This position paper, sponsored by the Asociación Española de Gastroenterología [Spanish Association of Gastroenterology], the Sociedad Española de Endoscopia Digestiva [Spanish Gastrointestinal Endoscopy Society] y the Sociedad Española de Anatomía Patológica [Spanish Anatomical Pathology Society], aims to establish recommendations for performing an high quality upper gastrointestinal endoscopy for the screening of Gastric Cancer Precursor Lesions (GCPL) in low-incidence populations, such as the Spanish population. To establish the quality of the evidence and the levels of recommendation, we used the methodology based on the GRADE system (Grading of Recommendations Assessment, Development and Evaluation). We obtained a consensus among experts using a Delphi method. The document evaluates different measures to improve the quality of upper gastrointestinal endoscopy in this setting and makes recommendations on how to evaluate and treat the identified lesions. We recommend that upper gastrointestinal endoscopy for surveillance of GCPL should be performed by endoscopists with adequate training, administering oral premedication and use of sedation. To improve the identification of GCPL, we recommend the use of high definition endoscopes and conventional or digital chromoendoscopy and, for biopsies, NBI should be used to target the most suspicious areas of intestinal metaplasia. Regarding the evaluation of visible lesions, the risk of submucosal invasion should be evaluated with magnifying endoscopes and endoscopic ultrasound should be reserved for those with suspected deep invasion. In lesions amenable to endoscopic resection, submucosal endoscopic dissection is considered the technique of choice.
Este documento de posicionamiento, auspiciado por la Asociación Española de Gastroenterología, la Sociedad Española de Endoscopia Digestiva y la Sociedad Española de Anatomía Patológica tiene como objetivo establecer recomendaciones para realizar una endoscopia digestiva (EDA) de calidad en la detección y vigilancia de lesiones precursoras de cáncer gástrico (LPCG) en poblaciones con incidencia baja, como la española. Para establecer la calidad de la evidencia y los niveles de recomendación se ha utilizado la metodología basada en el sistema GRADE (Grading of Recommendations Assessment, Development and Evaluation). Se obtuvo el consenso entre expertos mediante un método Delphi. El documento evalúa diferentes medidas para mejorar la calidad de la EDA en este contexto y hace recomendaciones de cómo evaluar y tratar las lesiones identificadas. Se recomienda que la EDA de vigilancia de LPCG sea realizada por endoscopistas con capacitación adecuada, administrando premedicación oral y uso de sedación. Para mejorar la identificación de LPCG se recomienda el uso de endoscopios de alta definición y cromoendoscopia convencional o digital y, para las biopsias, debe utilizarse el NBI para dirigirlas a las áreas más sospechosas de metaplasia intestinal. En cuanto a la evaluación de las lesiones visibles, el riesgo de invasión de la submucosa debe evaluarse con endoscopios de magnificación y reservar la ecoendoscopia para aquellas con sospecha de invasión profunda. En las lesiones susceptibles de resección endoscópica, la disección endoscópica submucosa se considera la técnica de elección.
Gastric cancer (GC) is the second most common type of gastrointestinal cancer. In Spain, as in most European countries, the incidence of GC is at a level considered low. In 2018, there were an estimated 7765 new cases of GC, with an incidence rate adjusted by age to the world population of 9.2 cases per 100,000 males and 4.3 cases per 100,000 females.1
Upper gastrointestinal endoscopy (UGE) is the gold standard for the diagnosis of GC and precursor lesions. Despite the growing interest in the issue of quality in UGE demonstrated by the appearance of different guidelines and position statements,2–5 currently, UGE quality is low according to the indicators available. Recent data show that in Western countries up to 14% of cases of GC were not diagnosed in a UGE in the previous three years, with this percentage being 9% in our setting.6,7 These data show the need to improve the quality of examinations.
The objectives of this position statement are:
- 1
To describe the minimum requirements for a high-quality UGE, including the requirements for the detection and surveillance of GC precursor lesions (GCPLs) and the detection of early GC (EGC).
- 2
To describe how to deal with a visible lesion.
The endoscopic evaluation of Barrett’s oesophagus and the recommendations on endoscopic surveillance of GCPLs (included in another consensus document) are outside the scope of this document.8 Also outside the scope of this document are the required contents of the pathology report in the evaluation of these lesions. This position statement is the fruit of a collaborative effort among the Asociación Española de Gastroenterología [Spanish Association of Gastroenterology] (AEG), the Sociedad Española de Endoscopia Digestiva (SEED) [Spanish Association of Gastrointestinal Endoscopy] and the Sociedad Española de Anatomía Patológica (SEAP) [Spanish Association of Pathology] to arrive at a set of unified criteria and recommendations for clinical gastroenterologists, endoscopists and pathologists.
