Ustekinumab (UST) is a monoclonal antibody against IL-12/23 approved in Spain (2017) to treat moderate/severe Crohn's disease.
ObjectiveTo evaluate the effectiveness and safety in real clinical practice in patients treated with UST in our centre.
MethodsThis is a prospective observational study including patients who started UST from 08/01/2017 to 02/28/2019 with follow-up up to that date. We analyse response and remission in weeks 16, 24 and 52, using “Crohn's Disease Activity Index” (response if 100 point decrease and remission if <150) and Physician's Global Assessment.
ResultsWe included 61 patients with a median duration of Crohn's disease of 14.6 years (0–36). The 83.6% of patients without steroids and 73.8% without associated immunosuppressors. Previously all patients had received anti-TNF and 14.8%, in addition, vedolizumab.
We observed a good correlation between Crohn's Disease Activity Index and Physician's Global Assessment (r=0.89, p<.001). In week 16 (n=45) 75.6% response (57.8% remission), in week 24 (n=35) 69.9% response (45.7% remission) and in week 52 (n=12) 75% response (58.3% remission). There were no statistically significant differences in the response/remission rates at week 16 or 24 depending on the reason for the onset of UST or the number of previous biologics. In 2 patients it was withdrawn due to toxicity (arthralgia/myalgia).
ConclusionUST is an effective and safe treatment in real clinical practice with high rates of clinical remission at week 16, 24 and 52 regardless of the order of biological used and the reason for starting UST.
Ustekinumab (UST) es un anticuerpo monoclonal frente a IL-12/23 aprobado en España (2017) para tratar el brote moderado/grave de enfermedad de Crohn.
ObjetivoEvaluar la efectividad y seguridad en práctica clínica real en los pacientes tratados con UST en nuestro centro.
MétodosEstudio prospectivo observacional unicéntrico incluyendo los pacientes que iniciaron UST desde el 1/08/2017 hasta el 28/02/2019 con seguimiento hasta esa fecha. Analizamos respuesta y remisión en semanas 16, 24 y 52, utilizando «Crohn's Disease Activity Index» (respuesta si descenso de 100 puntos y remisión si <150) y la «Valoración Global del especialista» traducción del «Physician's Global Assessment».
ResultadosIncluimos 61 pacientes con una mediana de duración de enfermedad de Crohn de 14,6 años (0-36). El 83,6% sin esteroides y el 73,8% sin inmunosupresores asociados. Previamente todos habían recibido anti-TNF y el 14,8%, además, vedolizumab.
Observamos buena correlación entre Crohn's Disease Activity Index y Valoración Global del especialista (r=0,89, p<0,001). En la semana 16 (n=45) un 75,6% de respuesta (57,8% remisión), en semana 24 (n=35) 69,9% respuesta (45,7% remisión) y en semana 52 (n=12) 75% respuesta (58,3% remisión). No se obtuvieron diferencias estadísticamente significativas en la tasa de respuesta/remisión en semana 16 ni 24 en función del motivo de inicio de UST o el número de biológicos previos. En 2 pacientes se retiró por toxicidad (artralgias/mialgias).
ConclusiónUST es un fármaco eficaz y seguro en práctica clínica real con altas tasas de remisión clínica en semana 16, 24 y 52 independientemente del orden de biológico utilizado y del motivo de inicio de UST.
The development of anti-TNF has heralded a revolution in the treatment and quality of life of patients with inflammatory bowel disease.1,2 However, approximately one third of patients do not respond initially to anti-TNF, and efficacy is eventually lost in a certain percentage of them or they present adverse events or intolerance, making its withdrawal necessary.2 Moreover, the efficacy diminishes when a second anti-TNF is used in both primary responders and in secondary failure.
Breakthroughs in our knowledge of the inflammatory pathways have made it possible to develop drugs with different TNF-alpha blockade mechanisms of action, thereby diversifying the therapeutic arsenal in Crohn's disease (CD). In 2015, the European Medicines Agency (EMA) approved vedolizumab, an anti-integrin drug, in moderate/severe CD, and in 2016 it did the same with ustekinumab (UST),12 which has been available in Spain since 2017.
UST is a monoclonal antibody against the p40 subunit which is part of IL-12 and IL-23. It is indicated for active moderate/severe CD with an unsuitable response, loss of response, intolerance or contraindication for conventional treatment or anti-TNF. UST had already been approved in other indications (plaque psoriasis and psoriatic arthritis3), although in CD it is the only entity in which endovenous (ev) induction is authorised.
Its efficacy and safety in induction was demonstrated in the UNITI-1 and UNITI-2 randomised, double-blind and placebo-controlled phase 3 trials. These trials recruited elderly patients with moderate/severe CD (CDAI 220-450), diagnosed at least 3 months previously and who had previously been given treatment with anti-TNF (UNITI-1) or conventional treatment (UNITI-2), and either this had failed (loss of primary or secondary response) or they presented intolerance or adverse drug reactions.
UST's efficacy and safety in the maintenance of response was demonstrated in the IM-UNITI phase 3 clinical trial.4
However, the evidence for UST using ev induction in clinical practice is still somewhat scant. Most of the studies published predate the approval of the ev formulation and present design differences, limited numbers of patients and different pathologies and form of administration of UST. Moreover, patients in real clinical practice usually differ from those included in clinical trials on account of their strict inclusion criteria.5–9
For this reason, we are presenting this clinical practice study, which analyses the characteristics of all patients with CD started on UST (ev induction) at our hospital, evaluating its efficacy and safety in induction and maintenance.
Material and methodsStudy design and participantsWe performed a single-centre prospective observational study including all patients with CD treated with UST at the Complejo Hospitalario de Navarra between August 2017 and February 2019, completing follow-up on that date. In all cases, induction was performed with ev UST.
