Hepatitis C virus (HCV) infection is a global health problem that can results in cirrhosis, hepatocellular carcinoma and even death. HCV infection is 3–20-fold more prevalent among patients with versus without severe mental illness (SMI), such as major depressive disorder, personality disorder, bipolar disorder and schizophrenia. Treatment options for HCV were formerly based on pegylated interferon alpha, which is associated with neuropsychiatric adverse events, and this contributed to the exclusion of patients with SMI from HCV treatment, elimination programmes, and clinical trials. Moreover, the assumption of poor adherence, scant access to healthcare and the stigma and vulnerability of this population emerged as barriers and contributed to the low rates of treatment and efficacy.
MethodsThis paper reviews the literature published between December 2010 and December 2020 exploring the epidemiology of HCV in patients with SMI, and vice versa, the effect of HCV infection, barriers to the management of illness in these patients, and benefits of new therapeutic options with pangenotypic direct antiviral agents (DAAs).
ResultsThe approval of DAAs has changed the paradigm of HCV infection treatment. DAAs have proven to be an equally efficacious and safe option that improves quality of life (QoL) in patients SMI.
ConclusionsKnowledge of the consequences of the HCV infection and the benefits of treatment with new pangenotypic DAAs among psychiatrists can increase screening, referral and treatment of HCV infection in patients with SMI.
La infección por el virus de la hepatitis C (VHC) es un problema de salud mundial que puede provocar cirrosis, carcinoma hepatocelular e incluso la muerte. La infección por el VHC es de 3 a 20 veces más prevalente entre los pacientes con enfermedades mentales graves (EMG), como el trastorno depresivo mayor, el trastorno de personalidad, el trastorno bipolar y la esquizofrenia. Las opciones de tratamiento para el VHC se basaban anteriormente en el interferón pegilado alfa, que se asocia con efectos adversos neuropsiquiátricos, y esto contribuyó a la exclusión de los pacientes con EMG del tratamiento del VHC, tanto de los programas de eliminación como de los ensayos clínicos. Además, la mala adherencia terapéutica, el escaso acceso de los pacientes a la asistencia sanitaria y el estigma y la vulnerabilidad de esta población surgieron como barreras y contribuyeron a las bajas tasas de tratamiento y eficacia.
MétodosEn este trabajo se revisa la literatura publicada entre diciembre de 2010 y diciembre de 2020 en la que se explora la epidemiología del VHC en pacientes con EMG, y vice versa, el efecto de la infección por VHC, las barreras para el manejo de la enfermedad en estos pacientes y los beneficios de las nuevas opciones terapéuticas con agentes antivirales directos pangenotípicos (AAD).
ResultadosLa aprobación de los AAD ha cambiado el paradigma del tratamiento de la infección por VHC. Los AAD han demostrado ser una opción igualmente eficaz y segura que mejora la calidad de vida (QoL) en los pacientes SMI.
ConclusionesEl conocimiento de las consecuencias de la infección por el VHC y los beneficios del tratamiento con los nuevos AAD pangenotípicos entre los psiquiatras puede aumentar el cribado, la derivación y el tratamiento de la infección por el VHC en pacientes con EMG.
Hepatitis C virus (HCV) infection is a global public health problem and the leading cause of chronic liver disease.1,2 It can lead to hepatic diseases such as cirrhosis or hepatocellular carcinoma1,2 and is the primary reason for liver transplants.2 According to the World Health Organization (WHO), nearly 400,000 patients died in 2016 from HCV-related liver disease.3
In 2015, after the introduction of direct-acting antivirals (DDAs), the WHO announced a global strategy with the goal of eliminating HCV by 2030.4 Like other health institutions and governments,5 Spain has positioned itself as one of the pioneer countries in the pursuit of the WHO goals through the National Strategic Plan for Hepatitis C (Plan Estratégico para el Abordaje de la Hepatitis C).5,6 Despite these goals, however, it was estimated that in 2017 and 2018, 76,839 people in the Spanish primary care setting presented with active infection. Moreover, the diagnosis rate of HCV remained unsatisfactory, and approximately 22,500 adults were unaware of their infection.5,7
Patients presenting with mental illness comorbid with HCV have largely been ineligible for clinical trials and HCV treatment programmes,8,9 limiting the information about the effects of antiviral therapy in this population. The aim of this review was to compile the most relevant evidence regarding HCV infection in patients with severe mental illness (SMI) in terms of epidemiology, effect of infection, barriers to management and treatment benefits.
MethodsA literature search was conducted in the PubMed database that included articles with abstracts published between December 11, 2010, and December 11, 2020, using multiple search queries to gather all publications concerning the prevalence, effect and management of HCV in the population with SMI, including schizophrenia, bipolar disorder, dual disorder, major depressive disorder (MDD) and borderline personality disorder. The search was restricted to studies in humans conducted in European and Western (the United States and Australia) populations, written in either English or Spanish. The search included clinical studies, clinical trials, controlled clinical trials, pragmatic clinical trials, randomized controlled trials, guidelines, practice guidelines, meta-analyses, multicentre studies, observational studies, reviews and systematic reviews.
