 72°C 20s for 32 cycles. The primers for the lysozyme were 5′-caagccatacaatgtgcgaagagag-3′ and 5′-tgttggtttgaggggaaagcaag-3′, and the cycling condition was 94°C 5min, 94°C 30s, 57°C 30s, and 72°C 20s for 30 cycles. After the 1.5% agarose gel electrophoresis, the bands for the PCR products of RD-5, sPLA2, lysozyme and GAPDH were analyzed by the image analysis software Gel-Pro 3.1, and the average “A” value for each band was calculated. The relative mRNA expressing levels of RD-5, sPLA2 and lysozyme were expressed as the ratios of each target to GAPDH.
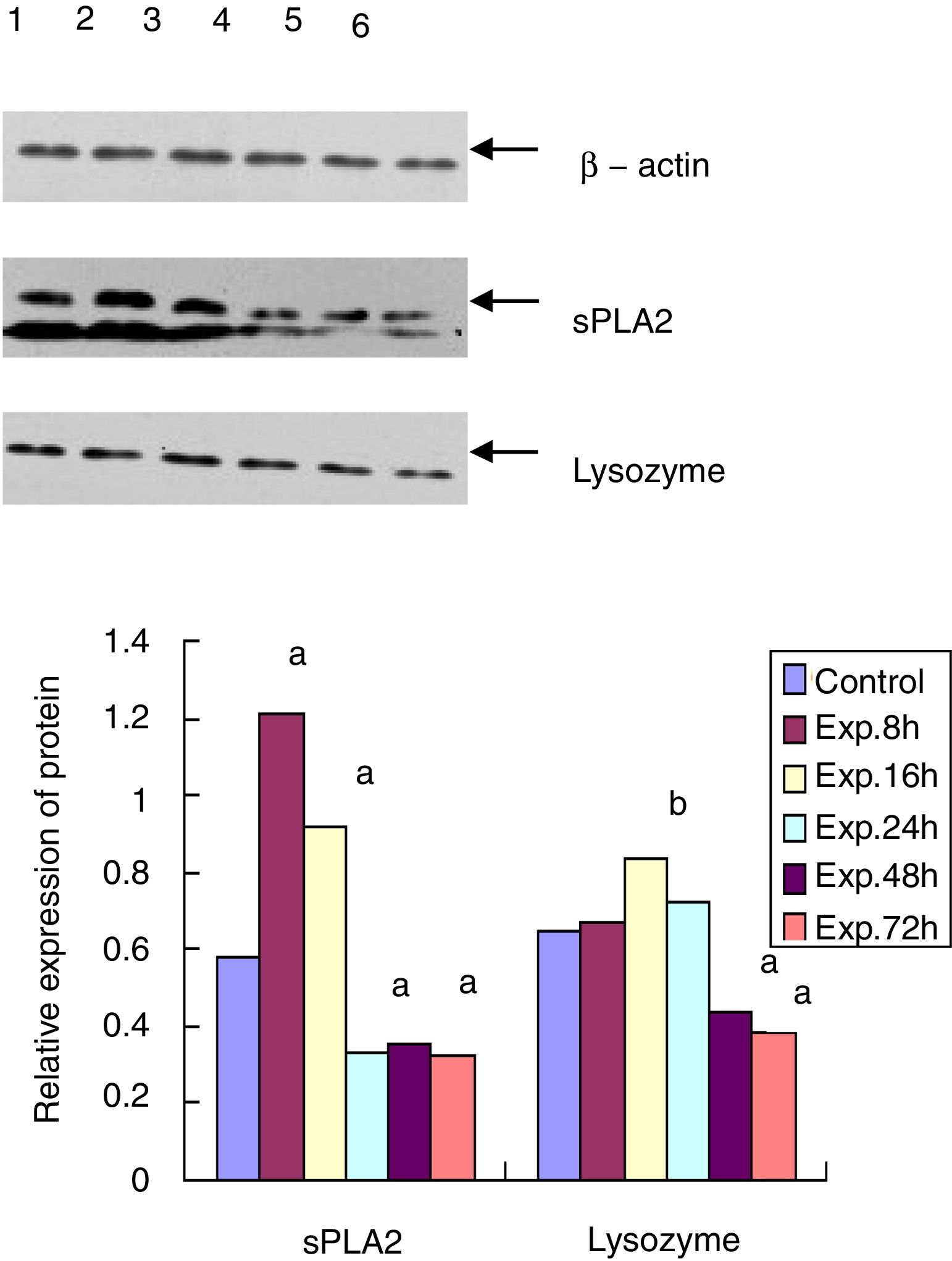
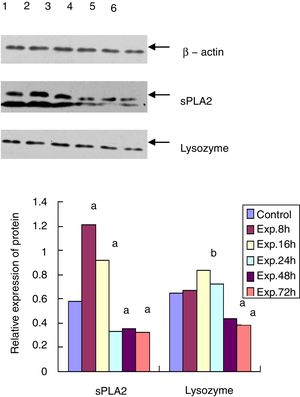
Western blot for protein expression of sPLA2 and lysozyme in the terminal ileumThe sample was lysed in the lysis buffer (20mmol/l NaCl, 10mmol/l Tris, 5mmol/l EDTA, 10% glycerol and 1% NP-40) and then centrifuged at 18,000×g for 15min at 4°C. A BCA assay was used to determine the protein concentration of the isolated supernatant. A total of 20μg of protein was mixed with 5× SDS loading buffer at a ratio of 1:4, boiled for 10min, and then cooled for 10min. After 15% of the SDS-PAGE gel electrophoresis and transfer, the PVDF membrane was blocked with 5% non-fat milk for 20min at room temperature. The membrane was then incubated with goat anti-mouse sPLA2 and lysozyme primary antibody at 1:500 dilution at 4°C overnight. After washing three times, the membrane was incubated with HRP conjugated mouse anti-goat secondary antibody (1:5000) for 3h at room temperature. After another 3 washes and incubation with the substrate, the membrane was exposed to X-ray film. β-actin was used as the internal control. The expression level of each protein was analyzed by Quantity One software.