MethodsA working group made up of experts from these associations and methodologies was set up to search and review the evidence. The working group put forward the clinically significant questions that are addressed in the statement. The main objective was to describe the characteristics of UGE for optimal detection of GCPLs.
The search for articles was conducted based on the different questions according to the following strategy: priority was given to identifying systematic reviews and other documents that critically synthesised the scientific literature; in a second phase, individual studies, randomised clinical trials (RCTs) and observational studies were searched. Regarding the electronic databases consulted, we focused on MEDLINE (accessed through PubMed).
To establish the levels of evidence and the grades of recommendation for the different questions addressed, we used the methodology based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group system.9 In order to establish the level of the recommendations, we considered not only the quality of the evidence, but also the risk–benefit balance, costs, and people’s values and preferences.
Once the recommendations were made, agreement among the members of the working group was assessed. A consensus was achieved using the Delphi method. The recommendations were evaluated by the panel members using a Likert scale (1: strongly disagree; 2: disagree; 3: doubtful or with qualms; 4: agree; and 5: strongly agree). In the event of disagreement, the recommendations were reformulated and voted on again. Those that obtained a final average agreement ≥4 are presented and substantiated. The first round of the Delphi consensus was done in November 2019 using the REDCap electronic data capture tools hosted at the AEG (www.aegastro.es).10 After reformulating the recommendations with agreement <4, a second round was held in a videoconference on 14 October 2020. The final recommendations, together with the evaluation of the evidence, the strength of the recommendation, and the degree of agreement, are shown in Table 1. An appendix is also included with representative images of GCPLs and EGC. This position statement was approved by the boards of directors of the three scientific associations involved.
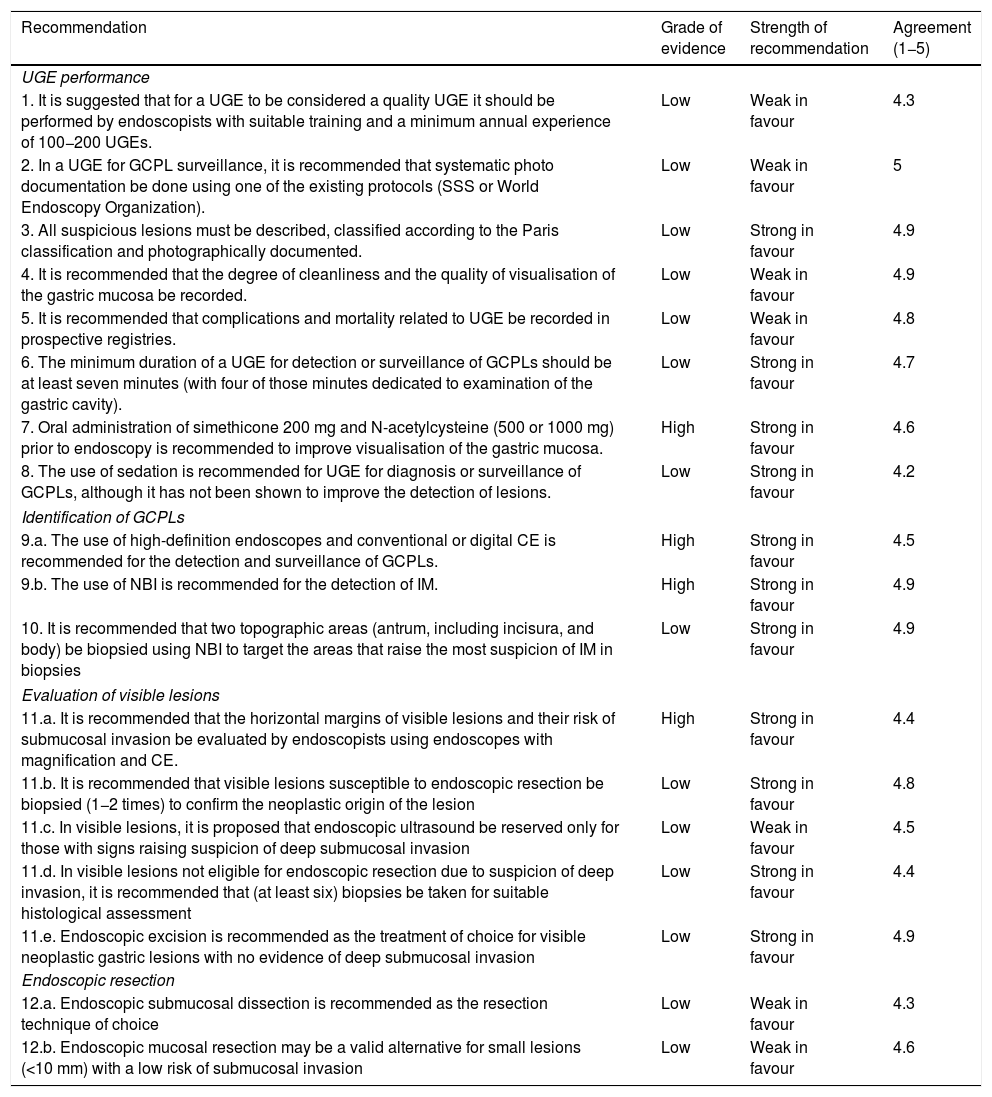
Summary of recommendations.
