Helicobacter pylori infection affects approximately 70% of the Chilean population. It is a public health problem whose eradication treatment is part of the explicit health guarantees in Chile.
ObjectivesCharacterize the most widely used H. pylori first-line eradication therapies in our environment and evaluate their efficacy.
MethodsA retrospective observational study was carried out where, in patients with certified H. pylori infection, the eradication therapy indicated by the treating physician, its efficacy, adherence and adverse effects, in addition to the eradication certification method used, were evaluated.
Results242 patients and 4 main therapies were analyzed: standard triple therapy, dual therapy, concomitant therapy, and bismuth quadruple therapy. Eradication rates of 81.9% (95% CI 74.44–87.63), 88.5% (95% CI 73.13–95.67), 93.7% (95% CI 78.07–98.44) and 97.6% (95% CI 84.81–99.67) were observed respectively, with concomitant therapy (RR: 1.14; 95% CI 1.01–1.29; p = 0.028) and quadruple therapy with bismuth (RR: 1.19; 95% CI 1.09–1.31; p < 0.001) being significantly more effective than standard triple therapy. Regarding the rate of reported adverse effects, it was 58.5% (95% CI 50.66–65.92), 35.4% (95% CI 24.6–48.11), 22.9% (95% CI 81–37.14) and 63.4% (95% CI 47.8–76.64), having the dual and concomitant therapy significantly fewer adverse effects compared with standard therapy.
ConclusionsQuadruple therapies are superior to standard triple therapy and should be considered as first-line treatment in Chile. Dual therapy is promising. More studies will be required to determine which therapies are most cost-effective.
La infección por Helicobacter pylori afecta aproximadamente al 70% de la población chilena. Es un problema de salud pública cuyo tratamiento de erradicación forma parte de políticas públicas en Chile.
ObjetivosCaracterizar los esquemas de erradicación de primera línea de H. pylori más utilizados en nuestro medio y evaluar su eficacia.
MétodosSe realizó un estudio observacional retrospectivo donde, en pacientes con infección por H. pylori certificada, se evaluó el esquema de erradicación indicado por el médico tratante, su eficacia, adherencia y efectos adversos.
ResultadosSe analizaron 242 pacientes y 4 esquemas de erradicación; terapia triple estándar, terapia dual, concomitante y cuadriterapia con bismuto. Se observaron tasas de erradicación de 81,9% (IC 95% 74,44–87,63), 88,5% (IC 95% 73,13–95,67), 93,7% (IC 95% 78,07–98,44) y 97,6% (IC 95% 84,81–99,67) respectivamente, siendo más eficaces la terapia concomitante (RR: 1,14; IC 95% 1,01–1,29; p = 0,028) y cuadriterapia con bismuto (RR: 1,19; IC 95% 1,09–1,31; p < 0,001) que la triple terapia estándar. La tasa de efectos adversos reportados fue de 58,5% (IC 95% 50,66–65,92), 35,4% (IC 95% 24,6–48,11), 22,9% (IC 95% 11,81–37,14) y 63,4% (IC 95% 47,8–76,64), para la terapia triple estándar, dual, concomitante, y cuádruple con bismuto, respectivamente. La terapia dual y concomitante tuvieron menos efectos adversos en comparación con la terapia estándar.
ConclusionesLas cuadriterapias son superiores a la triple terapia estándar por lo que deberían ser consideradas como tratamiento de primera línea en Chile. La terapia dual es promisoria. Más estudios serán requeridos para determinar qué esquemas son más costo-efectivos.
Helicobacter pylori is a Gram-negative spiral-shaped bacterium usually found on the surface of the gastric epithelium that induces chronic inflammation in the underlying mucosa.1 Thus it is associated with various diseases such as chronic gastritis, peptic ulcers (1%–10%), mucosa-associated lymphoid tissue (MALT) lymphoma (0.01%) and gastric cancer (0.1%–3%).2,3 The latter is a very significant disease in Chile, as it is among the leading causes of death in men and women.4,5
Infection with this bacteria has a prevalence of approximately 50% of the global population, varying according to geography, race/ethnicity, demographics and socioeconomic factors.6,7 In Chile it is a very common problem, with studies referring to a prevalence ranging from 60% to 79%.8
The mechanism of transmission is not fully understood, but the most commonly agreed-upon route of transmission is person-to-person, through oral–oral contact, faecal–oral contact or both, probably during childhood. Some reported factors in transmission are low socioeconomic status, having a family member who is a carrier, cramped conditions and use of non-potable water.8
Due to the high prevalence of H. pylori infection in the population and its relationship to various diseases, treatment to eradicate the pathogen and certification of the effectiveness of the treatment carry a certain importance. Around the world there is a high degree of geographic heterogeneity in the management of this infection; this, coupled with the fact that the eradication rates of multiple regimens are going down, in association with an increase in the rate of antimicrobial resistance, represents a public health problem.9 In Chile, the most commonly used regimen is OAC, which consists of proton-pump inhibitors (PPIs), amoxicillin and clarithromycin.8 This regimen falls under the public policies implemented by the Chilean Garantías Explícitas en Salud [Explicit Health Guarantees] (GES) programme, thus ensuring access to treatment within established periods. The effectiveness of this regimen could depend on antimicrobial resistance. Rates of resistance to clarithromycin around 30% in Italy and Japan, 40% in Turkey and 50% in China have been reported.9 The OAC regimen becomes ineffective in populations with resistance to clarithromycin over 15% and should be stopped in this context. In these geographic areas, quadruple therapies, with or without bismuth, and concomitant treatment are recommended.9 Preliminary data have suggested high rates of resistance to clarithromycin in Chile, with reports of rates of resistance of up to 22% in a Chilean cohort, in which the eradication rate achieved with standard triple therapy was 63.8%.10
In addition, strategies such as using high doses of antibiotics or stronger proton-pump inhibitors, as well as adding bismuth to treatments, have been proposed to optimise eradication regimens.11 In particular, recommendations have been made to improve regimens based on clarithromycin.12 If suitable adherence to planned treatments were achieved, universal eradication could be attained.13