MethodThe dose of UST was as indicated in the summary of product characteristics. Induction consisted of a single and initial ev dose calculated according to weight: <55kg: 260mg, 55–85kg: 390mg, >85kg: 520mg; followed by a dose of 90mg of subcutaneous (sc) UST at week 8.3
The response to induction was evaluated at week 16, and a maintenance dose of 90 mg sc was given every 8 weeks. The decision to intensify treatment by reducing the interval between doses (every 4–6 weeks) was left up to the criteria of the treating specialist. In no cases was reinduction with a new ev dose of the drug addressed.
We analysed the demographic and descriptive variables, treatments and the reason for beginning UST. All the patients were seen in consultation at least in weeks 16, 24 and 52. The following clinical activity indices were calculated: Crohn's Disease Activity Index (CDAI) and Harvey-Bradshaw Index (HBI), and inflammation markers such as C-reactive protein (CRP) and faecal calprotectin (FC). A Physician's Global Assessment (PGA) which included anamnesis, exploration and analytical parameters was performed.
Response was defined according to the PGA, which classifies it as no response, response (RP) or remission, and CDAI, regarding RP as a reduction of 100 points from the baseline and remission as a score of <150 points.
Statistical analysisWe used the SPSS programme, version 20, for the statistical analysis. Initially, we performed a descriptive epidemiological and disease analysis up until the beginning of treatment. Proportion was used in the categorical variables and the mean and standard deviation or median and interquartile range were used in the quantitative variables, as applicable.
Subsequently, we analysed response by using two of the aforementioned criteria (CDAI and PGA) and the analytical parameters at weeks 16, 24 and 52.
We performed a stratified analysis depending on the reason for starting with UST and the number of previous biologicals. We analysed the comparability of the groups by means of the chi-squared test or Fisher's exact test, as appropriate for qualitative variables, and by means of the student t-test for independent samples or the Wilcoxon test for quantitative variables.
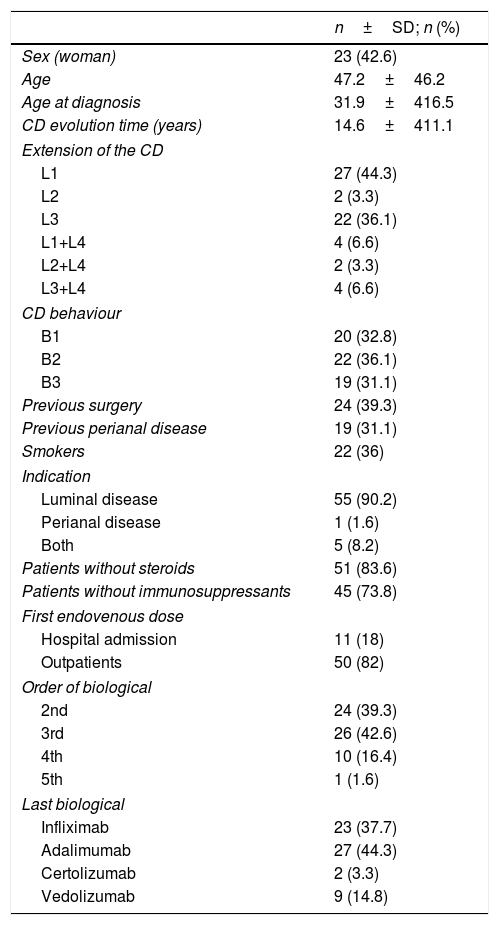
ResultsSixty-one patients, whose baseline characteristics are described in Table 1, were included. It should be highlighted that although 31.1% (n=19) had perianal disease, the indication of UST was active luminal disease in 90.2% (n=55), active luminal and perianal disease in 8.2% (n=5) and the indication was exclusively active perianal disease in only one patient (1.6%). At the time of induction with ev UST, 83.6% of the patients were not receiving steroids and 73.8% were without combined immunosuppressants. Moreover, 39.2% had a record of prior resective surgery.
Demographic baseline characteristics of the patients.
| n±SD; n (%) | |
|---|---|
| Sex (woman) | 23 (42.6) |
| Age | 47.2±46.2 |
| Age at diagnosis | 31.9±416.5 |
| CD evolution time (years) | 14.6±411.1 |
| Extension of the CD | |
| L1 | 27 (44.3) |
| L2 | 2 (3.3) |
| L3 | 22 (36.1) |
| L1+L4 | 4 (6.6) |
| L2+L4 | 2 (3.3) |
| L3+L4 | 4 (6.6) |
| CD behaviour | |
| B1 | 20 (32.8) |
| B2 | 22 (36.1) |
| B3 | 19 (31.1) |
| Previous surgery | 24 (39.3) |
| Previous perianal disease | 19 (31.1) |
| Smokers | 22 (36) |
| Indication | |
| Luminal disease | 55 (90.2) |
| Perianal disease | 1 (1.6) |
| Both | 5 (8.2) |
| Patients without steroids | 51 (83.6) |
| Patients without immunosuppressants | 45 (73.8) |
| First endovenous dose | |
| Hospital admission | 11 (18) |
| Outpatients | 50 (82) |
| Order of biological | |
| 2nd | 24 (39.3) |
| 3rd | 26 (42.6) |
| 4th | 10 (16.4) |
| 5th | 1 (1.6) |
| Last biological | |
| Infliximab | 23 (37.7) |
| Adalimumab | 27 (44.3) |
| Certolizumab | 2 (3.3) |
| Vedolizumab | 9 (14.8) |
SD: standard deviation; CD: Crohn's disease.