The IHC for sPLA2 and lysozyme in the terminal ileum of the control group and the experimental 72h groupThe sections were fixed in acetone, washed with phosphate buffer, and incubated with a 2nd antibody at room temperature for 10min; the experimental group was incubated with the primary antibody at a dilution of 1:200 at 37°C for 30min, and this step was skipped for the control group. After treatment overnight at room temperature, the sections were incubated with DAB for the development of colour.
Statistical analysisSPSS 18.0 was used for the statistical analysis and the results were expressed as the mean±SD (x¯±s). All of the data were analyzed by a one-way ANOVA and post-tested with LSD for the data having equal variance or with Tamhane's T2 for the data having unequal variance. p<0.05 was considered statistically significant.
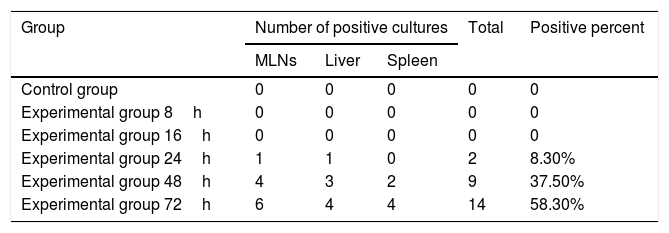
ResultsBacterial cultureNo bacteria grew in either the 8h or 16h experimental groups or in the cultures of mesenteric LNs, liver and spleen from the control group. The positive rates of 24h, 48h and 72h in the experimental groups were 8.3%, 37.5% and 58.3%, respectively (Table 1).
Results of bacterial cultures of MLNs, liver and spleen.
| Group | Number of positive cultures | Total | Positive percent | ||
|---|---|---|---|---|---|
| MLNs | Liver | Spleen | |||
| Control group | 0 | 0 | 0 | 0 | 0 |
| Experimental group 8h | 0 | 0 | 0 | 0 | 0 |
| Experimental group 16h | 0 | 0 | 0 | 0 | 0 |
| Experimental group 24h | 1 | 1 | 0 | 2 | 8.30% |
| Experimental group 48h | 4 | 3 | 2 | 9 | 37.50% |
| Experimental group 72h | 6 | 4 | 4 | 14 | 58.30% |
Note: MLNs refers to mesenteric lymph nodes.