There are no known data from Chile regarding the frequency of use of the various regimens proposed for H. pylori eradication, their possible efficacy in the Chilean population, patient adherence to the different options and the adverse effects to which these treatments could lead. This is important in Chile and in other countries in Latin America, as primary resistance to some antibiotics used in eradication regimens is high in this region.14 Other factors could also influence the pharmacological efficacy of PPIs.15 The above highlights the need to prepare follow-up programmes, regulate the use of eradication regimens and implement public policies.14
The main objectives of this study were to characterise eradication rates as well as to determine the main adverse effects of the most commonly used H. pylori eradication regimens at two health centres in Santiago de Chile and compare them to standard triple therapy.
MethodsStudy designA retrospective observational study was conducted based on a registry of patients with a certified diagnosis of H. pylori infection and an indication for treatment established by their treating gastroenterologist at centres belonging to the UC-Christus health network and the Clínica Alemana [German Clinic] in Santiago, Chile. Both see significant numbers of patients belonging to the private health network in the country.
Patients over 18 years of age with a diagnosis of H. pylori made by means of a urease test, a stool antigen test or a breath test in whom an initial eradication regimen established by the treating physician was indicated and who had test results certifying eradication of the infection were considered eligible for inclusion in the study. Patients with an allergy to penicillin; patients with a history of partial gastrectomy due to gastric cancer, incipient gastric cancer resolved by endoscopic resection, bariatric surgery or recent gastrointestinal haemorrhage prior to certification of eradication; and patients with no test confirming eradication of the infection subsequent to the treatment term were excluded. Pregnant and breastfeeding women were also excluded, as were people having received a prior eradication regimen.
Patient recruitment was based on the clinical registry of the six total treating physicians. An invitation to participate and an informed consent form were sent by e-mail to patients, according to the previously established criteria. In patients with certified H. pylori infection, the eradication regimen indicated by the treating physician, the method of certification of eradication used, the eradication rate and the onset of adverse effects associated with treatment, along with adherence to the regimen, were recorded. The latter information was obtained based on a review of clinical files and telephone follow-up according to protocol at three months. Patients were recruited between July 2017 and August 2020. Both the eradication regimen and the manner of certification of eradication were established by the treating physician, with no intervention on the part of the research team. The data were compiled and tabulated in a Microsoft Excel workbook.16
Given the multiple possible treatment regimens, in order to select the most commonly used regimens and thus analyse their effectiveness and associated adverse effects, along with methods of certification of eradication, a survey was conducted among gastroenterology department physicians and internal medicine residents belonging to the UC-Christus health network (25 physicians from the gastroenterology department and 64 internal medicine residents altogether).
Data analysisThe data obtained from the patients were stored confidentially and a code was assigned to the data collected such that the patients' identities could not be traced. Descriptive statistics were estimated using averages for continuous variables with a normal distribution and medians for those with a skewed distribution. For categorical variables, relative and absolute frequencies were obtained and their 95% confidence intervals (95% CIs) were estimated.
A univariate analysis was performed by means of binomial regression, considering outcomes, H. pylori eradication and having at least one adverse effect to correspond to dichotomous variables. Binomial regression was used to estimate and compare the likelihood of eradication and having adverse effects among the different regimens through measures of effect such as relative risk (RR). This type of regression model was chosen over logistic regression as logistic regression estimates odds ratios (ORs). ORs have been found to overestimate measures of effect in cases with a sample with a large proportion of positive events (in this study, eradication). In binomial regression models, comparisons of the regimens analysed in the registry were made taking standard treatment as a benchmark, regardless of the PPI used. Prevalences of adverse effects were graphed using a forest plot, which showed the overall proportion of each specific adverse event and that which resulted when they were grouped within each arm. This was done with the goal of identifying heterogeneity in adverse effects and their overall prevalence, thus facilitating comparison thereof between treatment arms. Thus an overall estimate of the proportion of adverse events of each regimen, with their respective 95% CIs, was obtained. In addition, Cochran's Q test was used to compare the proportions of the adverse effects reported on each regimen. The results are represented in a forest plot, and the inverse variance method was used in the estimates; this method is recommended when analysing proportions. Data analysis was performed using the STATA software program, version 16.17
Ethical considerationsThe research protocol for the study was approved by the ethics committee of the Pontificia Universidad Católica de Chile [Pontifical Catholic University of Chile] with ID 190801002. The study entailed no risks or additional costs for the patients by virtue of participating in it. The data were handled in a confidential manner, and patients who had any questions about the protocol were contacted by the principal investigators of the study. Additionally, an informed consent waiver was requested to gain access to information for patients recruited in related projects, both within the UC-Christus health network and outside of it, since 2017. This request was approved by the ethics committee of the Pontificia Universidad Católica de Chile with ID 200524003.