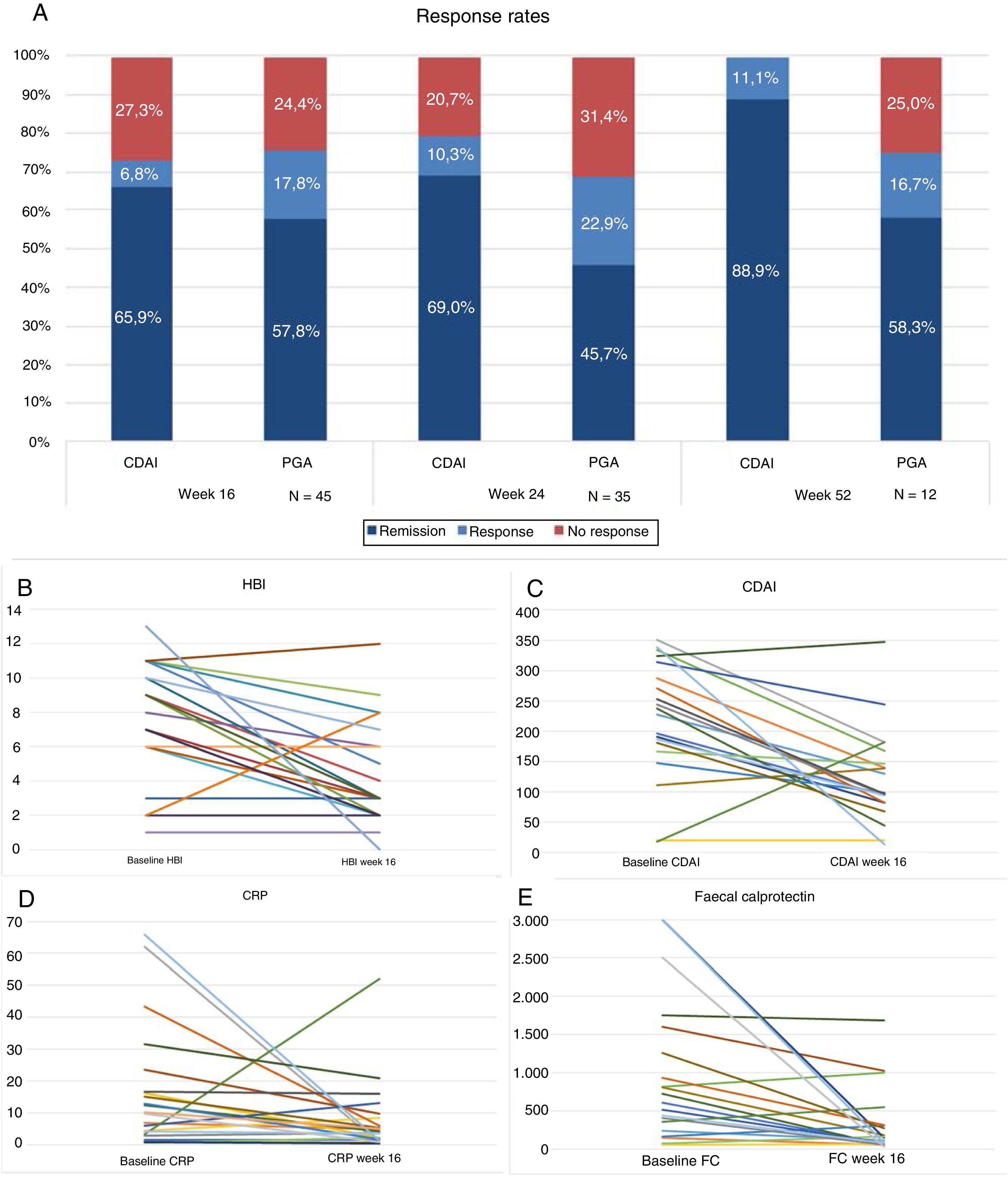
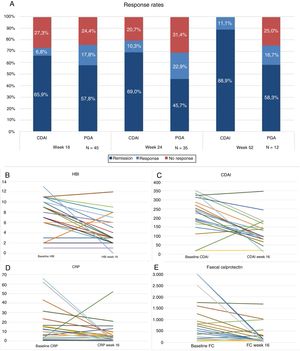
Forty-five patients reached week 16 in the study. At that time, according to the PGA, 57.8% (n=26) were in steroid-free remission, 17.8% (n=8) in response and 24.4% (n=11) had not responded to the drug (Fig. 1A). The majority of the patients were given the drug every 8 weeks, only 4 (8.8%) were intensified and were given UST (90mg sc) every 4 weeks. The difference in medians between the first ev infusion and week 16 was statistically significant for the HBI (p<0.001), CDAI (p<0.001), CRP (p=0.026) and FC (p=0.001) (Fig. 1B–E).
(A) Response rates, remission or no response at weeks 16, 24 and 52. CDAI: Crohn's Disease Activity Index; PGA: Physician's Global Assessment. (B) Variation in Harvey-Bradshaw Index (HBI) between baseline situation and week 16. (C) Variación in CDAI between baseline situation and week 16. (D) Variation in C-reactive protein (CRP) between baseline situation and week 16. (E) Variation in faecal calprotectin (FC) between baseline situation and week 16.
Thirty-five patients reached week 24, of whom 45.7% (n=16) were in steroid-free remission, 22.9% (n=8) in response and 31.4% (n=11) had failed to respond. Of the total, 17% (n=6) were intensified: 4 patients were receiving UST (90mg sc) every 4 weeks and 2 received it every 6 weeks. The difference in medians between the first ev infusion and week 24 was statistically significant for the HBI (p=0.01), CDAI (p=0.001) and FC (p=0.006). The differences were not statistically significant for CRP (p=0.13).
Twelve patients reached week 52. Of these, 58.3% (n=7) maintained steroid-free remission, 16.7% (n=2) continued with response and 25% (n=3) had failed.
The difference in medians between the first ev infusion and week 52 was statistically significant for the HBI (p<0.007) and CDAI (p<0.001), but not for FC (p=0.6) or for CRP (p=0.5).