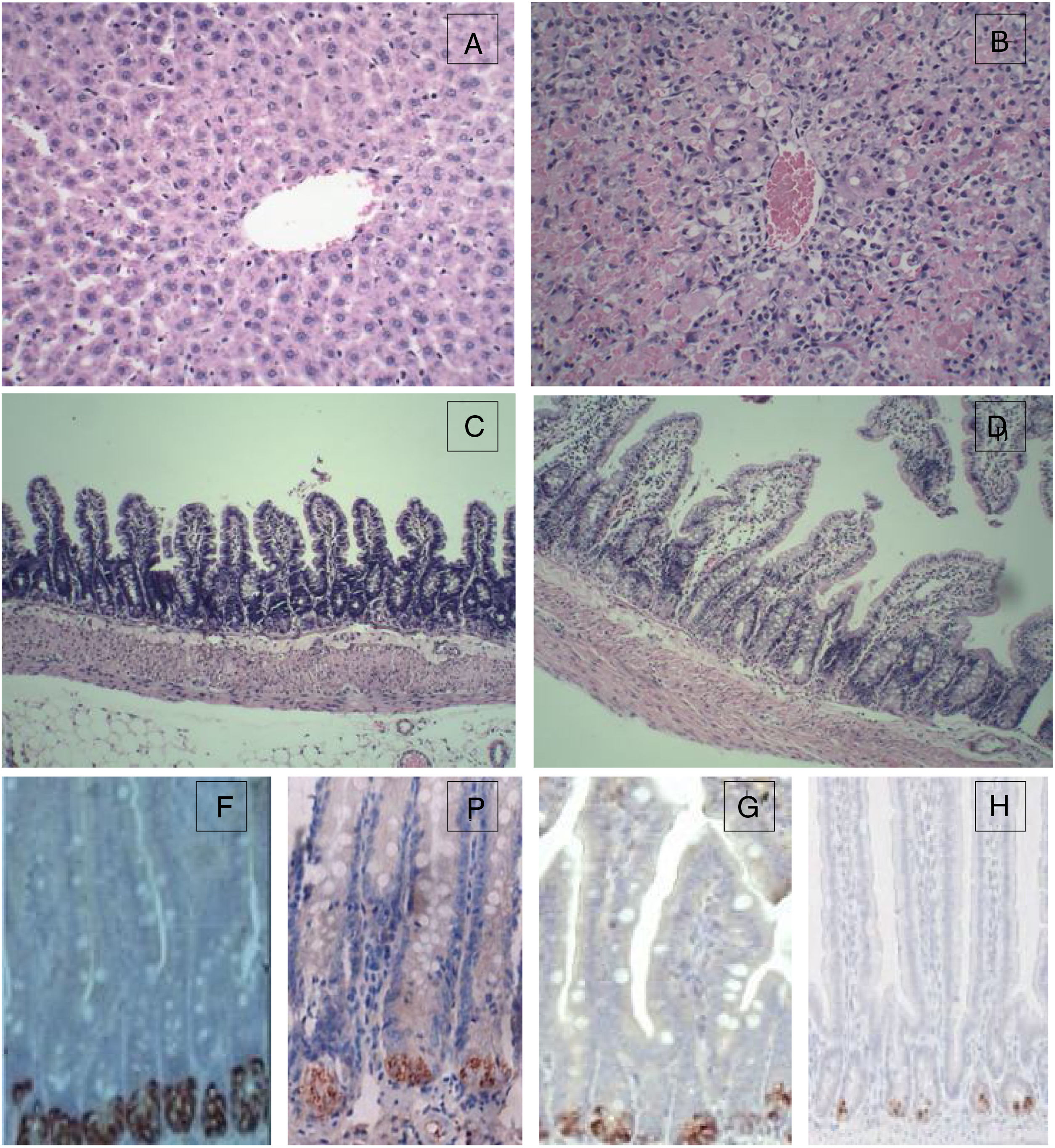
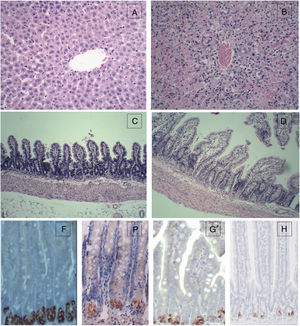
For the control group, the structure of hepatic lobules is intact, and hepatocytes, the portal area and the epithelial cells of bile ducts are in good condition without obvious inflammation, degeneration and necrosis. However, for the 72h experimental group, the normal structure of the liver was lost with huge area of necrosis (more than 2/3 of the whole section) and few live hepatocytes left. Massive inflammatory cell infiltration was observed in the portal area and necrotic area, and the sinus hepaticus had congestion and haemorrhaging. The terminal ileum of the control group was intact without inflammation, necrosis and cell detachment; for the 72h experimental group, the structure of terminal ileum was still fine without obvious detachment or necrosis of the mucosal epithelial cells. However, interstitial oedema in the villus mucosa and submucosa, and massive neutrophil infiltration was also observed (Fig. 1).
Histopathological examination (HE) of the liver and terminal ileum, and sPLA2 and lysozyme protein expression in the terminal ileum. (A) Liver of the control group (HE 200×); (B) liver of the experimental 72h group (HE 200×); (C) terminal ileum of the control group (HE 200×); (D) terminal ileum of the experimental 72h group (HE 200×); (E) lysozyme protein expression in the ileum of the control group (DAB 400×); (F) lysozyme protein expression in the ileum of the experimental 72h group (DAB 400×); (G) sPLA2 protein expression in the ileum of the control group (DAB 400×); (H) sPLA2 protein expression in the ileum of the experimental 72h group (DAB 400×).