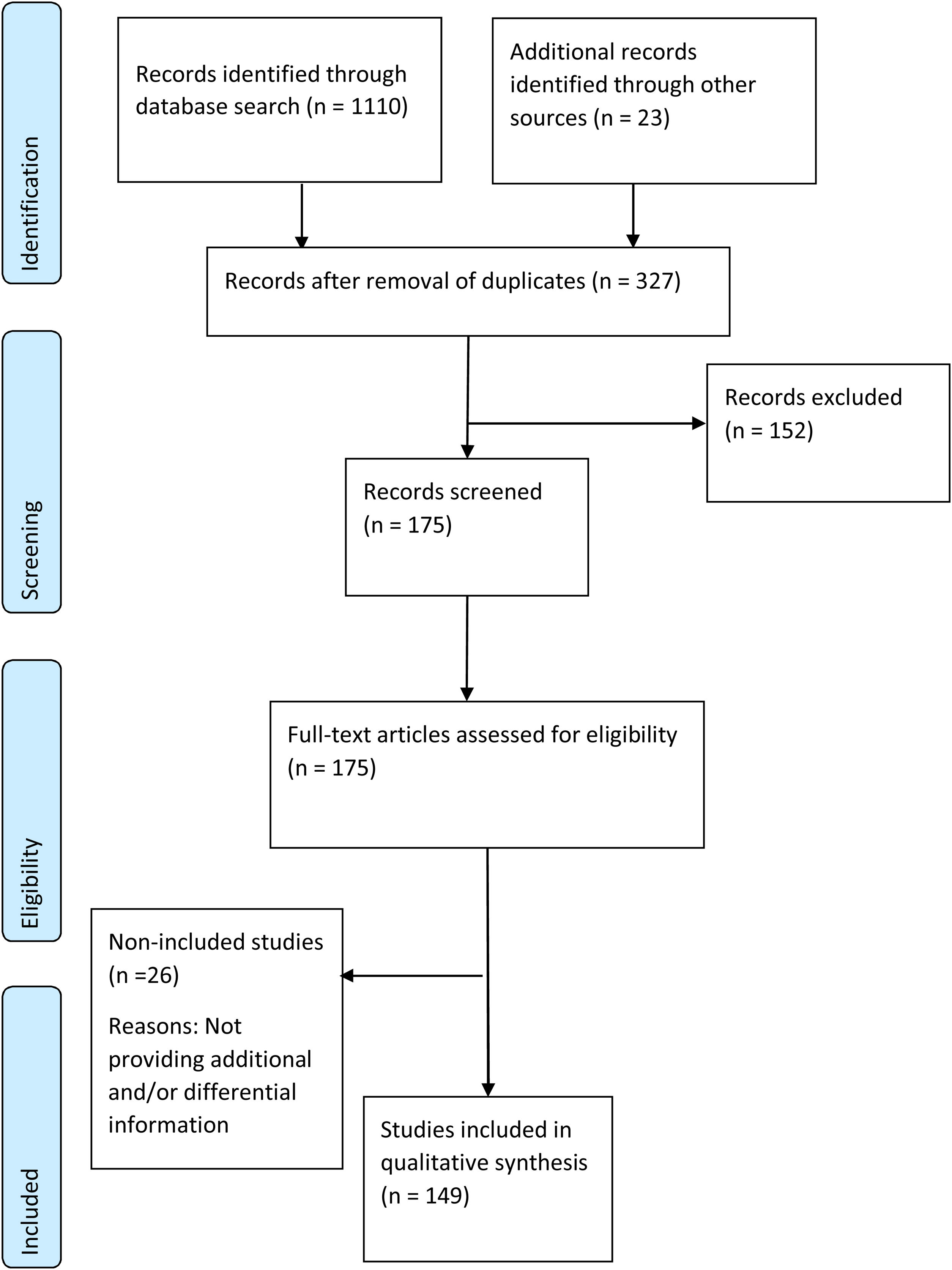
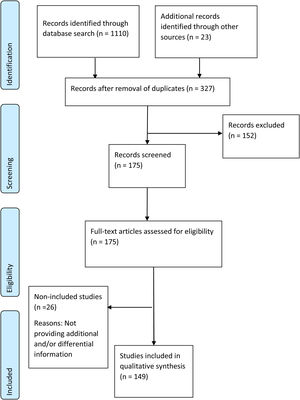
Duplicates were removed, and the title and abstract of all the identified publications were screened by two reviewers for relevance, with disagreements resolved by a third reviewer. After a full-text review of all the selected publications, all relevant information for the predefined topics of this review was systematically extracted by both reviewers. A PRISMA flow diagram was used to describe the results of the study selection process (Fig. 1).
The results of this search are presented narratively and include data obtained in the publications selected as well from an ascendant search from the references included in the selected publications.
ResultsPRISMA flow of publications reviewedA total of 1110 publications were identified. Duplicates were excluded, and 23 records, identified through ascendant search, were included. 327 references were retained and screened based on title and abstract, and 152 references were excluded. The remaining175 references were selected for full-text review, after which 26 were excluded owing to lack of new information. Finally, 149 references were included in the review (Fig. 1).
EpidemiologyHepatitis C virus infection in the general populationAccording to the WHO Global Hepatitis Report published in 2017, 71 million people were living with chronic HCV infection in 2015.10 This accounted for 1% of the worldwide population, with the highest prevalence reported in the Eastern Mediterranean and European regions. In Spain, according to data from the Seroprevalence Study of the Ministry of Health, 0.85% of the 20–80-year-old population in 2017 and 2018 had antibodies against HCV and 0.22% presented with active infection.7
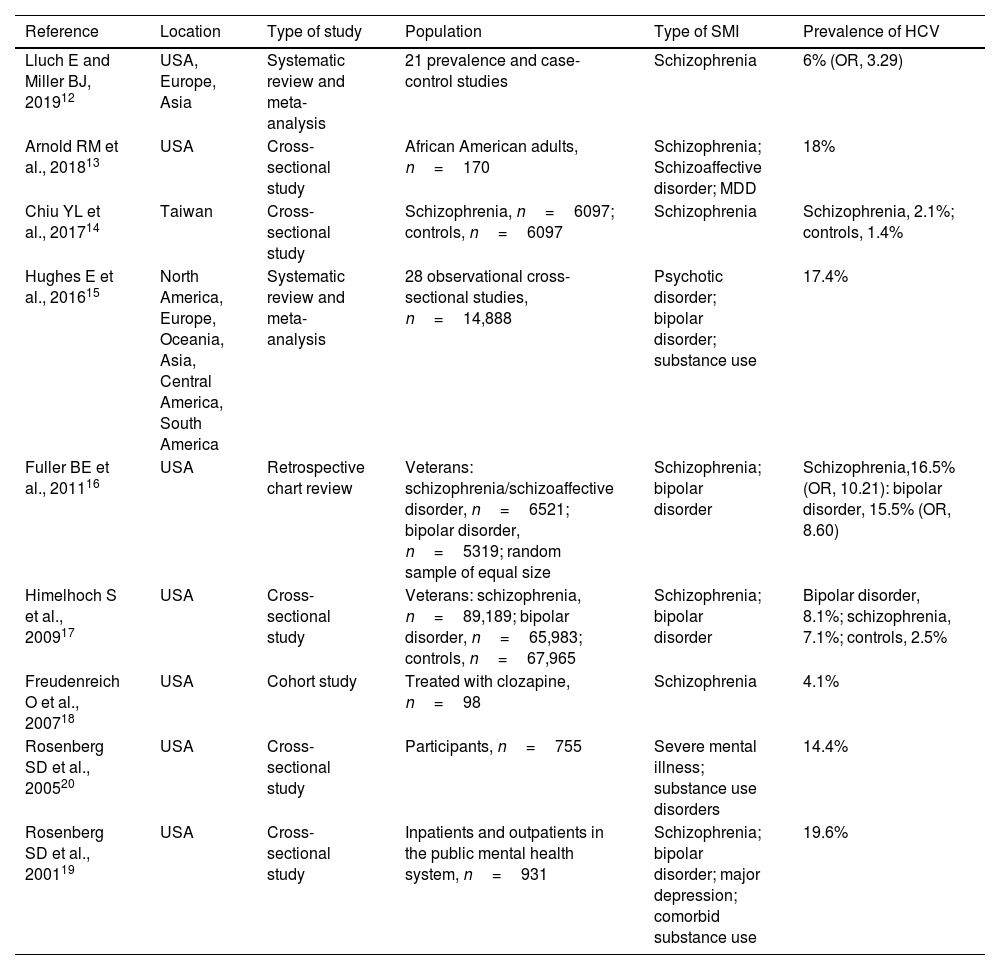
Hepatitis C virus infection in patients with severe mental illnessThe prevalence of HCV infection is higher among patients with versus without SMI. Some studies have estimated the prevalence of HCV infection to be 8–30% in patients with a mental illness diagnosis,11 with a 3–20-fold increased risk of infection versus those without a diagnosis (Table 1).12–20 These statistics do not include drug-dependent patients, for whom the prevalence of HCV was as high as 60–70%.21
Results of studies estimating the prevalence of HCV among patients with psychiatric disorders.
| Reference | Location | Type of study | Population | Type of SMI | Prevalence of HCV |
|---|---|---|---|---|---|
| Lluch E and Miller BJ, 201912 | USA, Europe, Asia | Systematic review and meta-analysis | 21 prevalence and case-control studies | Schizophrenia | 6% (OR, 3.29) |
| Arnold RM et al., 201813 | USA | Cross-sectional study | African American adults, n=170 | Schizophrenia; Schizoaffective disorder; MDD | 18% |
| Chiu YL et al., 201714 | Taiwan | Cross-sectional study | Schizophrenia, n=6097; controls, n=6097 | Schizophrenia | Schizophrenia, 2.1%; controls, 1.4% |
