Alendronate sodium (Fosamax®) is an oral osteoclast inhibitor which reduces bone resorption and is indicated for the treatment of Paget's disease and osteoporosis.1 In 1996, after the drug had been available on the market for only a year, the United States Food and Drug Administration (FDA) put out an alert about the need for preventive measures when administering the drug to prevent oesophagogastric lesions, in particular erosive or ulcerative oesophagitis. The mechanism behind the injury is unknown, but it has been suggested that direct contact with the mucosa may be involved. Consequently, those prescribed this drug should take it 30 min after their first meal of the day with plenty of water, and remaining standing or sitting up for at least 30 min.2
We report the case of a 62-year-old female smoker with osteoporosis who was admitted from the Accident and Emergency Department with a 24-h history of retrosternal pain, odynophagia and dysphagia for solids and liquids. She denied previous heartburn, dysphagia and general adaptation syndrome. She had been taking one 70-mg tablet of alendronic acid in an effervescent formulation every seven days for three weeks. Physical examination revealed no findings of note, with no crepitus of the neck or chest, abdomen non-tender on palpation and no masses or organomegaly. Blood testing showed leucocytosis of 15,000 with a neutrophil count of 11,600 and a C-reactive protein (CRP) level of 50. The patient subsequently had a spike of fever at 38°C, with no microbiological isolation. She was started on empirical treatment with intravenous analgesia and intravenous omeprazole 40 mg every 12 h.
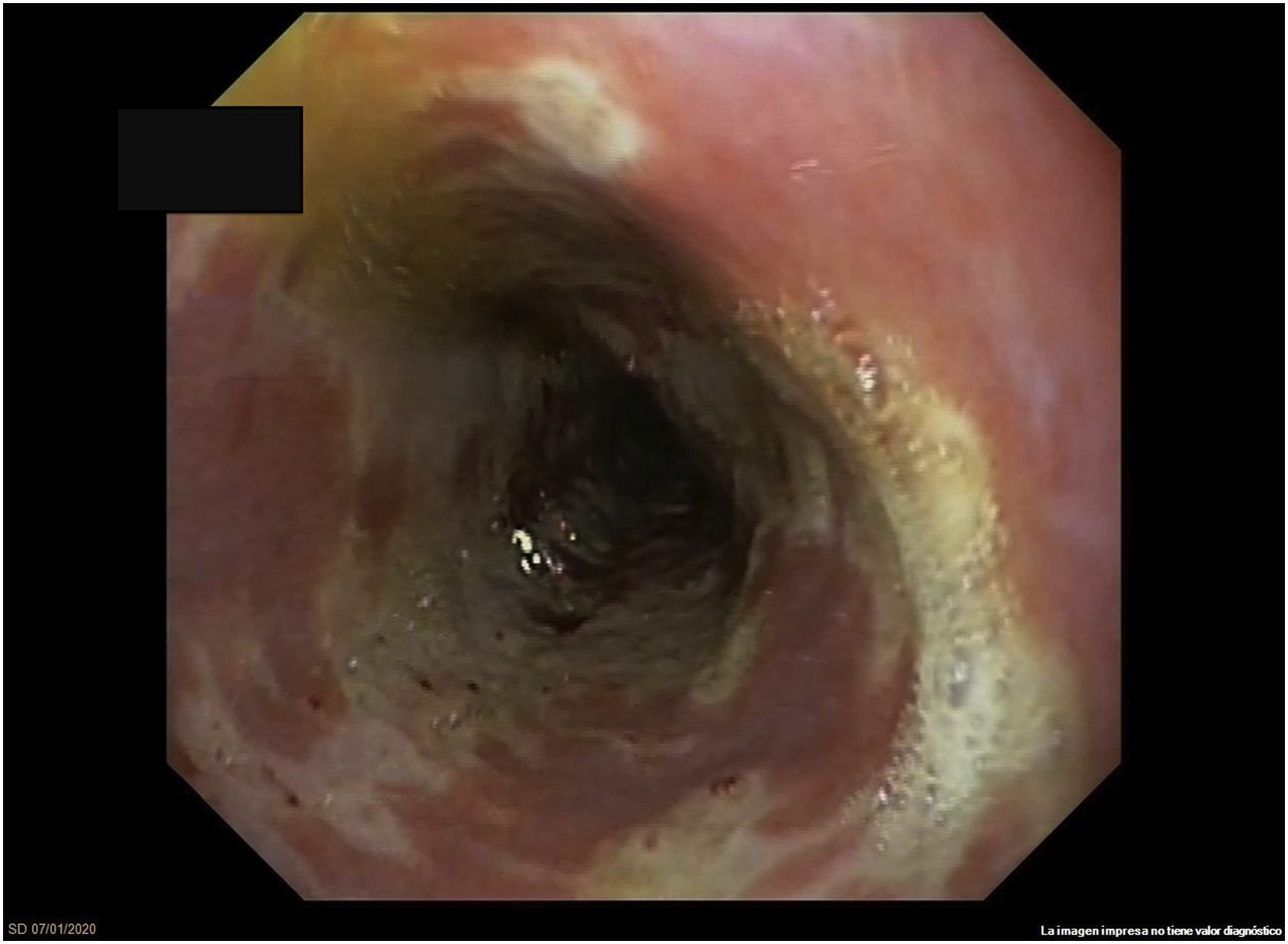
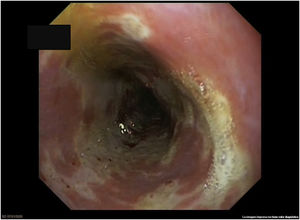
Gastroscopy identified friable mucosa, whitish exudate and ulceration covering the entire circumference in the distal segment of the oesophagus, suggestive of severe oesophagitis (Fig. 1). Biopsies were negative for cytomegalovirus (CMV) and for herpes simplex virus (HSV) types I and II. Histology showed sheets of flat, non-keratinised, multi-layered epithelium particular to the oesophagus and remnants of ulcerated tissue with fibrin, in addition to polymorphonuclear cells. Periodic acid–Schiff (PAS) and GROCOTT staining were performed to rule out the presence of fungi, with negative results. Immunohistochemistry for CMV and HSV was also performed and also found to be negative.