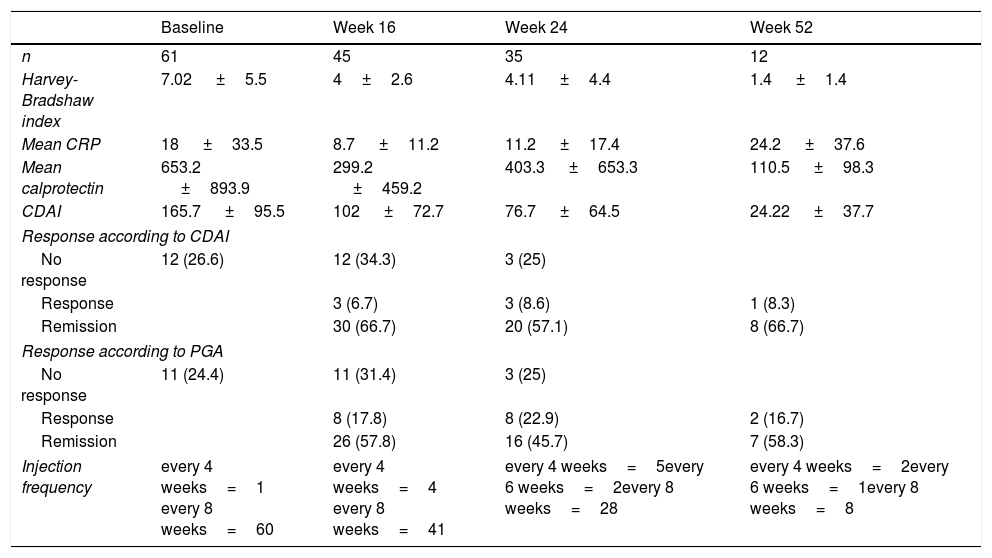
The inflammatory parameters, global response rates and injection frequency are shown in Table 2.
Clinical baseline characteristics of activity and biochemical parameters.
| Baseline | Week 16 | Week 24 | Week 52 | |
|---|---|---|---|---|
| n | 61 | 45 | 35 | 12 |
| Harvey-Bradshaw index | 7.02 ±5.5 | 4±2.6 | 4.11 ±4.4 | 1.4 ±1.4 |
| Mean CRP | 18 ±33.5 | 8.7 ±11.2 | 11.2 ±17.4 | 24.2 ±37.6 |
| Mean calprotectin | 653.2 ±893.9 | 299.2 ±459.2 | 403.3 ±653.3 | 110.5 ±98.3 |
| CDAI | 165.7 ±95.5 | 102 ±72.7 | 76.7 ±64.5 | 24.22 ±37.7 |
| Response according to CDAI | ||||
| No response | 12 (26.6) | 12 (34.3) | 3 (25) | |
| Response | 3 (6.7) | 3 (8.6) | 1 (8.3) | |
| Remission | 30 (66.7) | 20 (57.1) | 8 (66.7) | |
| Response according to PGA | ||||
| No response | 11 (24.4) | 11 (31.4) | 3 (25) | |
| Response | 8 (17.8) | 8 (22.9) | 2 (16.7) | |
| Remission | 26 (57.8) | 16 (45.7) | 7 (58.3) | |
| Injection frequency | every 4 weeks=1 every 8 weeks=60 | every 4 weeks=4 every 8 weeks=41 | every 4 weeks=5every 6 weeks=2every 8 weeks=28 | every 4 weeks=2every 6 weeks=1every 8 weeks=8 |
CDAI: Crohn's Disease Activity Index; CRP: C-reactive protein; PGA: Physician's Global Assessment.
There was an excellent correlation between the CDAI score and the PGA, with a Pearson's coefficient of r=0.898, which was statistically significant (p<0.001).
In our series, the drug had to be withdrawn in 2 patients (3.27%) on account of adverse reactions, both of them arthralgia and myalgia, and in another 4 (6.55%) for lack of efficacy. During follow-up, 11 of the 61 patients (18%) required hospitalisation: 6 for scheduled surgery (54.5%), 2 (18.2%) for symptoms of intestinal sub-occlusion/obstruction, and one case (9.1%) for severe infection due to urinary sepsis.
Of the 6 surgical patients, 4 were subsequently restarted on UST for the prevention of post-surgical recurrence, and one of them for an associated rheumatological condition as well. In the other 2 patients, anti-TNF was indicated as recurrence prevention.
Stratified analysisAll the patients had previously received anti-TNF; 39.3% (24) only one biological, 42.6% two biologicals and 18% three or more biologicals prior to the beginning of UST.
Nine (14.7%) were due to prior failure with anti-TNF and also with an anti-integrin drug.
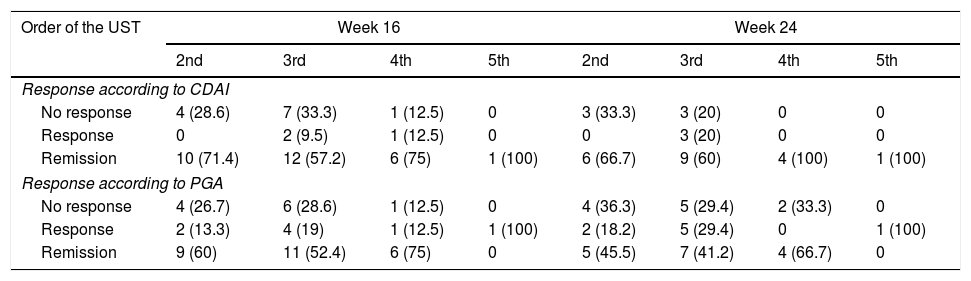
The stratified analysis according to the number of previous biologicals is displayed in Table 3. No statistically significant differences were observed in the response rate or remission at week 16 or at week 24.