| Hughes E et al., 201615 | North America, Europe, Oceania, Asia, Central America, South America | Systematic review and meta-analysis | 28 observational cross-sectional studies, n=14,888 | Psychotic disorder; bipolar disorder; substance use | 17.4% |
| Fuller BE et al., 201116 | USA | Retrospective chart review | Veterans: schizophrenia/schizoaffective disorder, n=6521; bipolar disorder, n=5319; random sample of equal size | Schizophrenia; bipolar disorder | Schizophrenia,16.5% (OR, 10.21): bipolar disorder, 15.5% (OR, 8.60) |
| Himelhoch S et al., 200917 | USA | Cross-sectional study | Veterans: schizophrenia, n=89,189; bipolar disorder, n=65,983; controls, n=67,965 | Schizophrenia; bipolar disorder | Bipolar disorder, 8.1%; schizophrenia, 7.1%; controls, 2.5% |
| Freudenreich O et al., 200718 | USA | Cohort study | Treated with clozapine, n=98 | Schizophrenia | 4.1% |
| Rosenberg SD et al., 200520 | USA | Cross-sectional study | Participants, n=755 | Severe mental illness; substance use disorders | 14.4% |
| Rosenberg SD et al., 200119 | USA | Cross-sectional study | Inpatients and outpatients in the public mental health system, n=931 | Schizophrenia; bipolar disorder; major depression; comorbid substance use | 19.6% |
HCV, hepatitis C virus; MDD, major depressive disorder; OR, odds ratio; SMI, severe mental illness.
The increased risk of HCV infection among those with mental illness diagnoses has been associated with several factors. This population is more likely than the general population to engage in higher-risk habits, such as the use of injectable drugs, having multiple sexual and high-risk partners and infrequent condom use.19,22,23 Moreover, the presence of SMI is associated with poverty, risky environments, and poor access to health and medical care.24
A higher risk of HCV reinfection has been described in injectable drug users.25 Because impulsive behaviour is frequently associated with illicit drug use,26 some authors have suggested evaluating the connection between impulsive behaviour and chronic HCV infection.27 Substance abuse and the use of alcohol are higher in the HCV-infected population, with a prevalence of 29–86%, depending on the population screened.28–31 On the other hand, people who injected drugs have a high prevalence of HCV infection (more than 80%)32 and a high prevalence of mental illness (more than 67%), so dual diagnosis patients can be considered to be those who have may constitute the group of major prevalence and risk for HCV transmission.32,33
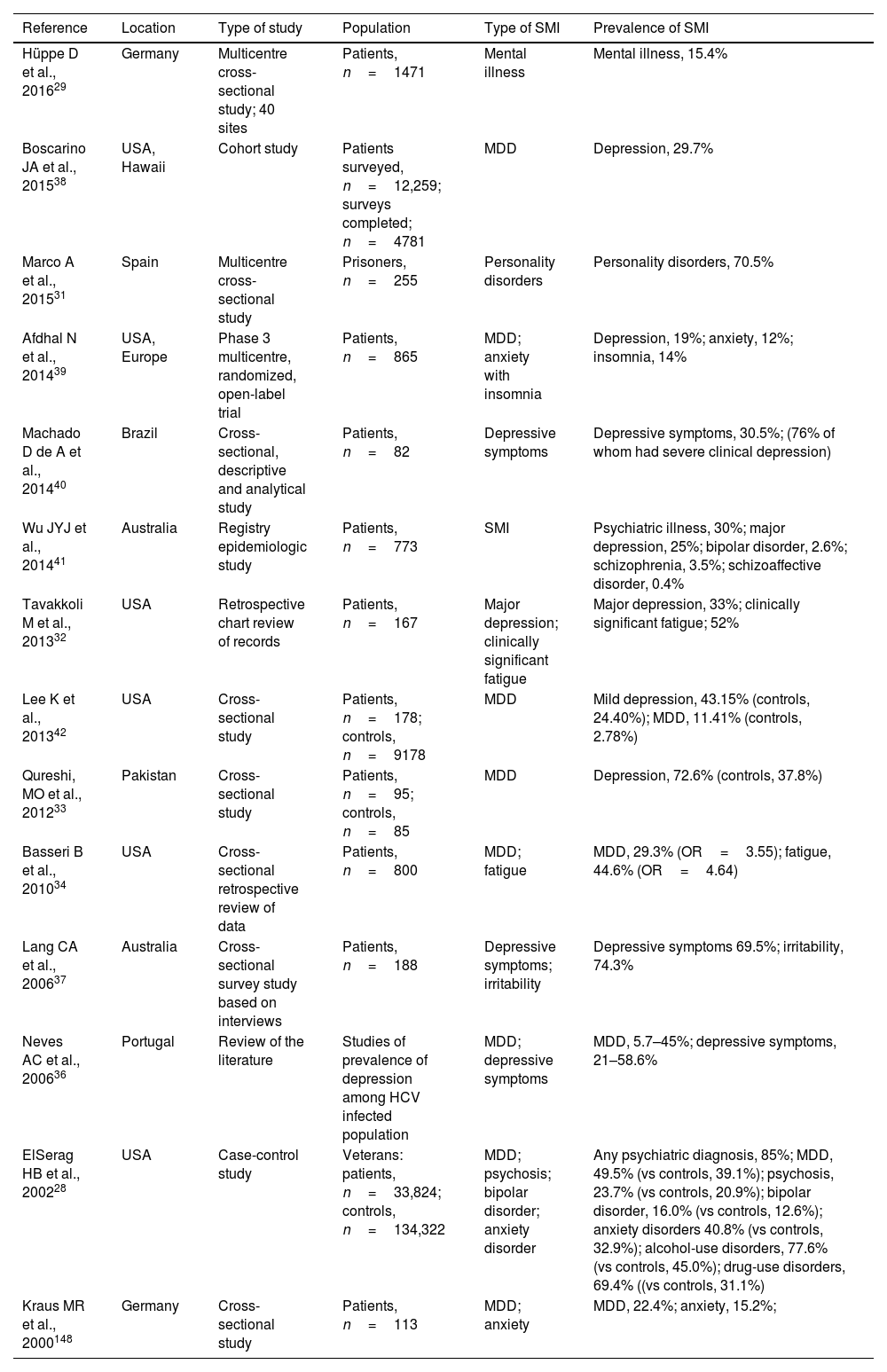
Severe mental illness in patients with hepatitis C virus infectionThe prevalence of mental illness and psychiatric disorders is higher in patients infected with HCV than the general population (Table 2).28,29,31–43 It has been estimated that up to 70% of patients with HCV may also have depressive disorders,28,44 with a prevalence of depression and fatigue 3–4 times higher in patients with HCV than the general population.34 A study reported that of 773 HCV-infected patients 30% had a pre-existing diagnosis of SMI: 25% depression, 2.6% bipolar disorder, 3.5% schizophrenia and 0.4% schizoaffective disorder.43