| Recommendation | Grade of evidence | Strength of recommendation | Agreement (1−5) |
|---|---|---|---|
| UGE performance | |||
| 1. It is suggested that for a UGE to be considered a quality UGE it should be performed by endoscopists with suitable training and a minimum annual experience of 100−200 UGEs. | Low | Weak in favour | 4.3 |
| 2. In a UGE for GCPL surveillance, it is recommended that systematic photo documentation be done using one of the existing protocols (SSS or World Endoscopy Organization). | Low | Weak in favour | 5 |
| 3. All suspicious lesions must be described, classified according to the Paris classification and photographically documented. | Low | Strong in favour | 4.9 |
| 4. It is recommended that the degree of cleanliness and the quality of visualisation of the gastric mucosa be recorded. | Low | Weak in favour | 4.9 |
| 5. It is recommended that complications and mortality related to UGE be recorded in prospective registries. | Low | Weak in favour | 4.8 |
| 6. The minimum duration of a UGE for detection or surveillance of GCPLs should be at least seven minutes (with four of those minutes dedicated to examination of the gastric cavity). | Low | Strong in favour | 4.7 |
| 7. Oral administration of simethicone 200 mg and N-acetylcysteine (500 or 1000 mg) prior to endoscopy is recommended to improve visualisation of the gastric mucosa. | High | Strong in favour | 4.6 |
| 8. The use of sedation is recommended for UGE for diagnosis or surveillance of GCPLs, although it has not been shown to improve the detection of lesions. | Low | Strong in favour | 4.2 |
| Identification of GCPLs | |||
| 9.a. The use of high-definition endoscopes and conventional or digital CE is recommended for the detection and surveillance of GCPLs. | High | Strong in favour | 4.5 |
| 9.b. The use of NBI is recommended for the detection of IM. | High | Strong in favour | 4.9 |
| 10. It is recommended that two topographic areas (antrum, including incisura, and body) be biopsied using NBI to target the areas that raise the most suspicion of IM in biopsies | Low | Strong in favour | 4.9 |
| Evaluation of visible lesions | |||
| 11.a. It is recommended that the horizontal margins of visible lesions and their risk of submucosal invasion be evaluated by endoscopists using endoscopes with magnification and CE. | High | Strong in favour | 4.4 |
| 11.b. It is recommended that visible lesions susceptible to endoscopic resection be biopsied (1−2 times) to confirm the neoplastic origin of the lesion | Low | Strong in favour | 4.8 |
| 11.c. In visible lesions, it is proposed that endoscopic ultrasound be reserved only for those with signs raising suspicion of deep submucosal invasion | Low | Weak in favour | 4.5 |
| 11.d. In visible lesions not eligible for endoscopic resection due to suspicion of deep invasion, it is recommended that (at least six) biopsies be taken for suitable histological assessment | Low | Strong in favour | 4.4 |
| 11.e. Endoscopic excision is recommended as the treatment of choice for visible neoplastic gastric lesions with no evidence of deep submucosal invasion | Low | Strong in favour | 4.9 |
| Endoscopic resection | |||
| 12.a. Endoscopic submucosal dissection is recommended as the resection technique of choice | Low | Weak in favour | 4.3 |
| 12.b. Endoscopic mucosal resection may be a valid alternative for small lesions (<10 mm) with a low risk of submucosal invasion | Low | Weak in favour | 4.6 |
The expert group selected and answered the following questions:
Endoscopist’s experience- 1
What experience should an endoscopist have in order to perform a quality UGE?
It is suggested that for a UGE to be considered of quality it should be performed by endoscopists with suitable training and a minimum annual experience of 100−200 UGEs.