Response rates depending on whether ustekinumab was the second, third, fourth or fifth biological.
| Order of the UST | Week 16 | Week 24 | ||||||
|---|---|---|---|---|---|---|---|---|
| 2nd | 3rd | 4th | 5th | 2nd | 3rd | 4th | 5th | |
| Response according to CDAI | ||||||||
| No response | 4 (28.6) | 7 (33.3) | 1 (12.5) | 0 | 3 (33.3) | 3 (20) | 0 | 0 |
| Response | 0 | 2 (9.5) | 1 (12.5) | 0 | 0 | 3 (20) | 0 | 0 |
| Remission | 10 (71.4) | 12 (57.2) | 6 (75) | 1 (100) | 6 (66.7) | 9 (60) | 4 (100) | 1 (100) |
| Response according to PGA | ||||||||
| No response | 4 (26.7) | 6 (28.6) | 1 (12.5) | 0 | 4 (36.3) | 5 (29.4) | 2 (33.3) | 0 |
| Response | 2 (13.3) | 4 (19) | 1 (12.5) | 1 (100) | 2 (18.2) | 5 (29.4) | 0 | 1 (100) |
| Remission | 9 (60) | 11 (52.4) | 6 (75) | 0 | 5 (45.5) | 7 (41.2) | 4 (66.7) | 0 |
CDAI: Crohn's Disease Activity Index; PGA: Physician's Global Assessment.
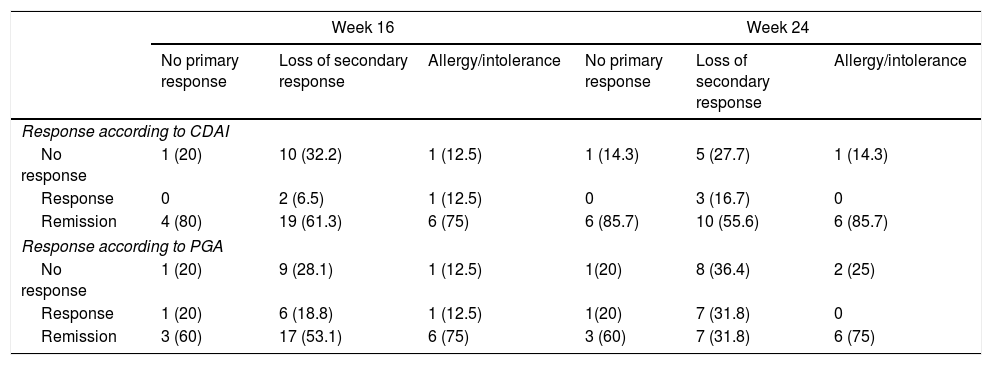
The stratified analysis according to reason for withdrawal of the anti-TNF (primary or secondary failure or toxicity) is shown in Table 4. No statistically significant differences were observed in the response rate or remission at week 16 or at week 24.
Response rates according to the reason for indication of ustekinumab (no primary response, loss of secondary response or allergy/intolerance of previous biological).
| Week 16 | Week 24 | |||||
|---|---|---|---|---|---|---|
| No primary response | Loss of secondary response | Allergy/intolerance | No primary response | Loss of secondary response | Allergy/intolerance | |
| Response according to CDAI | ||||||
| No response | 1 (20) | 10 (32.2) | 1 (12.5) | 1 (14.3) | 5 (27.7) | 1 (14.3) |
| Response | 0 | 2 (6.5) | 1 (12.5) | 0 | 3 (16.7) | 0 |
| Remission | 4 (80) | 19 (61.3) | 6 (75) | 6 (85.7) | 10 (55.6) | 6 (85.7) |
| Response according to PGA | ||||||
| No response | 1 (20) | 9 (28.1) | 1 (12.5) | 1(20) | 8 (36.4) | 2 (25) |
| Response | 1 (20) | 6 (18.8) | 1 (12.5) | 1(20) | 7 (31.8) | 0 |
| Remission | 3 (60) | 17 (53.1) | 6 (75) | 3 (60) | 7 (31.8) | 6 (75) |
CDAI: Crohn's Disease Activity Index; PGA: Physician's Global Assessment.
In the UNITI-1 and UNITI-2 clinical trials, the primary endpoint was the evaluation of clinical response at week 6 with an ev dose of 6mg/kg. At week 6, 34% of the patients in the UNITI-1 and 55% in the UNITI-2 were in remission (CDAI <100), with these figures being 38% and 58%, respectively, at week 8.4,10
With regard to maintenance, in the IM-UNITI study, doses of 90mg sc were used every 8 or 12 weeks. The percentage of clinical remission (CDAI <150) at week 44 was significantly superior to placebo (48.8% every 12 weeks and 53.1% every 8 weeks). Although the response rates with the regimen every eight weeks were higher, no statistically significant differences were observed.4,10
The induction and maintenance doses in our patients are the same as those indicated in the summary of product characteristics and the UNITI studies: 6mg/kg ev for induction and 90mg sc (every 8 and 12 weeks) in maintenance.
Moreover, as expounded in the EFIFECT study on treatment with anti-TNF, up to half of the patients with IBD treated in real clinical practice are not represented in the clinical trials. This explains, at least partly, that clinical trial results tend to underestimate the response observed in real clinical practice.11 Analysing the UNITI-1 inclusion criteria, only 29.5% of our patients would have been included, and 0% in the UNITI-2.
In our series, there was a lack of response in 27.3% at week 16, well below the no-response percentage in the UNITI study, which was 52.6% (according to the CDAI). If we compare the IM-UNITI study at week 52 to our data, the no-response rates were 40.6% and 25%, respectively. If we analyse the percentage of remission at week 52, the difference is not so pronounced, being 53.1% in the IM-UNITI and 58.3% in our study.4
Different real clinical practice studies were published prior to the approval of the drug (5–9), most of them with heterogeneous populations with differences in the subcutaneous induction regimens.