Results of studies estimating the prevalence of psychiatric disorders among HCV-infected population.
| Reference | Location | Type of study | Population | Type of SMI | Prevalence of SMI |
|---|---|---|---|---|---|
| Hüppe D et al., 201629 | Germany | Multicentre cross-sectional study; 40 sites | Patients, n=1471 | Mental illness | Mental illness, 15.4% |
| Boscarino JA et al., 201538 | USA, Hawaii | Cohort study | Patients surveyed, n=12,259; surveys completed; n=4781 | MDD | Depression, 29.7% |
| Marco A et al., 201531 | Spain | Multicentre cross-sectional study | Prisoners, n=255 | Personality disorders | Personality disorders, 70.5% |
| Afdhal N et al., 201439 | USA, Europe | Phase 3 multicentre, randomized, open-label trial | Patients, n=865 | MDD; anxiety with insomnia | Depression, 19%; anxiety, 12%; insomnia, 14% |
| Machado D de A et al., 201440 | Brazil | Cross-sectional, descriptive and analytical study | Patients, n=82 | Depressive symptoms | Depressive symptoms, 30.5%; (76% of whom had severe clinical depression) |
| Wu JYJ et al., 201441 | Australia | Registry epidemiologic study | Patients, n=773 | SMI | Psychiatric illness, 30%; major depression, 25%; bipolar disorder, 2.6%; schizophrenia, 3.5%; schizoaffective disorder, 0.4% |
| Tavakkoli M et al., 201332 | USA | Retrospective chart review of records | Patients, n=167 | Major depression; clinically significant fatigue | Major depression, 33%; clinically significant fatigue; 52% |
| Lee K et al., 201342 | USA | Cross-sectional study | Patients, n=178; controls, n=9178 | MDD | Mild depression, 43.15% (controls, 24.40%); MDD, 11.41% (controls, 2.78%) |
| Qureshi, MO et al., 201233 | Pakistan | Cross-sectional study | Patients, n=95; controls, n=85 | MDD | Depression, 72.6% (controls, 37.8%) |
| Basseri B et al., 201034 | USA | Cross-sectional retrospective review of data | Patients, n=800 | MDD; fatigue | MDD, 29.3% (OR=3.55); fatigue, 44.6% (OR=4.64) |
| Lang CA et al., 200637 | Australia | Cross-sectional survey study based on interviews | Patients, n=188 | Depressive symptoms; irritability | Depressive symptoms 69.5%; irritability, 74.3% |
| Neves AC et al., 200636 | Portugal | Review of the literature | Studies of prevalence of depression among HCV infected population | MDD; depressive symptoms | MDD, 5.7–45%; depressive symptoms, 21–58.6% |
| ElSerag HB et al., 200228 | USA | Case-control study | Veterans: patients, n=33,824; controls, n=134,322 | MDD; psychosis; bipolar disorder; anxiety disorder | Any psychiatric diagnosis, 85%; MDD, 49.5% (vs controls, 39.1%); psychosis, 23.7% (vs controls, 20.9%); bipolar disorder, 16.0% (vs controls, 12.6%); anxiety disorders 40.8% (vs controls, 32.9%); alcohol-use disorders, 77.6% (vs controls, 45.0%); drug-use disorders, 69.4% ((vs controls, 31.1%) |
| Kraus MR et al., 2000148 | Germany | Cross-sectional study | Patients, n=113 | MDD; anxiety | MDD, 22.4%; anxiety, 15.2%; |
HCV, hepatitis C virus; MDD, major depressive disorder; SMI, severe mental illness.