ResultsSurvey results on regimens usedOf the total population surveyed, 39 responses were obtained, within which the regimens reported as the most commonly used ones were: standard triple therapy: clarithromycin, amoxicillin and PPIs (57%); concomitant: clarithromycin, amoxicillin, metronidazole and PPIs (15%); dual therapy: amoxicillin at high doses and PPIs (15%); and quadruple therapy with bismuth: amoxicillin, metronidazole, PPIs and bismuth subsalicylate (8%). Consultation with specialist physicians, prior to review of clinical files, facilitated the preparation of the registry as it enabled compilation of information about the most representative treatment regimens. These regimens were also the most commonly used ones in the study population.
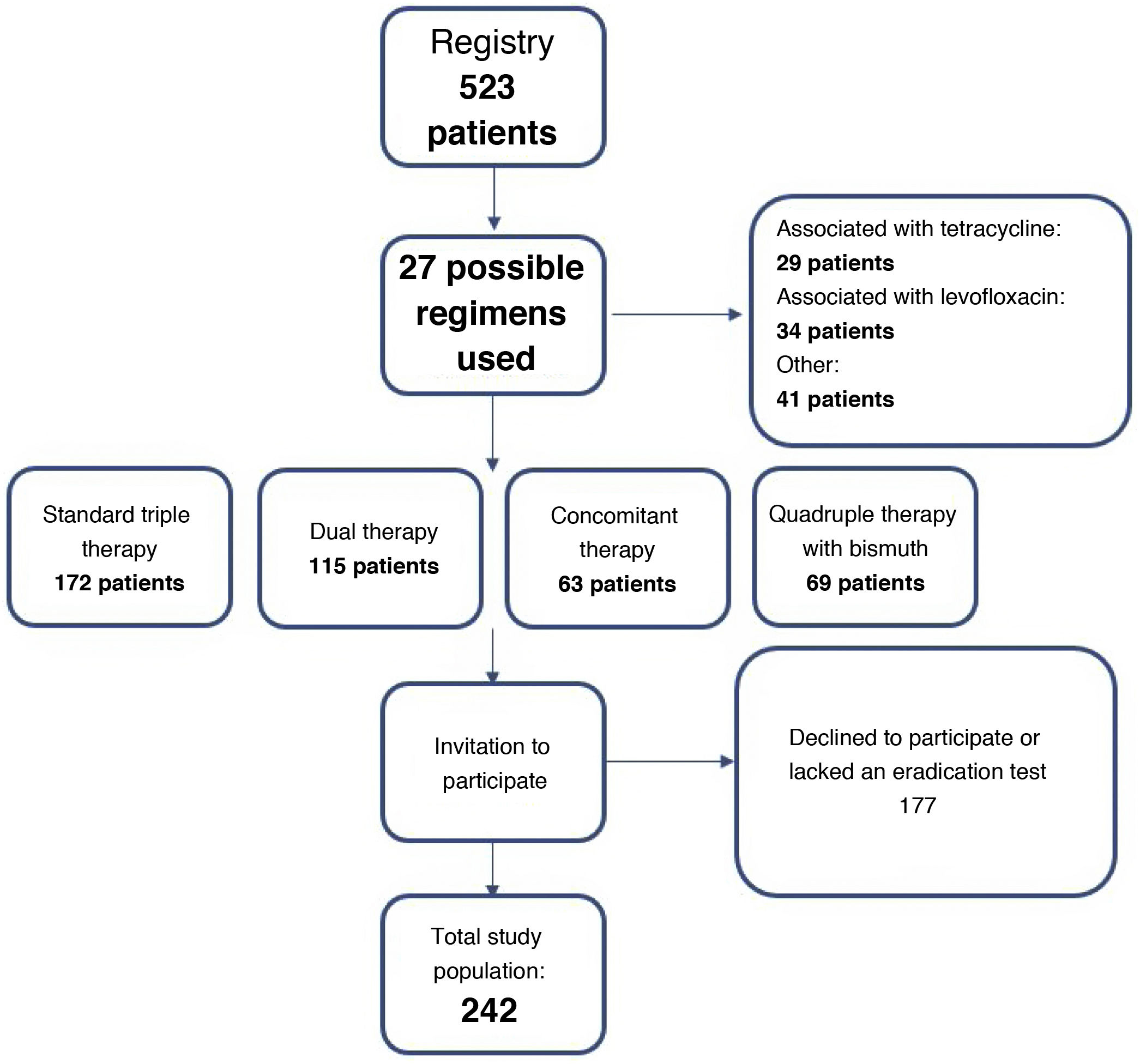
Description of the sampleAltogether, 523 patients and 27 different treatment regimens were identified. Selection of patients in whom any of the four regimens to be analysed was indicated yielded a total of 419 potential participants. Of these, 177 declined to participate in the study or lacked a test certifying eradication of the infection; hence, the data analysed corresponded to 242 patients from two health centres (UC-Christus health network and Clínica Alemana in Santiago). Fig. 1 describes the algorithm for recruiting the patients in the study.