Quality of evidence: low. Grade of recommendation: weak in favour.
Agreement: 4.3.
EvidenceA quality gastroscopy must begin with the proper training of the endoscopist. This includes knowledge of the indications, a good technique and systematic examination, and the drafting of a full report that reflects the findings or the absence of these. It must be borne in mind that lesions in the upper gastrointestinal tract are more difficult to detect than in the colon and more complex to identify and characterise. Therefore, the number of upper endoscopies performed by the endoscopist for a high-quality UGE should be greater than that required for colonoscopy. In the literature, there is no consensus on what the minimum recommended number of UGEs to achieve and maintain competence in UGE should be. The recommended minimum number of endoscopies varies widely depending on the studies performed and ranges from 100 to 200 examinations.11,12 In the case of colonoscopy, some data show that a disruption in learning yields a worsening of competence.13 By extrapolation, it seems reasonable to recommend the continuous performance of UGEs and a minimum of annual high-quality diagnostic UGEs to maintain competence.
Furthermore, GCPL detection has great interobserver variability when only white light is used. In recent years, it has been shown that the use of virtual chromoendoscopy (CE) not only improves detection but also reduces this variability.14 An online training programme with 200 videos and suitable feedback achieved an improvement in the diagnosis of both intestinal metaplasia (IM) and dysplasia, attaining a specificity greater than 80% and 90%, respectively, at the end of the training. These results were seen in dysplasia even after the first 20 videos.15 The value of online training for the diagnosis of EGC was demonstrated in an international multicentre RCT in which 332 endoscopists from 35 countries participated.16
Expert opinionAlthough there are few data on the number of gastroscopies that is considered necessary to guarantee the performance of a quality UGE, it seems reasonable to recommend a minimum of 100−200 per year. It has already been noted that a quality UGE begins with suitable training of the endoscopist, but it is also very important that all the details of the examination are included in the report. On this matter, the Sociedad Española de Patología Digestiva (SEPD) [Spanish Association of Gastrointestinal Pathology] recently published a guide that describes all the steps of UGE.17
Regarding mastery of the CE technique, there are no specific recommendations, but it seems that online training with images and suitable feedback could be sufficient. Courses must be organised both locally and internationally and would be well-received.
Photo documentation- 2
What images should be obtained in a UGE for GCPL surveillance?
In a UGE for GCPL surveillance, it is recommended that systematic photo documentation be done using one of the existing protocols (systematic screening protocol for the stomach [SSS] or World Endoscopy Organization [WEO]).
Quality of evidence: low. Grade of recommendation: weak in favour.