Although HCV infection is easily diagnosed through blood tests,45 it often goes undiagnosed in the early stages. Because only mild flu-like symptoms are observed at the beginning of the infection,46 acute HCV frequently goes undiagnosed, with the majority of HCV cases not detected until the chronic phase.46 In 2008, it was estimated that 60–90% of the 5–10 million chronically infected people in Europe were undiagnosed.47 These figures have probably fallen lately, as they have in the United States, where undiagnosed HCV diminished from 70% to 50%, mostly as a result of enhanced screening for HCV initiated in 2012.48
Undiagnosed HCV is particularly high among patients with SMI. In a study in California, HCV screening rates of 4.7% were reported for patients receiving care in mental health clinics versus 12.7% for the overall US population.49 One possible explanation is that some mental health professionals may be reluctant to assume responsibility for referral and treatment, have doubts about diagnostic testing, lack medical knowledge, be under time constraints or be reluctant to inquire about risk factors.45 Given the high prevalence of HCV among people with SMI, undiagnosed HCV represents a serious public health concern.
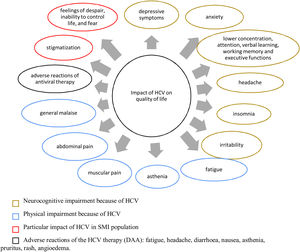
Effect of hepatitis C virus infection on patients with severe mental illnessQuality of lifeActive HCV infection is generally associated with impaired quality of life (QoL) in all dimensions: physical, mental and social.50 Extrahepatic symptoms linked to reduced health-related QoL (HRQoL), such as fatigue, asthenia, irritability, general malaise, musculoskeletal and joint pain, headaches, anorexia, myalgia, vomiting, abdominal pain, anhedonia and insomnia as well as depression, anxiety and increased sensitivity to pain have been reported in patients with chronic HCV.50–53 Some of these symptoms contributing to the decrease in QoL may have been caused by some antiviral drugs that are no longer used, such as pegylated interferon alpha (PEG-IFN-α), ribavirin (RBV), and even protease inhibitors, such as telaprevir and boceprevir.54
The effect of HCV infection is greater in patients with psychiatric disorders55 than the general population. The combination of chronic viral hepatitis with other chronic diseases has been associated with feelings of despair, inability to control life and fear of loss of capacities and death.56 Although a direct effect of HCV infection on HRQoL exists, the presence of psychiatric comorbidities, the diagnosis of HCV and the anxiety generated by prognosis, treatment and stigmatization have also been described as determinant.57–60 The presence of MDD, for instance, has been found to be more determinant for the HRQoL of patients with HCV than the grade of liver fibrosis. Consequently, the psychological effects of the disease deserve special attention in this population, and the implementation of integrated medical, psychiatric, and psychological care may be helpful.61
Patients with end-stage liver disease caused by HCV are at greater risk of depression or mood disorders than other patients with the disease.62 Even HCV-infected patients with mild liver disease have shown increased levels of depression and reduced concentration, attention, verbal learning, working memory, executive functions and psychomotor abilities.57
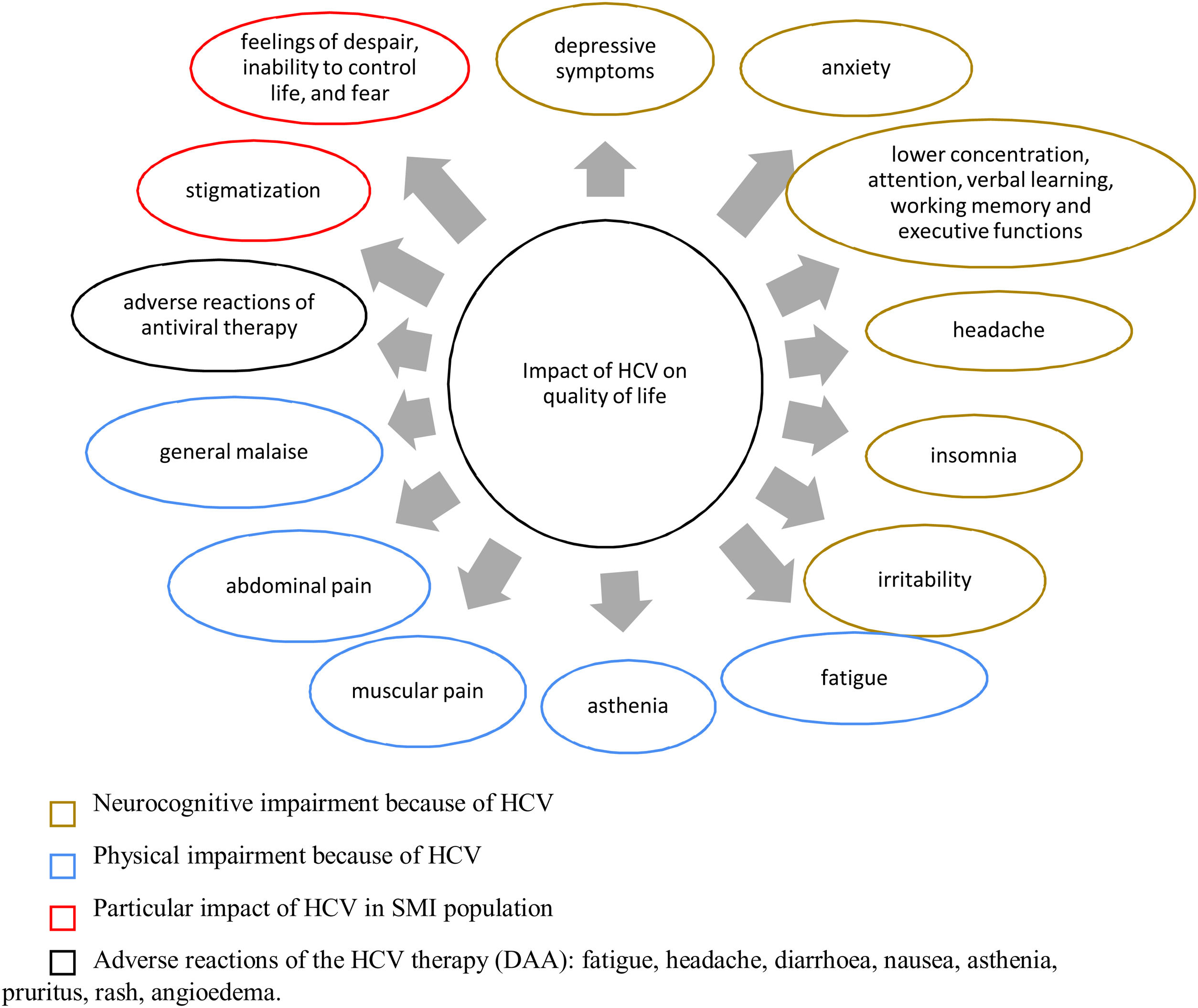
Evidence about the QoL dimensions affected by HCV infection are summarized in Fig. 2, both in the general population and in patients with SMI.