Of the 242 patients analysed with complete follow-up, 138 (56.94%) were women. The average age in the sample was 48.31 years (standard deviation [SD] 15.24). The most common comorbidities were hypertension (24.76%), hypothyroidism (12.38%), gastro-oesophageal reflux disease (11.9%) and cirrhosis (11.42%). The above is valid for a total of 210 patients, as data on comorbidities in patients corresponding to the concomitant regimen (n = 32) could not be compiled. Within the sample, 12.04% of the patients were seen to have used PPIs in the two weeks prior to the test certifying eradication of the infection and 1.02% of patients were seen to have used antibiotics or bismuth in the four weeks prior to the confirmation test. The most common indication for eradication of H. pylori infection was abdominal pain/dyspepsia, in 36.36% of cases (Table 1).
Characteristics of the patients analysed in the study.
| Characteristics | Descriptive statistics |
|---|---|
| Average age | 48.31 years (SD 15.24) |
| Sex | 144 women (59.5%) |
| Comorbidities | |
| Hypertension | 52 (21.49%) |
| Hypothyroidism | 27 (11.16%) |
| Gastro-oesophageal reflux disease | 26 (10.74%) |
| Cirrhosis | 24 (9.92%) |
| Use of PPIs two weeks prior to eradication test | 76 (31.4%) |
| Use of antibiotics or bismuth four weeks prior to confirmation test | 3 (1.23%) |
| Indication for eradication | |
| Abdominal pain/dyspepsia | 88 (36.36%) |
| Atrophic gastritis | 17 (7.02%) |
| Peptic ulcer | 8 (3.31%) |
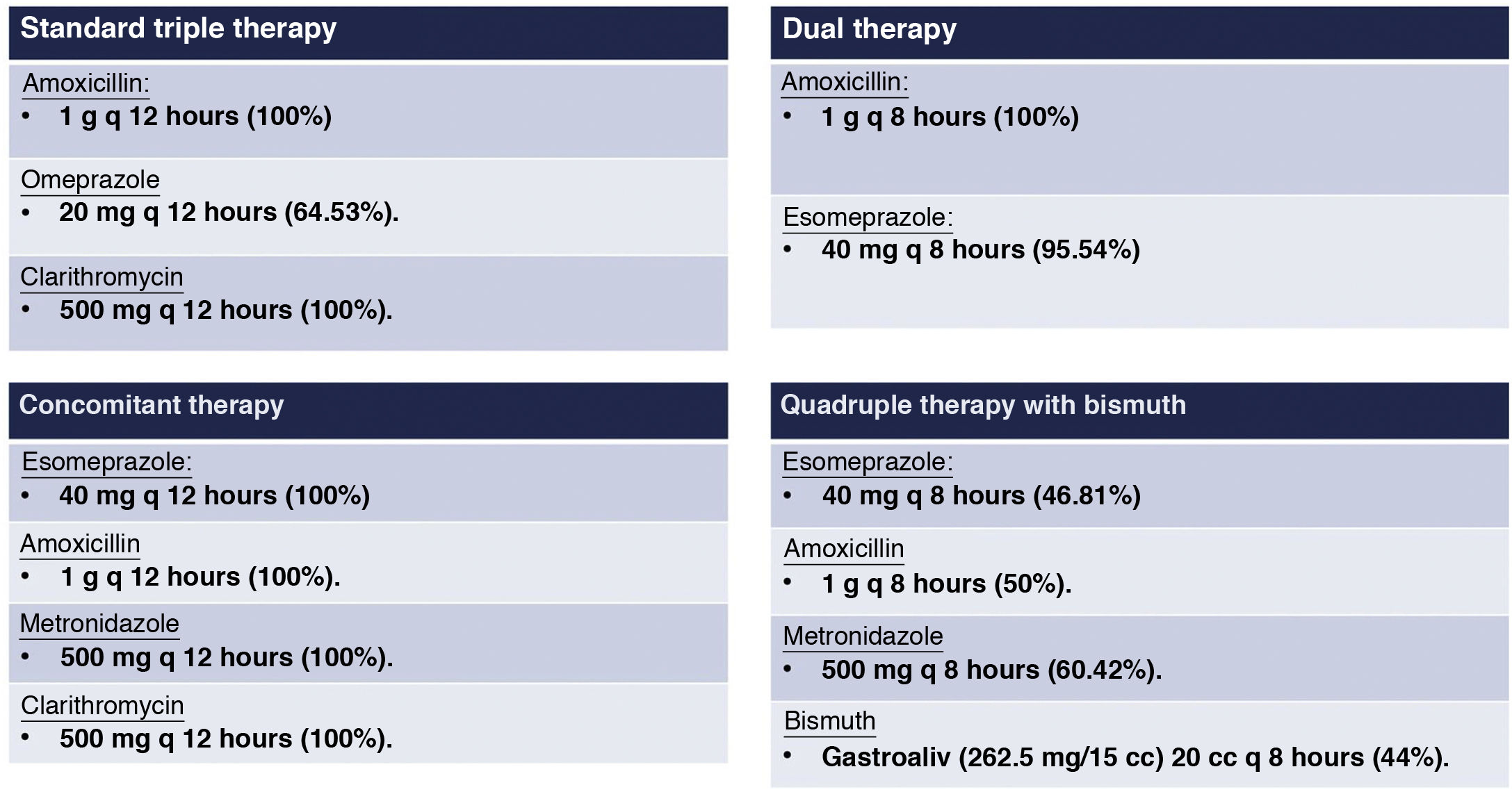
A total of 133 patients (54.95%) belonged to the standard triple therapy group, 42 (17.36%) belonged to the quadruple therapy with bismuth group, 32 (13.22%) belonged to the concomitant therapy group and 35 (14.46%) belonged to the dual therapy group. Although the drugs that comprise each regimen had been established in advance, the dose had not. Thus, on standard triple therapy, there was a great deal of heterogeneity in terms of PPIs used; omeprazole 20 mg every 12 h was most common, while on quadruple therapy with bismuth the doses of all the components of the therapy were variable. In addition, in both patients managed with concomitant therapy and patients managed with dual therapy, high homogeneity in treatment doses was detected. Fig. 2 shows the details of the regimens and the most commonly used drug doses.