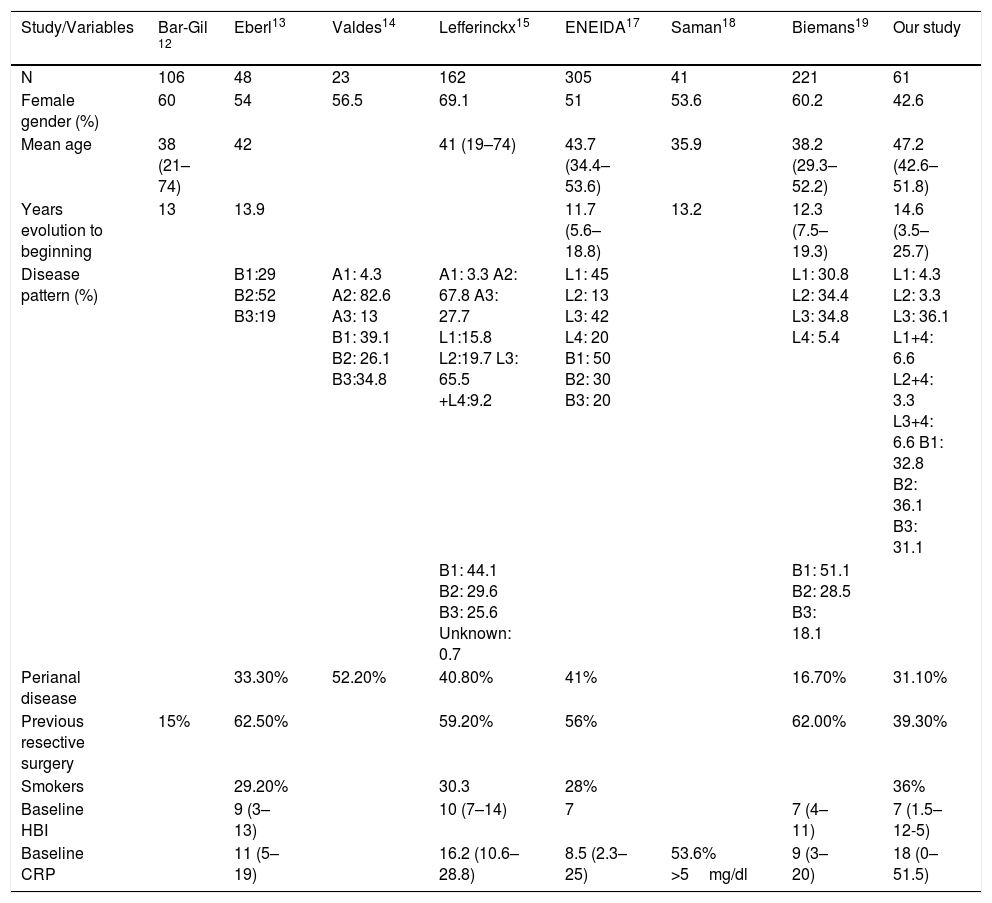
Focusing on studies with ev USF induction, there are several articles that evaluate its efficacy in clinical practice. Part of the evidence comes from studies presented at international meetings of organisations such as the European Crohn's and Colitis Organisation or United European Gastroenterology, whereas others have been published more recently. They all include patients with active CD and a weight-adjusted ev induction regimen followed by a dose of 90mg sc at week 8. The baseline characteristics of all of them are shown in Table 5.
Summary of the baseline characteristics of the different clinical practice studies.
| Study/Variables | Bar-Gil 12 | Eberl13 | Valdes14 | Lefferinckx15 | ENEIDA17 | Saman18 | Biemans19 | Our study |
|---|---|---|---|---|---|---|---|---|
| N | 106 | 48 | 23 | 162 | 305 | 41 | 221 | 61 |
| Female gender (%) | 60 | 54 | 56.5 | 69.1 | 51 | 53.6 | 60.2 | 42.6 |
| Mean age | 38 (21–74) | 42 | 41 (19–74) | 43.7 (34.4–53.6) | 35.9 | 38.2 (29.3–52.2) | 47.2 (42.6–51.8) | |
| Years evolution to beginning | 13 | 13.9 | 11.7 (5.6–18.8) | 13.2 | 12.3 (7.5–19.3) | 14.6 (3.5–25.7) | ||
| Disease pattern (%) | B1:29 B2:52 B3:19 | A1: 4.3 A2: 82.6 A3: 13 B1: 39.1 B2: 26.1 B3:34.8 | A1: 3.3 A2: 67.8 A3: 27.7 L1:15.8 L2:19.7 L3: 65.5 +L4:9.2 | L1: 45 L2: 13 L3: 42 L4: 20 B1: 50 B2: 30 B3: 20 | L1: 30.8 L2: 34.4 L3: 34.8 L4: 5.4 | L1: 4.3 L2: 3.3 L3: 36.1 L1+4: 6.6 L2+4: 3.3 L3+4: 6.6 B1: 32.8 B2: 36.1 B3: 31.1 | ||
| B1: 44.1 B2: 29.6 B3: 25.6 Unknown: 0.7 | B1: 51.1 B2: 28.5 B3: 18.1 | |||||||
| Perianal disease | 33.30% | 52.20% | 40.80% | 41% | 16.70% | 31.10% | ||
| Previous resective surgery | 15% | 62.50% | 59.20% | 56% | 62.00% | 39.30% | ||
| Smokers | 29.20% | 30.3 | 28% | 36% | ||||
| Baseline HBI | 9 (3–13) | 10 (7–14) | 7 | 7 (4–11) | 7 (1.5–12-5) | |||
| Baseline CRP | 11 (5–19) | 16.2 (10.6–28.8) | 8.5 (2.3–25) | 53.6% >5mg/dl | 9 (3–20) | 18 (0–51.5) |
HBI: Harvey Bradshaw Index; CRP: C-reactive protein.