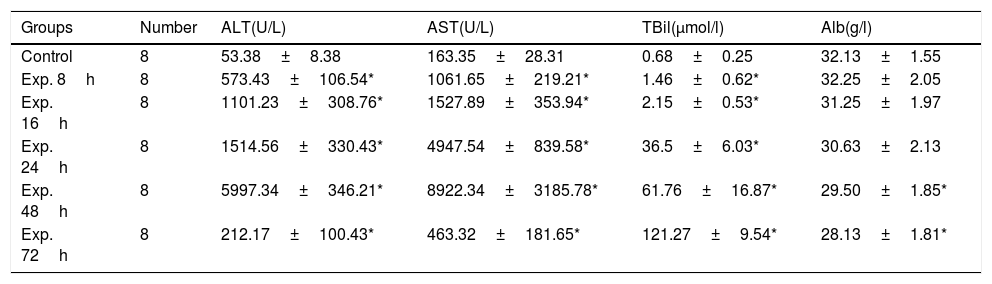
ALT and AST gradually increased in the experimental group and peaked 48h after induction of ALT. During the same period of time, the total bilirubin also increased. Then, aminotransferase was decreased but total bilirubin continued to rise. The difference between the control group and the experimental group was statistically significant with regard to ALT, AST and TBil levels at all of the time points examined except Alb (Table 2).
Serological markers of the control and the experimental groups at different time points (x¯±s).
| Groups | Number | ALT(U/L) | AST(U/L) | TBil(μmol/l) | Alb(g/l) |
|---|---|---|---|---|---|
| Control | 8 | 53.38±8.38 | 163.35±28.31 | 0.68±0.25 | 32.13±1.55 |
| Exp. 8h | 8 | 573.43±106.54* | 1061.65±219.21* | 1.46±0.62* | 32.25±2.05 |
| Exp. 16h | 8 | 1101.23±308.76* | 1527.89±353.94* | 2.15±0.53* | 31.25±1.97 |
| Exp. 24h | 8 | 1514.56±330.43* | 4947.54±839.58* | 36.5±6.03* | 30.63±2.13 |
| Exp. 48h | 8 | 5997.34±346.21* | 8922.34±3185.78* | 61.76±16.87* | 29.50±1.85* |
| Exp. 72h | 8 | 212.17±100.43* | 463.32±181.65* | 121.27±9.54* | 28.13±1.81* |
Note: ALT, alanine transaminase; AST, aspartate aminotransferase; TBil, total bilirubin; Alb, albumin.