Regarding adherence to each of the regimens, six patients on standard triple therapy, six patients on quadruple therapy with bismuth, three patients on dual therapy and one patient on concomitant therapy did not complete the entire regimen.
Eradication rateThe eradication rate was highest on quadruple therapy with bismuth, at 97.62% (84.81%–99.67%). The second most effective treatment was concomitant therapy, at 93.75% (78.07%–98.44%). Quadruple therapy with bismuth (RR: 1.19; 95% CI 1.09−1.31; p < 0.001) and concomitant therapy (RR: 1.14; 95% CI 1.01−1.29; p = 0.028) were significantly more effective than standard triple therapy (Table 2). However, there were no significant differences in terms of effectiveness between standard triple therapy and dual therapy (RR: 1.08 95% CI 0.94−1.25; p = 0.288).
Comparison of rates of effectiveness of standard triple therapy versus dual therapy, concomitant therapy and quadruple therapy with bismuth.
| Regimen | Eradication rate (%) (95% CI) | Relative risk(95% CI) | p value |
|---|---|---|---|
| Standard triple therapy (n = 133) | 81.95 (74.44−87.63) | Standard | |
| Dual therapy (n = 35) | 88.57 (73.13−95.67) | 1.08 (0.94−1.25) | 0.288 |
| Concomitant therapy (n = 32) | 93.75 (78.07−98.44) | 1.14 (1.01−1.29) | 0.028 |
| Quadruple therapy with bismuth (n = 42) | 97.62 (84.81−99.67) | 1.19 (1.09−1.31) | <0.001 |
The regimen with the highest rate was quadruple therapy with bismuth, at 63.41% (95% CI 47.80–76.64) (Table 3). The rate of adverse effects was 58.49% (50.66−65.92) for standard triple therapy, 35.48% (95% CI 24.6−48.11) for dual therapy and 21.95% (95% CI 11.81−37.14) for concomitant therapy. Comparison to standard triple therapy revealed a lower risk of adverse effects on both concomitant therapy (RR: 0.38; 95% CI 0.21−0.68; p < 0.01) and dual therapy (RR: 0.61; 95% CI 0.42−0.87; p < 0.01). Examination of standard triple therapy versus quadruple therapy with bismuth revealed no significant differences in terms of adverse effects (RR: 1.08; 95% CI 0.88–1.42; p = 0.55).
Comparison of rates of adverse effects of standard triple therapy versus dual therapy, concomitant therapy and quadruple therapy with bismuth.