Effect of HCV infection on quality of life. DAA, direct-acting antivirals; HCV, hepatitis C virus; SMI, severe mental illness. References: Depression,28,39,54,55,42,139 anxiety,28,39,55,139 lower concentration attention, verbal learning, working memory and executive functions,55 headache,49,50,51 insomnia,39 irritability,49,50,51 fatigue,49,51,52,55,139 asthenia,50 muscular pain,50,139 abdominal pain,52 general malaise,50 adverse reactions of antiviral therapy,52 stigmatization,55 feelings of despair, inability to control life and fear.54
The presence of mental health disorders has been reported as a primary reason for excluding HCV-infected patients from interferon (IFN)-based antiviral therapy, which is associated with neuropsychiatric adverse events.28 However, there is evidence that patients treated and achieving a sustained viral response (SVR) have a better QoL than those who do not achieve SVR.63
Neurocognitive impairmentA clear association between neurocognitive impairment and HCV infection has been found in the literature. Several studies have concluded that HCV infection leads to changes in verbal recall, working memory, processing speed, attention, concentration, fine motor skills, executive function, learning, memory and cognitive performance.64–68
Neurocognitive impairment is one of the most common extrahepatic manifestations of HCV, independent of either the degree of fibrosis65 or the presence of depression or encephalopathy.69 Attention and concentration deficit have been reported in up to 50% of non-cirrhotic patients.70,71 Cognitive dysfunction has been shown to affect approximately one-third of patients with HCV infection without decompensated cirrhosis.72 Although neurocognitive impairment has been found in patients infected with HCV and mild liver disease,73,74 it is more marked in patients with advanced liver fibrosis.75
The pathogenesis of HCV-related neurocognitive dysfunction appears to be associated with HCV-driven chronic mild inflammation affecting the brain, especially the white matter. Some evidence supports the idea that HCV can cross the blood-brain barrier.61 In fact, replicative forms of HCV virus have been found in the brain of some infected patients in autopsy studies.76 HCV can replicate in the mononuclear cells of the immune system and within brain cells, causing an inflammation which, while usually mild, may lead to cognitive dysfunction.77 There is also growing evidence of a genetic basis for QoL outcomes in patients with HCV, and an immune activation of the brain induced by a pro- and anti-inflammatory cytokine imbalance may be an explanation.78
Barriers to the management of patients with hepatitis C virus infection and severe mental illnessThe presence of SMI in HCV-infected patients has an influence on their management and prognosis.28 Here we review some of the barriers and challenges observed in this regard.
Lack of treatment, treatment delay and treatment failureAntiviral treatment was considered to be contraindicated in patients with HCV comorbid with certain psychiatric disorders until 201179 because IFN-based therapy, the gold standard treatment, was associated with neuropsychiatric adverse events. In fact, most of the existing concerns about antiviral treatment, in terms of neuropsychiatric side effects, were primarily related to IFN-based therapies.80 Treatment with PEG-IFN-α and RBV was contraindicated in patients with a clinical history or a current diagnosis of depression.81
The presence of substance abuse or mental disease in patients with HCV has been associated with lower treatment rates in many studies.43,82–84 Substance abuse and psychiatric disorders were considered predictors of non-treatment, with odds ratios of 17.68 and 9.45, respectively.84 In another study, the exclusion rate from HCV therapy due to SMI was 44%.83 A third study reported that patients with versus without psychiatric disorders (mainly, schizophrenia and schizoaffective disorder, followed by bipolar affective disorder and MDD) are 1.41 times less likely to receive antiviral treatment.43 Moderate to severe depressive symptoms have also been associated with antiviral treatment delay. Similarly, untreated depression or generalized anxiety disorder have been shown to be related to HCV treatment failure.85 Patients with HCV infection and psychiatric comorbidities may be undertreated owing to fears of worsening the underlying condition due to side effects, although compliance concerns may also play a role.43,86,87
Poor adherenceAdequate compliance is crucial to achieving an SVR and successful HCV treatment. A systematic review showed that non-adherence to IFN-based antiviral therapy is linked to viral response failure.88 This is important because lack of adherence can favour the development and spread of resistant HCV mutations.89 Before the approval of DAAs, the use of PEG-IFN-α was associated with side effects, which was one of the main reasons for treatment discontinuation, dose reductions and lack of compliance.90
Substance abuse and psychiatric disorders are associated with cognitive impairment in HCV-infected patients and can lead to poor adherence and lack of treatment efficacy.91 Some studies have suggested that psychiatric comorbidity and drug abuse are risk factors for non-adherence and not reaching SVR,92–94 whereas other studies have not found significant differences.95–99 Discontinuation due to psychiatric adverse events, such as fatigue, depression, irritability and insomnia, have been found in 10–14% of patients receiving IFN-based therapy.100,101 Nevertheless, a European expert consensus on HCV, antiviral treatment and mental health stated that when proper treatment and monitoring are provided, psychiatric pathology does not entail an increased risk of poor adherence.102
Stigma and vulnerabilityBoth HCV infection and SMI contribute to patient stigmatization and vulnerability. The diagnosis of HCV infection usually produces a feeling of fear of transmitting the virus, affects social life and increases anxiety levels.65,103 According to some authors, psychiatric morbidity associated with HCV appears, in part, from coping with stigma and prejudice.104 During advanced disease stages, patients may even be unable to hold a job, which can have financial, socioeconomic and QoL-related implications.105 Moreover, the stigma felt among people with HCV might discourage them from seeking assistance from friends or family, further increasing the feeling of exclusion.106
There is also a stigma connected with SMI, with a consequent effect on patients, providers and payers in terms of receiving and offering proper treatment.45 In particular, patients with HCV with psychiatric disorders or substance abuse are frequently associated lack of education, insufficient social support systems, homelessness and stigmatization.30 As a consequence, the coexistence of HCV and SMI signifies complex social challenges.