RD-5 and sPLA2 mRNA gradually increased at the early time points in the ileum of the experimental group and peaked 16h after the induction of ALF (1.291±0.153 and 1.131±0.128), which was significantly different from the control group (0.725±0.116 and 0.722±0.112, t=69.25 and 95.71, p<0.01). Then, RD-5 and sPLA2 mRNA gradually decreased, and by 72h after the induction, their expression levels (0.415±0.104 and 0.425±0.076) were much lower than the control group (t=31.55 and 44.98, p<0.01); the lysozyme mRNA level of the control group was 0.853±0.093. For the experimental group, lysozyme mRNA also increased, and peaked at the 8h point (1.211±0.107) then gradually decreased. By 72h after ALF induction, lysozyme mRNA (0.704±0.103) was much lower than the control group (t=9.224, p<0.05) (Fig. 2).
Western blot for the protein expression of sPLA2 and lysozyme in terminal ileumThe sPLA2 protein level of the 8h experimental group was 1.215±0.243, which was significantly different from the control group (0.583±0.121, t=135.38, p<0.01). The sPLA2 protein level of the 72h experimental group was 0.327±0.086, which was much lower than that of the control group (t=12.28, p<0.01). Lysozyme expression in the experimental group peaked at 16h after ALF induction (0.835±0.153), which was statistically different from that of the control group (0.650±0.093, t=6.87, p<0.05). The lysozyme protein level of the 72h experimental group was 0.382±0.057, which was much lower than that of the control group (t=15.83, p<0.01) (Fig. 3).
IHC for sPLA2 and lysozyme in the terminal ileumsPLA2 and lysozyme were stained positive in the Paneth cells in the intestinal crypt. The sPLA2 had a strong positive staining in the Paneth cells in the ileum of the control group while the staining in the 72h experimental group was much weaker with only a few brown spotting signals; lysozyme also had a strong positive staining in all of the Paneth cells in the ileum of the control group, whereas the staining in the 72h experimental group was positive only in some Paneth cells and was much weaker with a sporadic staining pattern (Fig. 3).
DiscussionAcute liver failure is a life-threatening disease. In our country, the most common cause is hepatitis B infection. The loss of liver function often leads to many complications, including hepatic encephalopathy, gastrointestinal bleeding, spontaneous peritonitis and hepatorenal syndrome, among which peritoneal infection (spontaneous peritonitis) has the highest incidence and is related to disease progression and mortality.6,7 The aetiological pathogens causing peritoneal infection translocate from the intestine, as shown by the assay involving green fluorescent protein labelled bacteria.8 The mechanism behind bacterial translocation that occurs in ALF is still unknown. The fact that the majority of the translocating bacteria are the normal commensal flora in the intestine suggests that infection results from comprised intestinal immunity that is insufficient in preventing the normal flora from translocation.