Agreement: 5.
EvidenceIn order to achieve a complete examination of the upper gastrointestinal tract, a series of reference points must be visualised. The procedure must begin in the upper oesophageal sphincter and end in the second portion of the duodenum. The upper oesophagus, gastroesophageal junction, fundus, gastric body, incisura, antrum, duodenal bulb and distal duodenum should be visualised. A retroversion manoeuvre must be performed to examine the fundus in 100% of patients. In an analysis of 111,962 endoscopies, photo documentation of the ampulla of Vater was independently associated with a higher rate of detection of neoplastic lesions (odds ratio [OR] 1.33; 95% confidence interval [CI] 1.03–1.70).18 A retrospective study included 54,889 patients who underwent gastroscopy for GC screening in order to identify indicators of UGE quality related to the finding of EGC. In the multivariate analysis, the percentages of detection of gastric subepithelial lesions and gastric diverticula were independently associated with the visualisation of EGC.19
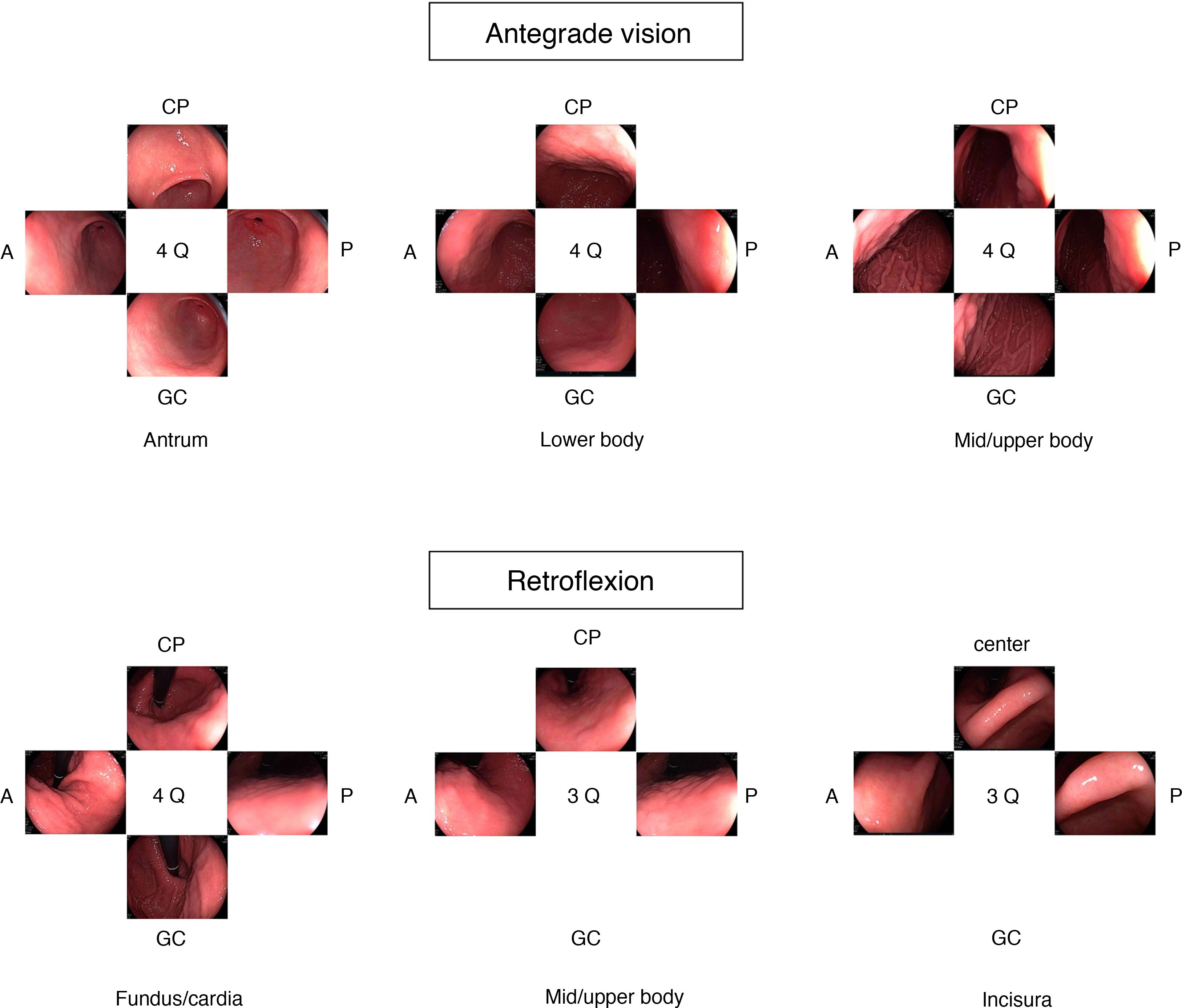
Various guidelines suggest that acquisition of images promotes careful examination and cleansing of the mucosa, ensuring a thorough examination.3,4 When GCPL surveillance is being done, it is recommended that a more extensive photographic mapping be performed according to the SSS (Fig. 1), which includes 22 photographs with a well-distended, clean stomach.20 More recently, the WEO published a position statement in which it proposes a very similar protocol with 21 photographs in total.21 In the case of an initial UGE outside the context of surveillance, the European guidelines recommend taking five photographs of the gastric cavity as well as of the abnormalities detected.3
Expert opinionAlthough there are no data to support the notion that routine imaging improves the diagnostic performance of endoscopy, it is reasonable to think that comprehensive imaging involves a more detailed examination of the gastric mucosa. In Japan, where the incidence of EGC is high, systematic examination has been performed for many years to visualise the entire mucosa and avoid leaving unexamined areas. This systematic examination consists of three phases: distending well with insufflation, removing mucus adhered to the mucosa by repeated washing with water and a defoaming agent, and finally mapping the entire mucosa with the SSS. More recently, the WEO proposed another documentation system with photographs of the stomach that is very similar to the SSS but takes fewer photos of the incisura. As there is no scientific evidence available to recommend one strategy or the other, in UGE for GCPL surveillance it is believed that the important thing is to follow any of the systematic proposals in the strategies.
Documentation of lesions- 3
How should endoscopic lesions be documented?
All lesions must be described, classified according to the Paris classification and photographically documented.
Quality of evidence: low. Grade of recommendation: strong in favour.
Agreement: 4.9.
EvidenceNone.