Access and links to healthcarePatients with HCV face barriers to care at every stage of the disease. A model for HCV treatment in the United States based on a meta-analysis estimated that only half of patients with HCV received screening and education; chronic infection was verified in only 25%; and fewer than 10% achieved SVR.107 Some barriers to treatment access have been identified at the patient level (patient preference, alcohol abuse, missed appointments), provider level (reluctance to treat past substance abusers) and system level (referral-associated delays).108
Patients with HCV with psychiatric disorders or substance abuse frequently do not have medical insurance or sufficient access to medical care.30 Similarly, they have poor access to HCV education, screening, diagnostic confirmation and treatment.45
In view of these data, HCV care needs to be enhanced, especially within the subpopulation of those with SMI. A pragmatic cascade for the care of patients with HCV has been proposed by some authors, addressing issues such as testing, links to care, liver fibrosis assessment, treatment uptake, adherence and cure of HCV.107
Some studies have also highlighted the importance of strict and comprehensive monitoring of patients with HCV infection and psychiatric diseases or substance abuse.86,102,109 A study by Sockalingam and colleagues stated that patients with HCV, psychiatric disorders and substance abuse benefit from a community-based interdisciplinary model of psychosocial support and a harm-reduction approach.86
Change of paradigm in hepatitis C virus infection treatmentPrevious interferon-based treatmentInterferon-based therapy is associated with side effects that include depressive symptoms,102–110 fatigue, irritability, anxiety and cognitive and sleep disturbances.102 A history of psychiatric disease is a strong risk factor for developing depression, anxiety and other psychiatric disorders during IFN-based therapy.77,111–115
Suicidal thoughts can arise from a combination of depressive symptoms, anxiety, agitation, and irritability,46 representing the worst complication of IFN-induced depression.113 The incidence of suicidal ideation in patients with HCV has proven to be higher among those treated with INF-based therapy.116
Neurobehavioral symptoms associated with IFN-based therapy have been reported to reduce HRQoL and compromise compliance, thereby reducing its antiviral efficacy.117–119 With INF-based therapy, initiation rates ranged from 14% to 29% in different study populations. The presence of decompensated liver disease, comorbidities, psychiatric disorders and concerns about side effects as well as the lack of access to care were the main reasons for these low treatment rates.82,108,120–123 Nevertheless, both European and American guidelines formerly recommended a multidisciplinary approach with immediate access to specialized management instead of excluding HCV-infected patients with psychiatric comorbidity from IFN-based therapy79,102,124
Benefits of current treatment with direct-acting antiviral agentsThe approval of DAAs has triggered a change of paradigm in the treatment and management of patients with HCV.125 DAAs allow short-term administration, are suitable for almost all kinds of patients with HCV and have shown excellent tolerance and efficacy rates.126 Thanks to the availability of these safe, effective and well-tolerated options, the WHO established the elimination of HCV infection as an objective for 2030.4 In Spain, almost 144,000 HCV-infected patients were treated with DAAs between 2015 and 2020, representing almost all patients diagnosed and followed up.6
Approval of pangenotypic DAA regimens in 20164 has eliminated the universal requirement for pre-treatment HCV genotyping, although it is still recommended in patients with prior treatment failure because the DAA treatment regimen and duration may differ according to the genotype. Pangenotypic DAA regimens have greatly simplified HCV antiviral therapy administration by reducing treatment duration.124
Current pangenotypic DAAs are effective and safe options. They are indicated for patients with chronic HCV infection. An 8-week glecaprevir plus pibrentasvir regimen is recommended for all treatment-naive patients without cirrhosis or with compensated cirrhosis. For treatment-experienced patients (previously treated with PEG-IFN+RBV±sofosbuvir, or sofosbuvirvRBV), treatment varies between 8 to 16 weeks. A 12-week regimen of sofosbuvir+velpatasvir is recommended in patients without cirrhosis or with compensated cirrhosis (plus RBV in genotype 3 patients with compensated cirrhosis) and 12 weeks plus RBV in decompensated cirrhosis. In the case of coexisting HIV infection, the indication of both DAAs is the same as in patients with HCV alone.127,128
Adherence rates with new pangenotypic treatments have proved to be as high among patients with psychiatric disorders (95.4%) as in patients without them (96.7%), with the lowest rate of 89.5% in patients with bipolar disorder.129 Similarly, in clinical trials, adherence with DAA regimens have proven to be high among patients at risk of non-adherence, such as patients with psychiatric disorders, with injectable drug use or on stable opioid substitution therapy.80,130,131
EfficacySustained viral response rates higher than 95% have been reported in patients treated with DAAs at week 12 post-treatment (SVR12). The use of all-oral DAA combinations provides a significantly improved short- and long-term prognosis for HCV-infected patients.132 A retrospective study including 833 patients showed that SVR12 rates in HCV-infected patients with mental illness and/or substance abuse treated with DAAs were greater than 95% and statistically significant differences for patients without mental illness or substance abuse were not perceived.11 Thus, DAAs are an effective option for treating HCV infection regardless of the presence of underlying mental illness or substance abuse, with good efficacy and minimal psychiatric side effects, an important finding for patients previously excluded from treatment.133
Safety, tolerability and drug–drug interactionsDirect-acting antivirals have few adverse effects and are well tolerated, particularly compared with IFN-based regimens. Because adverse events, such as fatigue, headache or nausea, were mild during clinical trials, patients were more likely to adhere to and complete treatment.45,134 DAAs have also been associated with a lower incidence of depressive episodes than IFN-based regimens.135 In real-world practice, DAAs have not increased symptoms of depression or sleep disturbances in patients with HCV and psychiatric comorbidity or drug abuse. In fact, symptoms of depression were significantly reduced 12 weeks after treatment.133