The patient subsequently followed a good clinical course with oral omeprazole and sucralfate. She regained suitable tolerance to an oral diet and so was discharged home with outpatient follow-up. Gastroscopy one month later showed mucosal healing, and biopsies were normal.
Oesophagitis associated with the use of alendronate is a type of drug-induced oesophagitis. Its prevalence is at least 1.5%, but can be much higher if preventive measures are not properly taken during drug administration.1 It can present with odynophagia, dysphagia for solids and liquids, retrosternal pain, epigastric pain, fever and even haematemesis, sometimes requiring hospital admission.1,2 Oesophageal strictures may also occur at a later stage.1 Symptoms can appear two to four weeks after starting treatment; however, in our case, they occurred only four days later.3
Characteristic endoscopic findings are large erosions with variable morphology and dense whitish exudate in the distal and medial segments.1
While there are no pathognomonic pathology findings for this condition, in two published reviews, one by Abraham et al. and the other by Ribeiro et al., histopathology findings generally consisted of granulation tissue and inflammatory exudate. In addition, it is common to find polarisable foreign material with a crystalline, clear, refractive appearance mixed with inflammatory exudates, probably related to prolonged contact between the drug and the mucosa.1,4 Other possible findings are multinucleated giant cells with inflammatory exudate and reactive squamous cells close to the ulcers. In our case, no polarisable material was identified, but inflammatory cells were. These changes can be mistaken for HSV oesophagitis; hence, immunohistochemistry techniques are important for distinguishing between them. In our case, these tests were negative.1,2,4
A thorough medical history, upper gastrointestinal endoscopy and histology are essential for diagnosing this disorder. Infectious oesophagitis, mainly herpes or candida, must be ruled out as it would require specific treatment; candida is more common in immunosuppressed patients. To conclude, this disease is not to be overlooked, as it can be prevented with hygiene and dietary measures when administering the drug.
Please cite this article as: Zabalza San Martín L, Saldaña Dueñas C, Gómez Alonso M, Aisa Ribera G. Intolerancia oral por esofagitis secundaria a ácido alendrónico. Gastroenterol Hepatol. 2021;44:27–28.