Expert opinionWhen a lesion suggestive of GCPL/EGC is located, at least the anatomical location of the lesion and the distance to a fixed reference point (such as the dental arch) should be reflected in the endoscopy report. The number of lesions and the size and morphology of each of them should also be described, according to the recommendations of the Minimal Standard Terminology (MST 3.0) (www.worldendo.org/guidelines).22 Any additional mucosal abnormalities should also be described. Standardisation in endoscopy reports aids in decision-making. Therefore, we recommend that, when a malignant lesion is detected, its morphology be described according to the Paris classification23 and it be imaged.
Degree of cleanliness- 4
Is it necessary to record the degree of cleanliness and the quality of the UGE visualisation?
It is recommended to record the degree of cleanliness and the quality of visualisation of the gastric mucosa.
Quality of evidence: low. Grade of recommendation: weak in favour.
Agreement: 4.9.
EvidenceIn order to diagnose GCPLs/EGC in the gastric mucosa, it is necessary to inspect it in the absence of bubbles, mucus and debris. Unlike colonoscopy, the quality of gastric preparation is not usually collected routinely and, in fact, there are no validated scales used at the care level. In the studies conducted to evaluate the effect of premedication on the visibility of the gastric mucosa, the scale proposed by Kuo and later modified by Chang has been used.24–26 This scale measures the cleanliness of the stomach in four segments: the antrum, the lower gastric body, the upper gastric body and the fundus. For each segment, it establishes a score between 1 (perfect cleanliness) and 4 (large amount of mucus that requires more than 50 mL of water to remove it), yielding a total score of 4–16.
Expert opinionGastric cleansing should be classified according to a validated scale and should be reflected in the endoscopy report. Studies are needed to define and validate the most appropriate cleansing scale. Similar to the Boston scale used in colonoscopy, assessment of the degree of cleanliness should be performed after washing to reflect the degree of visibility of the mucosa (level 1: absence of adhered mucus and foam and visibility of the entire mucosa; level 2: thin layer of mucus and some foam that do not impede visibility; level 3: presence of mucus and foam that prevent evaluation of some areas of the mucosa; level 4: mucosa cannot be assessed due to food debris, mucus and/or foam). This assessment should be done by segments or areas and the percentage of mucosal surface that cannot be assessed could be added as additional information. Lastly, if it is impossible to achieve a complete and correct visualisation of the gastric mucosa after washing, a recommendation should be made as to whether the procedure must be repeated.4
Recording of complications- 5
Should complications of UGE be documented?
It is recommended that complications and mortality related to UGE be collected in prospective registries.
Quality of evidence: low. Grade of recommendation: weak in favour.
Agreement 4.8.
EvidenceSerious complications related to diagnostic UGE are rare and include cardiopulmonary incidents and infections.27 Perforation and bleeding are more common in therapeutic UGEs, especially in endoscopic submucosal dissection (ESD) as it is the most technically demanding technique. According to a recent meta-analysis on outcomes of gastric ESD in Europe,28 the overall rate of complications is 9.5%, including bleeding (5.8%), perforation (3.4%) and stenosis (0.35%). A total of three patients (0.26%) died in the month after the dissection, but in no case was there a direct relationship with the procedure.
Expert opinionOne of the keys to managing the quality of a centre’s endoscopy unit is keeping a record of complications, and for this it is necessary to have prospective registries. The American Society of Gastrointestinal Endoscopy (ASGE) proposes that complications be recorded as a post-procedure quality parameter,27 not only for immediate complications but also for delayed ones (between 14 and 30 days). It would also be highly desirable to audit the specific rate of complications of each endoscopist on the unit, with a view to pursuing training and corrective measures when this rate is not as expected. Since the most common complications (perforation and bleeding) occur in the context of therapeutic UGE, it is unlikely that this type of complications will be found in a diagnostic UGE, but some incidents such as those related to sedation may occur.4
Visualisation time- 6
What should be the minimum visualisation time of the gastric cavity in a UGE for detection of GCPLs?
The minimum duration of a UGE for detection of GCPLs should be at least seven minutes, with four of those minutes dedicated to examination of the gastric cavity.
Quality of evidence: low. Grade of recommendation: strong in favour.
Agreement: 4.7.