The study by Bar-Gil12 et al. includes 106 patients from Israeli sites with a mean follow-up of 24 weeks. The main outcome variable was steroid-free clinical remission at week 24. In total, 80% of the patients came from failure with two biologicals (including anti-TNF and anti-integrins). Ten of the patients (9.4%) discontinued treatment due to lack of response and 4 (3.7%) for side effects. Of the total cohort, 91 patients reached week 24: 38 patients with clinical response (41.7%), of whom 21 (23%) achieved steroid-free clinical remission at week 24.
In the Finnish cohort of Eberl,13 48 patients were included, 46 (96% of whom had previously failed with a biological and 34 (71%) with 2 or 3 biologicals. 73% were on combined treatment (48% corticosteroids). The main indication for UST was failure to respond to previous treatment (90%). Other reasons were side effects to previous treatments (30%) or immunomodulator inefficacy (40%).
Forty-two patients reached week 16 with a maintenance regimen every 8 weeks (69%) and every 12 (31%). Clinical and endoscopic activity was evaluated at week 16, as well as “clinical benefit”, defined as the proportion of patients in remission/response. HBI or endoscopic data were not available for all the patients.
At week 16, 63% of the patients were in clinical remission and 55% at the end of follow-up. Clinical benefit was 83% at week 16 and 76% at the end of follow-up. 88% were steroid-free.
During follow-up, 8 patients discontinued treatment due to lack of response.
Valdés14 et al. included 23 patients who had failed with two biologicals (without specifying whether they were anti-TNF or anti-integrin) evaluating the response at week 12 and 24 as a reduction in the mean value of CDAI and HBI. Only 11 patients reached week 24.
The Belgian cohort of Lieffericckx15 included 163 patients initially. The patients were evaluated at week 8, 16 and 52. Activity was evaluated by means of HBI and biochemistry by means of CRP. A total of 152 patients were ultimately included (those excluded include those with HBI <4). All the patients except one (due to a background of cancer) had previously been given anti-TNF, 82% had received 2 anti-TNFs. At the beginning, 70% were on systemic corticosteroids and 44.7% budesonide or similar.
At week 8, 59.2% presented clinical response, including 28.2% in remission. Meanwhile, 38.2% and 19.7%, respectively, were steroid-free.
At week 16: 51.9% presented response and 30.9% (30.9% RE), with 45.4% and 26.9% steroid-free. Of the 62% patients without initial clinical response (at week 8), 24.2% achieved a late clinical response at week 16. After one year of follow-up: 42.1% (25.7% RE) presented response and 38.8% (24.3% RE) were steroid-free. A sub-analysis of variables associated with response and remission after one year of treatment was performed, the only statistically significant variable being bowel disease and BMI under a non-remission factor.
Moreover, 17 patients (10%) required surgery during follow-up for disease-related complications, with the most frequent indication (6 patients) being intestinal resection.
Santoni et al.16 included 100 French patients between 2014 and 2017, evaluating response at week 8. Of these, 74% achieved PR and 50% RE.
We also have the Spanish ENEIDA series.17 It is a multicentre retrospective study including 305 patients from 42 Spanish hospitals that participate in the Estudio Nacional en Enfermedad Inflamatoria Intestinal sobre Determinantes Genéticos y Ambientales [Nationwide study on genetic and environmental determinants of inflammatory bowel disease] (ENEIDA). It includes patients with active CD (HBI >4 or endoscopic activity associated with CRP >3mg/l and/or FC >250 mcg/g) whose baseline characteristics are shown in Table 5. RE and PR were evaluated at weeks 8 and 14. The patients who had not received the first two doses of UST were excluded, as were those with an indication of perianal disease, recurrence prevention or extraintestinal involvement.
64% of the patients had failed with 2 anti-TNFs and 29% had failed with vedolizumab. 36% were taking corticosteroids at induction and 40% were on immunosuppressants.
Two groups were considered for the outcome analysis: 88 patients (28.8%) had an HBI ≤4. In this group, remission was achieved in 83 patients (94%) at week 8 and in 80 patients (90%) at week 14. The remaining 217 patients (72%) had an HBI >4. In these, remission was achieved in 101 patients (47%) at week 8 and in 126 patients (58%) at week 14.
Of the 109 patients on initial corticosteroids, 48% were corticosteroid-free at week 14, and 9 of the 11 anti-TNF-naïve patients achieved clinical remission.
As predictors of clinical response at week 14, intolerance of previous drugs (21% of the patients) is regarded as a good response factor, whereas the number of previous anti-TNFs or severity in the endoscopy are regarded as proof predictors, with statistical significance.
Saman18 included 41 patients, evaluating clinical response as a reduction in 100 points in the CDAI, reduction in stools or clinical improvement or by the PGA. Remission was regarded as CDAI <150.
Three patients were treated with CDAI <150 due to loss of response to previous treatments or intolerance.
Two groups were evaluated: one with CDAI <150 and mild disease, and another with moderate-severe CD. The data for both are provided together in Table 5. Of the 41 patients included, 92.7% had previously received an immunomodulator and 68.3% at least one anti-TNF. At the beginning of UST, 36.6 were on concomitant corticosteroids.
Fourteen of the 41 (34.1%) were non-responders and 3 of the initial responders changed to non-responders due to worsening diarrhoea.
58.5% responded after the first 3 doses of UST: 34.1% of the total with remission and 24.4% with response. In the study, several secondary analyses were performed depending on CRP levels, previous failure with anti-integrins and anti-TNFs. Of all the 37 patients who had failed with anti-TNF, 17 were primary non-responders, 10 of whom (59%) responded to UST without losing response. Of those who had previously lost their response to anti-TNF (20/37), 11 (55%) responded and 3 (15%) lost response. The study concludes that primary failure with anti-TNF does not seem to be a predictive factor of a poor response to UST.