Direct-acting antivirals are associated with few drug-drug interactions, although some have been reported. The use of certain cytochrome and glycoprotein P-inducing agents (such as carbamazepine, phenytoin, phenobarbital, primidone, oxcarbamazepine, eslicarbazepine and St John's wort) are contraindicated with all DAA regimens owing to the risk of significantly reduced concentrations of DAAs and the consequent high risk of virologic failure.127,128,136,137 Treatment with these anticonvulsants for patients unable to switch remains problematic and further data regarding treatment for these patients are required. The European Association for the Study of the Liver guidelines137 have recommended the use of a free, regularly updated, public-access tool developed by the University of Liverpool to check key drug-drug interactions.138
Quality-of-life improvementSustained viral response has been associated with HRQoL improvements in patients receiving treatment for HCV139 even up to one year after the end of treatment.140,141 Because DAAs report SVR rates above 95% and a good safety profile, improved QoL in patients treated with DAAs is expected. Some studies have focused on the effect of DAAs on HRQoL, showing improvements in the mental health and emotional domains of patient-reported questionnaires.142,143 In general, patient-reported outcomes have been better with DAAs than with previous medications for the treatment of HCV infection.142,144
The improvements in HRQoL and in general health, fatigue, emotional well-being and physical functioning have been observed with sofosbuvir-based therapy, achieving SVR after 12 weeks of treatment, even in patients with cirrhosis.144–146 Other trials have demonstrated that, compared with IFN-based regimens, improvements in HRQoL occur within the first 4 weeks of DAA treatment, coincide with suppression of viral replication and continue during treatment and follow-up.147,148 Moreover, they also seem to be effective in decompensated cirrhotic patients for whom IFN-based treatment is contraindicated.147
Nardelli and co-authors have reported improvement in neuropsychological tests and in most HRQoL domains in patients treated with DAAs. Moreover, they observed a significant correlation between each psychological test and the summary components of the 36-item Short Form Health Survey HRQoL questionnaire.103 Similarly, a recent study reported that DAAs do not worsen mood symptoms or promote the onset of new psychiatric conditions in DAA-naive patients, even in those with a history of psychiatric illness.149
Taking all these data into account, DAAs have a very good safety profile and improve patient QoL. However, because addressing psychiatric variables might also have a positive effect on QoL, a combination of psychotherapy, cognitive intervention and support groups has been proposed for patients to achieve a better QoL and to reduce complaints about health status, mood and cognition.64
DiscussionIn summary, the prevalence of HCV infection is higher in patients with versus without SMI. Similarly, mental illness is more prevalent in the HCV-infected population than in the general population. HCV infection has an effect on QoL and neurocognitive function for many patients, but it is even more disrupting in patients with SMI. However, these patients have been excluded from treatment with HCV antiviral therapy because of the association of IFN-based therapy, formerly the gold standard, to neuropsychiatric adverse events.
The approval of pangenotypic DAAs in 2016 represented a paradigm shift, providing an effective and safe treatment for HCV infection regardless of the presence of underlying psychiatric illness or substance abuse. Moreover, patient HRQoL has been shown to improve after achieving SVR with pangenotypic DAAs.
A greater awareness among mental health physicians of the consequences of HCV infection and the benefits of effective treatment should help increase the screening and referral rate of their patients with SMI for treatment of HCV. In addition, barriers to administering and receiving HCV antiviral therapy (e.g., inadequate treatment, poor patient compliance, reluctance to treat past substance abusers, referral delays) will need to be overcome for patients with SMI. In this way, the coordinated work of specialists can contribute to achieving in the WHO global strategy to eliminate HCV by 2030.
Authors’ contributionsAll authors contributed to the study conception and design, and reviewed the queries for literature search. The first draft of the manuscript was written by the contracted medical writer and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availabilityThis research is a review article. All the data are extracted from the articles reviewed.
FundingThis work was supported by AbbVie. AbbVie reviewed the content of the publication. All content decisions remained with the authors. No honoraria or payments were made for authorship. Funding for open access charge: Universidad de Granada/CBUA.
Conflict of interestsLuis Gutiérrez-Rojas has been a speaker for, a consultant for and/or advisory board member of Janssen-Cilag, Lundbeck, AbbVie and Servier.
Jesús J. de la Gándara has been a speaker for Pfizer.
Luisa Garcia-Buey has served as an advisor and/or a speaker for Intercept Pharmaceuticals, MSD, AbbVie and Gilead.
Juan Isidro Uriz Otano has been a speaker for and/or advisory board member of AbbVie, Gilead and Intercept Pharmaceuticals.
Álvaro Mena has been a speaker for and/or a consultant for Janssen, Gilead, MSD, Viiv and AbbVie.
Carlos Roncero has received lecture fees from Janssen-Cilag, Indivior, Servier, GSK, Rovi, AstraZeneca, Gilead, MSD, Sanofi, Exeltis, AbbVie, Takeda, Rubio and Casen. He has received financial compensation for his participation as a consultant and a board member of AbbVie, Lundbeck, Gilead, MSD, Indivior, Exceltis, Martindale, Camurus, Gebro and Mundipharma. He has carried out the PROTEUS project, which was funded by a grant from Reckitt-Benckiser/Indivior and the COSTEDOPIA project, which was funded by Indivior. He received two medical educational grants from Gilead and medical writing support from AbbVie.
The authors thank Maite Artés, Gloria González and Montse Pérez from Adelphi Targis for their support in the literature review and Maite Artés from Adelphi Targis and Jemina Moreto on contract with Adelphi Targis for their medical writing contribution. These contributions were funded by AbbVie. All the authors reviewed the manuscript for medical and scientific accuracy as well as for intellectual property considerations.