EvidenceThe European Society of Gastrointestinal Endoscopy (ESGE) initiative to improve gastroscopy quality published in 2016 recommends dedicating at least seven minutes to performing a UGE from intubation to extubation.29 This recommendation is based on a retrospective cohort study that evaluated the relationship between examination time and diagnosis of lesions.30 In this study, 837 patients with gastrointestinal symptoms but without a history of GC or previous endoscopies were evaluated. The mean examination time was 6.6 min for gastroscopies without findings and without biopsies. Based on this result, the authors established a cut-off point at seven minutes and observed that “slow” endoscopists detected twice as many gastric lesions (intestinal metaplasia, atrophy, dysplasia or cancer) than “fast” ones and three times more dysplasia and cancer.30 Another subsequent cohort study corroborated the importance of time to increase the detection of lesions, although in this case the cut-off point was three minutes.31
No studies have evaluated whether a longer visualisation time during surveillance UGEs increases the detection of EGC. However, a recent study conducted in South Korea evaluated the impact of time in detecting synchronous lesions before endoscopic treatment.32 Of 83 patients with synchronous lesions, 46 were identified before resection of the index lesion. In this group, the mean duration of the endoscopy was 6.5 min, compared to 3.8 min for gastroscopies in which synchronous lesions were detected during the endoscopy performed a year later.
Expert opinionThe level of evidence supporting the optimal duration of a diagnostic gastroscopy is very low.30,31,33 Although no studies have evaluated the optimal duration of a surveillance gastroscopy, the recommendation of a duration of seven minutes seems reasonable based on the two studies mentioned above.30,32 Additionally, Veitch et al. recommend dedicating at least four minutes to examining a standard-size stomach (730 cm2) to rule out the existence of EGC.33 Given that the proposed increase in duration hardly affects the number of examinations that endoscopy units could perform, and the low risk it poses to the patient, this panel of experts opts for a recommendation strongly in favour.
Use of premedication with mucolytics and defoaming agents- 7
Does the use of defoaming agents and mucolytics improve the detection of GCPLs/EGC?
Oral administration of simethicone 200 mg and N-acetylcysteine (500 or 1000 mg) prior to endoscopy is recommended to improve visualisation of the gastric mucosa.
Quality of evidence: high. Grade of recommendation: strong in favour.
Agreement: 4.6.
EvidenceThe use of oral premedication in combinations of defoaming agents and mucolytics (simethicone plus N-acetylcysteine or simethicone plus pronase) improves visualisation of the gastric mucosa.25,34,35 According to the results of a clinical trial by Monrroy et al.,25 visualisation of the gastric mucosa improves with oral administration of simethicone 200 mg plus N-acetylcysteine prior to endoscopy. In the post-hoc analysis of this trial, which included five groups (three with premedication: simethicone, simethicone plus N-acetylcysteine 500 and simethicone plus N-acetylcysteine 1000; and two control groups: water and no intervention), a higher rate of detection of lesions in gastroscopy was observed when the premedication groups were compared to the control group with water alone (32% versus 14%; P = .02). This difference did not hold when the premedication and non-intervention groups were compared. However, these results should be interpreted with caution, since the study was not designed with the objective of detecting lesions. There were no differences in examination times or adverse effects. In another blinded RCT, conducted by Liu et al.36 in 2018, premedication with simethicone and pronase improved visibility of the gastric mucosa, but did not increase the rate of detection of preneoplastic lesions.
In a meta-analysis published by Chen et al.,37 simethicone increased visibility of the gastric mucosa. However, this visualisation was not improved by adding N-acetylcysteine or pronase to simethicone. Similarly, in a trial by Elvas et al.,38 no differences were detected regarding visualisation of the mucosa between the group on simethicone alone and the group on simethicone plus N-acetylcysteine. However, this association could reduce the need for washing with water during the procedure.39 Finally, in an RCT by Zhang et al.35 from 2018 (control group versus simethicone plus pronase), no differences were observed in terms of diagnosis of lesions <5 mm (erosions, polyps, ulcers, etc.), but the premedication group showed an increase in the number of patients in whom biopsies were performed (19.7% versus 31.1%; P = .001) and the number of diagnosed GCPLs.
Premedication is usually administered orally, with a volume of 100 mL administered 10−20 min before the examination.40 In studies in which simethicone is administered through the endoscope working channel, traces of this non-absorbable material have been shown to hinder endoscope processing and promote bacterial growth in the endoscope channel. Therefore, it is advisable to minimise its administration through the endoscope and use the lowest possible dose.41,42 No studies that demonstrated traces of simethicone when it is used as an oral premedication prior to the procedure, although it seems reasonable to exercise caution during its use as well.