| Regimen | Rate of adverse effects (%) | Relative risk | p value |
|---|---|---|---|
| (95% CI) | (95% CI) | ||
| Standard triple therapy (n = 133) | 58.49 (50.66−65.92) | Standard | |
| Dual therapy (n = 35) | 35.48 (24.6−48.11) | 0.61 (0.42−0.87) | <0.01 |
| Concomitant therapy (n = 32) | 22.95 (11.81−37.14) | 0.38 (0.21−0.68) | <0.01 |
| Quadruple therapy with bismuth (n = 42) | 63.41 (47.8−76.64) | 1.08 (0.83−1.42) | 0.553 |
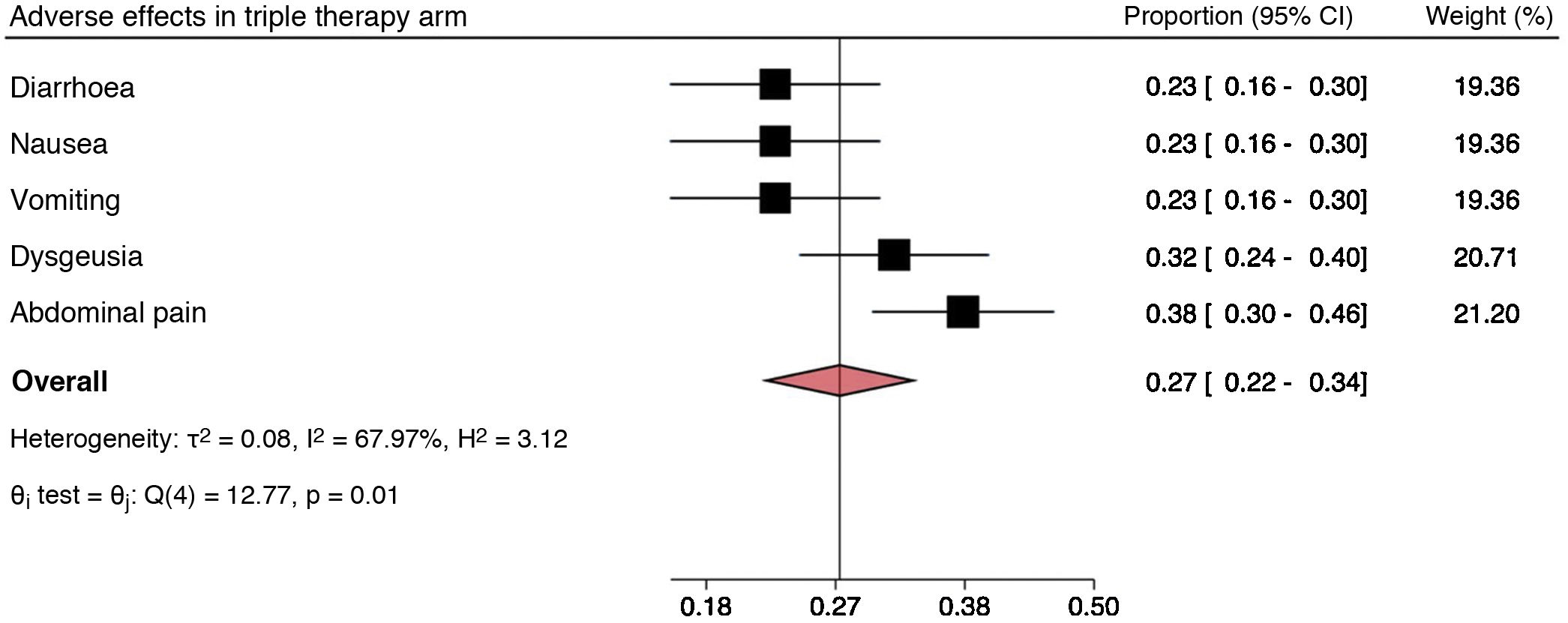
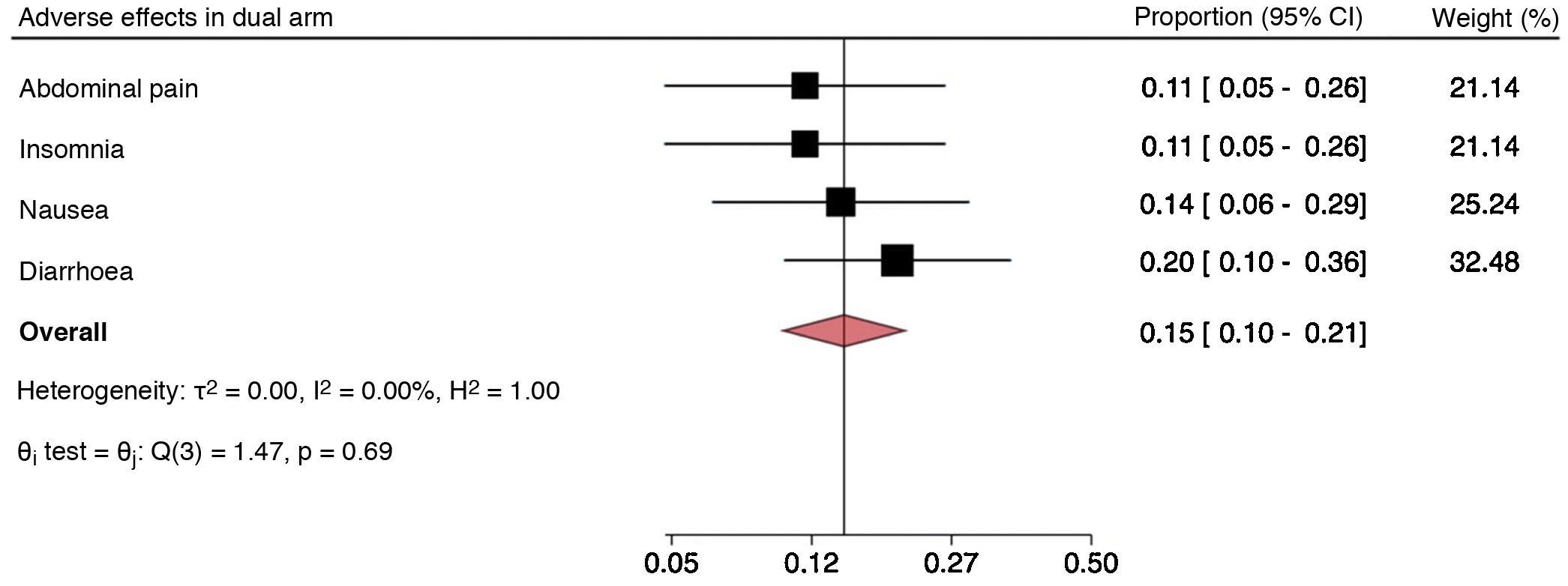
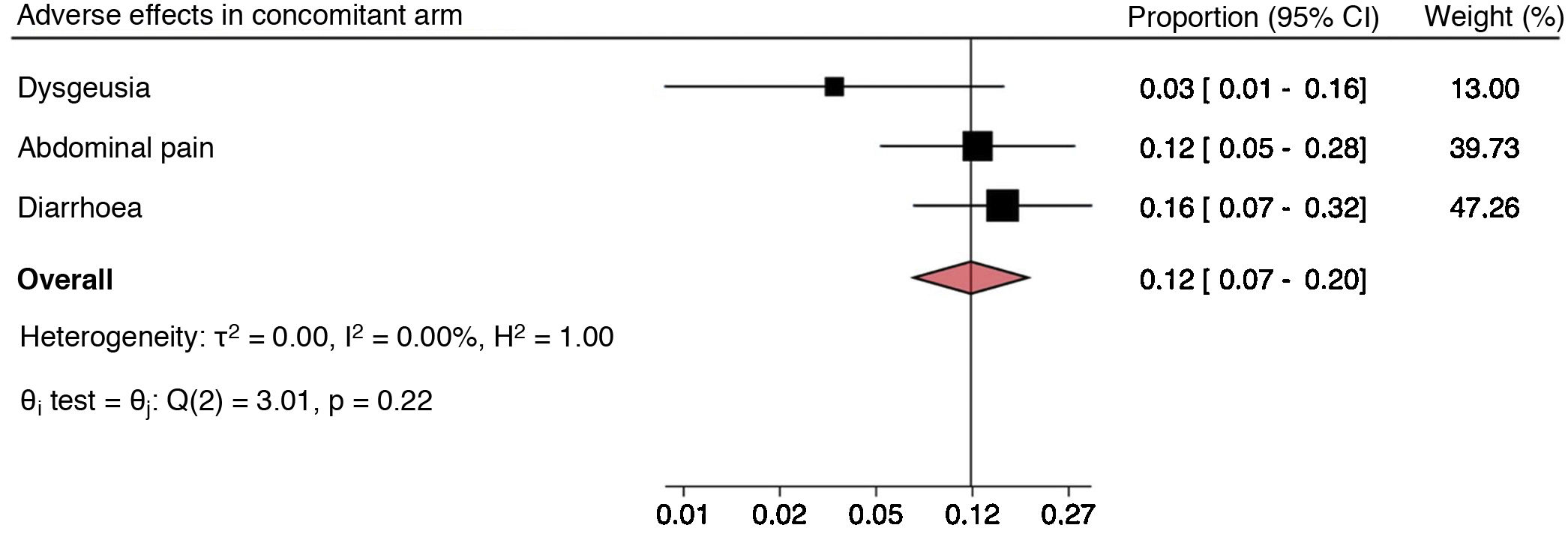
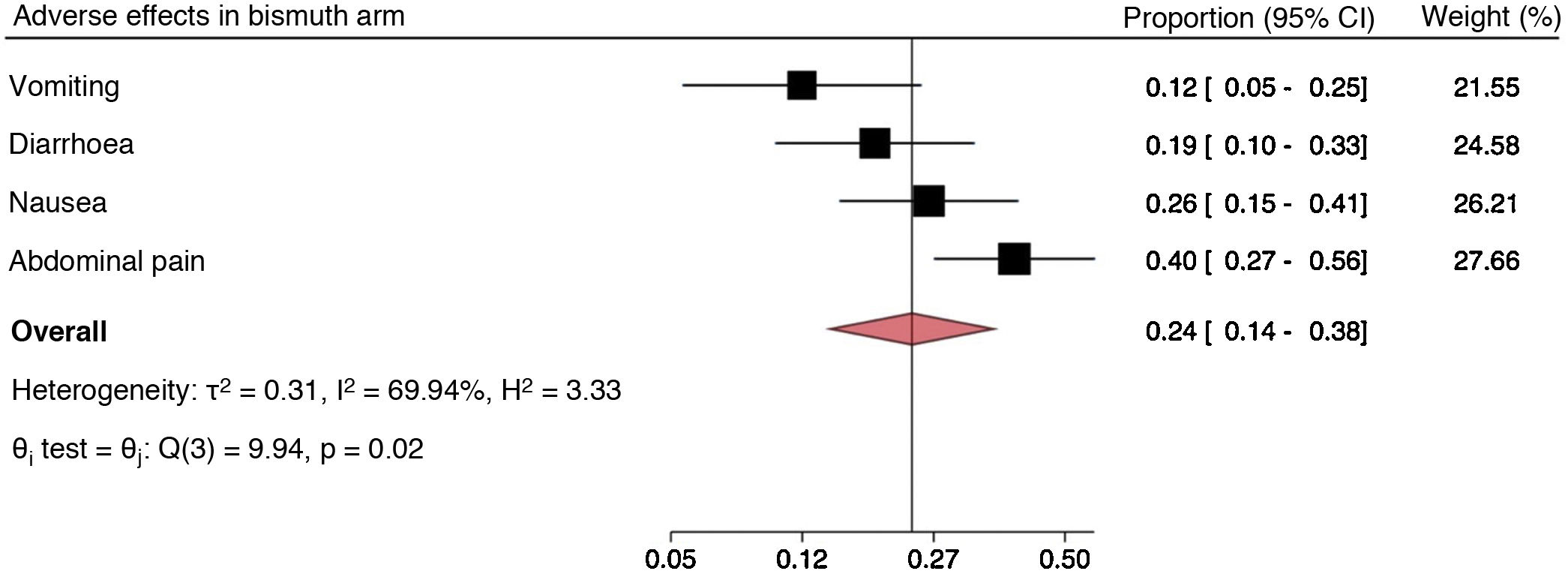
The most commonly reported adverse effects with standard triple therapy were diarrhoea, nausea, vomiting, dysgeusia and abdominal pain (Fig. 3). For dual therapy, they were abdominal pain, insomnia, nausea and diarrhoea (Fig. 4). For concomitant therapy, they were dysgeusia, abdominal pain and diarrhoea (Fig. 5). Finally, for quadruple therapy with bismuth, the most common adverse effects were nausea, vomiting, diarrhoea and abdominal pain (Fig. 6). The overall proportion of adverse effects for each regimen can be seen in the diamond of each forest plot. In addition, the Q test showed differences in the proportions of adverse effects reported on standard triple therapy, with notably higher rates of dysgeusia and abdominal pain. Something similar occurred on quadruple therapy with bismuth, with a higher proportion of reports of abdominal pain. On the dual and concomitant regimens there were no differences in terms of proportions of adverse effects reported.
DiscussionAnalysis of the results of this study revealed a great deal of heterogeneity in the regimens used to treat this infection and in the ways in which these regimens are implemented. Without going further, the European H. pylori registry18 recognised the failure of the efforts of gastroenterologists in that region to unify the criteria for management in clinical practice of this infection.19