They also analysed which patients would have fulfilled the inclusion criteria for the phase II/III clinical trials for UST, not being applicable in 39% of the patients, above all due to CDAI <220 (above all it is assumed that this subgroup of patients corresponds to those with previous intolerance or contraindication for anti-TNF).
Biemans19 included 221 patients. To evaluate effectiveness, the study only included patients with HBI >4.
The primary objective was steroid-free clinical remission at week 52. Clinical remission was defined as HBI <4 with response being a 3-point reduction in HBI versus the baseline. Biological remission CRP <5mg/l and CTF <200mg/l.
97.7% of the patients had previously received immunomodulators and 98.6% an anti-TNF (73.3% had received 2 anti-TNFs). 59.5% initiated UST in monotherapy. Sixty-eight patients presented HBI <5, of whom 86.8% presented activity data according to biomarkers, fistula, endoscopy or radiology. These patients were not included in the effectiveness data.
Data pertaining to response/remission/steroid-free remission, respectively: week 12 (47.7%/30.7%/24.2%), week 24 (46.1%/40.1%/38.2%) and week 52 (42.4%/39.4%/37.1%).
With regard to clinical factors associated with steroid-free remission, only BMI <18 appears as a poor response factor with statistical significance. Being anti-TNF-naïve does not appear to be a predictive factor of good response. Of the total, 46 patients required hospitalisation, 6 for induction, 14 required surgery (for whom follow-up data are available), and 9 remained on treatment, with 5 of them achieving steroid-free remission.
In real clinical practice, the CDAI tends to be somewhat unapplicable and the PGA probably reproduces the way that we act in the surgery better. In our case, the PGA, besides the anamnesis, included physical exploration data and analytical parameters. We thus achieved an excellent correlation between both indices (r=0.898).
The previously published studies did not perform a stratified evaluation of the results according to the reason for indication of UST (primary, secondary failure or toxicity), which is relevant because we are dealing with different clinical scenarios. In fact, this group of patients constitutes the population included in the UNITI-1, with no specific results reported for each one of the groups. In our series, no statistically significant differences were identified by stratifying the results for this reason. However, the response rates and remission in patients that initiated USD because of toxicity to anti-TNF were higher than the other indications (Table 4).
Another noteworthy aspect is that 4 of the 6 surgical patients restarted UST by way of prevention of post-surgical recurrence. In the other 2 patients, adalimumab was used, taking into account the natural history of the disease in each patient and response to previous treatments.
With regard to adverse effects, no anaphylactic reactions are described in the position paper for USD in CD. A risk of thrombosis was described, without being able to confirm causality. The main adverse effects described were asthenia and myalgias.3
According to the results of the UNITI studies, 3 severe infections were documented: one Listeria meningitidis and two oesophageal candidiases. Also, one case of active pulmonary tuberculosis. With regard to neoplasms, one case of multiple myeloma and one metastatic adenocarcinoma in the small intestine were reported in the UST group and one basocellular carcinoma in the placebo group.3,4
In the studies prior to the approval of ev UST, Khorrami described adverse events in 11 patients (9.5%): two coronary events and three infections merit particular mention.7 In the series of Wils,5 20 patients (16%), mainly myalgias (3%), infections (7%) requiring withdrawal in 4 of them (one for an allergic reaction). In the follow-up,6 the presence of an anal adenocarcinoma should be noted.
In the cohort of Ma,8 53 adverse events (31.1%) were recorded, mainly infections and arthralgias. In the series of Battat,9 50% presented minor adverse reactions and 4.8% major adverse reactions.
The following adverse events reported from real clinical practice data referred to above should be mentioned:
- -
Bar-Gil: 12 patients (11.3%) mainly arthralgias, weakness and skin lesions.12
- -
Eberl: 4 cases; two mild (rash, mouth discomfort) and two serious (abscess and possible allergic reaction), leading to the withdrawal of UST.13
- -
Lieffericckx: 11 patients (6.75%), including one discontinuation for arthralgias, one allergic reaction and one miscarriage.15
- -
Santoni: 11 patients (11%) and one patient died after five months from a serious adverse event.16
- -
ENEIDA study: 38 patients (12%), most of them systemic infections or local abscesses. Forty patients required hospitalisation (13%), 7 of them for adverse reactions: two for severe infections, three for obstruction, one for abdominal septic shock and one for a psoas abscess.17
- -
Saman: two patients (4.9%) arthralgias and skin lesions.18
- -
Biemans: 118 patients (60%), withdrawn in 8 for serious adverse effects (4 arthralgias, one reaction to the infusion, one vasculitis, severe headache and one for recurring infections). In addition, 6 severe infections, 4 of them gastrointestinal.19
The limitations of our study include the fact that the number of patients that reached week 52 is still low and that it is a refractory cohort who come from failure with two or more anti-TNF drugs. For this reason, current results must be interpreted in that light and may not be extrapolatable to biological-naïve patient cohorts.
Although there are broader series of patients, most of them include multicentre studies. Our study includes all the patients who initiated UST (prospective) with homogeneity of clinical criteria and posology, an aspect we regard as relevant when interpreting the results. The analysis stratified according to the reason for starting UST offers a more precise appraisal of the different scenarios of the effectiveness that we encounter in real practice.
Taking the results of our series into account, we may conclude that UST is an effective drug in real clinical practice with a good safety profile and that patients present high rates of clinical remission at week 16, 24 and 52, irrespective of the order of biological used. Moreover, we consider that stratifying outcomes according to the reason for beginning UST may help us to identify the scenarios in which the drug is most effective.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Saldaña Dueñas C, Rullán Iriarte M, Elosua González A, Rodríguez Gutiérrez C, Rubio Iturria S, Nantes Castillejo Ó. Ustekinumab en enfermedad de Crohn: efectividad y seguridad en práctica clínica. Gastroenterol Hepatol. 2020;43:497–505.