Recent studies have shown that certain antimicrobial peptides, including defensins, cathelicidins, lysozyme and sPLA2 produced by intestinal epithelial cells, play an important role in preventing intestinal damage and intestinal bacterial translocation.9 Among them, defensin-5 plays the most outstanding role in the innate immunity of the intestine; this peptide has the strongest anti-bacterial activity and is able to kill Gram positive and negative bacteria, fungi and viruses. Salzman et al.10,11 previously showed that in transgenic mice, defensin from Paneth cells has the ability of inhibiting BT. sPLA2 and lysozyme have a similar anti-bacterial spectrum with strong activity against most Gram positive bacteria and certain Gram negative bacteria such as E. coli and Salmonella.12 A study found that in a mouse model of parenteral nutrition, decreased PLA2 expression was closely related to the damaged intestine barrier function.13 These AMPs function by stopping bacteria from crossing over the intestinal epithelial cells and translocating after being produced and secreted onto intestine mucous layer while there are no AMPs in the intestinal content.14–18
In the current study, we investigated the expression of RD-5, sPLA2 and lysozyme produced by mucosal epithelial cells of the intestine and its relationship to bacterial translocation using RT-PCR, Western blot and IHC in the rat model of ALF. Our results showed that RD-5 and sPLA2 mRNA in the experimental group peaked 16h after ALF induction and then gradually decreased. By 48h and 72h after establishment of the model, the of the mRNA expression levels were lower than the control group; lysozyme mRNA peaked at 24h and 72h, and it was lower than that of the control group; sPLA2 protein expression peaked at 8h and then suddenly dropped, and at 24h its expression was lower than that of the control group. Lysozyme protein peaked at 16h and then gradually decreased, and by 48h and 72h, its expression was lower than that of the control group. During the early period of ALF, these three proteins increased instead of decreasing their expression levels, which suggests that increased expression of these proteins in the intestinal mucosa could be protective against external stimuli and the invasion of other pathogens, although their later expression was decreased. sPLA2 and lysozyme protein decreased at an earlier time point than the decrease in their mRNA levels, which suggests that during the middle to late period of ALF, the rate of sPLA2 and lysozyme protein consumption was faster than their synthesis, leading to decompensation and bacterial translocation.
The bacterial culture indicates that no bacteria grew in the cultures of the control group from either the 8h or 16h experimental groups. The positive rates of the 24h, 48h and 72h experimental groups were 8.3%, 37.5% and 58.3%, respectively. RD-5 and sPLA2 mRNA levels progressively decreased in the 24h, 48h and 72h experimental groups. At 24h, the sPLA2 protein level was lower than the control group and bacterial translocation emerged. At 48h and 72h, the sPLA2 protein remained low and the lysozyme protein decreased and was lower than the control group, which further boosted the occurrence of bacterial translocation. This result suggests that late in ALF RD-5, sPLA2 and lysozyme levels were not high enough to prevent bacterial translocation. One thing should be noted: despite the fact that the positive rate of the bacterial culture from the 72h experimental group was 58.3%, the structure of the terminal ileum was nearly intact without obvious cell detachment and necrosis of the mucosal epithelium, and only interstitial oedema in the villus mucosa and submucosa and massive neutrophil infiltration was observed. This result suggests that bacterial translocation has nothing to do with the intestinal structure but rather with the weakened intestinal immune barrier function.
In summary, in the healthy state, commensal flora actively interact with the intestinal mucosa, which maintains the balance of intestinal microecological environments and prevents the invasion of pathogens.19 The imbalance between host and microbe and the resultant bacterial translocation in ALF rats is related to the compromised immune barrier function due to insufficient expression of RD-5, sPLA2 and lysozyme in ileal Paneth cells. Our study shows that recovery of the host mucosal immune barrier function should play a key part in the future treatment of ALF. Therefore, the regulatory mechanism regarding the recovery of the host mucosal immune barrier function should play a key part in the future treatment of ALF, and the function of intestinal AMPs warrants further investigation, which certainly will provide new methods and theories for the treatment of peritoneal infection caused by ALF.
ConclusionOur study shows that the immune barrier function of the ileal mucosa in the rat model of acute liver failure was compromised as the decreased expression of RD-5, sPLA2 and lysozyme in Paneth cells were decreased. At the same time increased intestinal bacterial translocation. So that recovery of the host mucosal immune barrier function should play a key part in the future treatment of ALF.
Availability of data and materialThe data showed in the manuscript can support our conclusions, and there's no other supporting data.
Authors’ contributionsAll authors have contributed equally to this paper. All authors approved the final manuscript.
FundingThis work was supported by the National Science and Technology Major Project of China [grant number 2018ZX10302205] and the Personnel Plan of Jiangxi Science and Technology Department (No. 20161BCD40015).
Conflict of interestsThe authors declare that they have no competing interests.
Not applicable.