As mentioned above, both quadruple therapy with bismuth and concomitant therapy proved significantly more effective than standard triple therapy, with eradication rates greater than 90%. Our findings were consistent with those of international randomised studies in which the eradication rates achieved were as high as 98.4% with quadruple therapy with bismuth and 93.1% with concomitant therapy as a first-line treatment in per-protocol analysis.20 These findings could support their use as a reasonable first-line alternative to increase the eradication rate achieved.
In addition, eradication rates greater than 80% were found for both standard triple therapy and dual therapy in our population, with no significant differences with respect to effectiveness between the two. This could have been due in part to the fact that just 35 patients in this cohort received dual therapy, and a larger population might be sufficient to reveal differences. One reason to use dual therapy is that antibiotic resistance to amoxicillin is rare, with reported rates of resistance as low as 0%.21 In addition, a significantly lower rate of adverse effects was found in patients treated with dual therapy. Both findings were consistent with a recent meta-analysis that found that dual therapy was as effective as the standard of care, with fewer associated adverse effects (RR: 0.48).22 However, it must be taken into account that the dose and frequency of administration of amoxicillin has varied across studies, with some administering the drug three times per day and others administering it four times per day. Despite the above, the findings set out render it a reasonable alternative to standard triple therapy, with the advantage of lesser exposure to antibiotics on the part of the population.
The strengths of this study included its novel nature as a registry comparing the effectiveness and adverse effects of four regimens commonly used in real clinical practice by gastroenterologists and internists at two centres in our country, with a sample of 242 patients who had a prospective follow-up of three months. The limitations of the study included the variability of drug doses, on both quadruple therapy with bismuth and triple therapy, and the lesser degree of representation within the study population of regimens other than triple therapy. This was important, since even when different PPIs were used, differences in eradication rates could be seen.23 In addition, regimens based on other antibiotics such as levofloxacin and alternative treatment regimens for patients with allergies were not included. Finally, follow-up by telephone on a delayed basis of side effects and lack of treatment adherence might have resulted in underestimates of the corresponding data.
This study opens up a number of opportunities and makes it possible to imitate the European trend of a H. pylori treatment registry and evaluate the cost-effectiveness of the different treatments in order to influence public policies on a nationwide level.
ConclusionBased on our findings, we conclude that concomitant therapy and quadruple therapy with bismuth are more effective than standard triple therapy, and therefore are reasonable alternatives as a first-line treatment in Chile. In addition, dual therapy has similar rates of effectiveness, but a better profile of adverse effects, rendering it a good alternative to standard triple therapy.
FundingThis study was partly funded by the projects FONIS SA19/0188 (“Cost-effectiveness of new population screening strategies for gastric cancer control in Chile”) and LEGACy (“CeLac and European consortium for a personalized medicine approach to Gastric Cancer”; no. 825832 of the Horizon 2020 programme of the European Union).
Conflicts of interestThe authors declare that they have no conflicts of interest.