Expert opinionThe use of premedication with mucolytics and defoaming agents improves visualisation of the gastric mucosa, although there are discrepant results when evaluating the use of simethicone combinations compared to the use of simethicone alone. The study by Zhang et al.35 suggested that improved visualisation increases the number of biopsies and, therefore, the diagnosis of GCPLs. However, in the study, whether biopsies were performed was not protocolised and we do not know whether preparation with simethicone influenced whether targeted biopsies were performed. Meanwhile, a benefit in the detection of lesions has not been clearly demonstrated. There are also discrepancies regarding the most effective premedication regimen. Based on the RCT conducted by Monrroy et al.,25 the most effective combination seems to be simethicone 200 mg plus N-acetylcysteine (500 or 1000 mg) administered orally 20 min before gastroscopy. No complications secondary to oral premedication have been demonstrated during gastroscopy performed with deep sedation; therefore, the use of propofol would not contraindicate the use of pre-gastroscopy preparations to improve visualisation of the mucosa.35
Use of sedation- 8
Does sedation improve detection of GCPLs/EGC?
The use of sedation is recommended for UGE for diagnosis or surveillance of GCPLs, although it has not been shown to improve detection.
Quality of evidence: low. Grade of recommendation: strong in favour.
Agreement: 4.2.
EvidenceMost clinical guidelines recommend sedation for UGE.43,44 In a double-blind RCT,45 sedation with midazolam improved patients’ perception of the procedure, reducing their previous anxiety and increasing their cooperation and acceptance in the event of having to repeat it. Propofol is associated with greater endoscopist satisfaction and faster recovery, with no differences in patient satisfaction or in safety compared to other drugs.46,47 In another randomised and blinded study,48 when videos of UGEs were reviewed, it was observed that endoscopists perceived a better quality of gastroscopy in patients sedated with propofol versus midazolam. Of all the parameters evaluated, complete visualisation of the antrum and the lesser curvature in retroflexion were clearly superior in the group sedated with propofol. Furthermore, more time could be spent on endoscopic examination during the recording of the examinations due to the better tolerance of the patients to gastric distension.
The effect of sedation on the detection of lesions has only been evaluated in a retrospective study49 that observed a significant increase in the detection of gastric polyps, especially those located in the gastric body and fundus in patients sedated with propofol versus those not sedated. While recognising the weak scientific support regarding the influence of sedation on the detection of lesions, the Asian consensus on the diagnostic standards of UGE2 recommends the use of sedation to improve the detection rate of early oesophagogastric neoplasms, and the British Society of Gastroenterology (BSG) and the Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS)4 also advise, with a strong recommendation, the use of sedation as part of a quality UGE.
Expert opinionSedation allows for a longer examination, improves the patient’s and the endoscopist’s perception of the procedure, decreases patient anxiety, and increases acceptance of repeating the examination. This would make it possible to increase patient compliance with the follow-up recommendations advised and improve the efficacy of surveillance gastroscopies.
Visualisation technique- 9
What is the imaging technique of choice for the detection of GCPLs/EGC?
The use of high-definition endoscopes and conventional or digital chromoendoscopy is recommended for the detection and surveillance of GCPLs.
Quality of evidence: high. Grade of recommendation: strong in favour.
Agreement: 4.5.
The use of narrow-band imaging (NBI) is recommended for the detection of IM.
Quality of evidence: high. Grade of recommendation: strong in favour.
Agreement: 4.9.
EvidenceThere are two types of enhanced imaging techniques: staining techniques with the use of dyes (conventional chromoendoscopy [CE]) and specific (digital) systems included in the endoscopy equipment available on the market: NBI, flexible spectral imaging colour enhancement (FICE), i-Scan, blue laser imaging (BLI) and linked colour imaging (LCI). All of them have been studied both to detect and to characterise GCPLs and EGC.
Conventional CE in the detection of GCPLs/EGC. Both methylene blue and indigo carmine or acetic acid have been used for this purpose, as well as a combination of the latter two (bichromoendoscopy [biCE]).
Conventional CE showed a higher percentage of detection of gastric lesions when compared with white light imaging (WLI) in a meta-analysis50 that included 699 patients and 902 lesions. CE presented a sensitivity of 90% and a specificity of 82% for the diagnosis of both GCPLs and EGC. When the analysis was stratified by histology, the diagnostic precision was superior to WLI in both cases (GCPL: 98.4% versus 81%; EGC: 86.6% versus 54.9%).
Digital CE in the detection of GCPLs. For intestinal metaplasia (IM), specific optical patterns using digital chromoendoscopy without magnification have been reported: the ridge mucosal pattern and bluish-whitish areas. Both patterns have been reported with both NBI and BLI. With the addition of magnification to NBI, other signs have also been reported: light blue crests, marginal turbid bands and the presence of white opaque substance. In a meta-analysis that evaluated these markers for the diagnosis of IM, the highest sensitivity and specificity were obtained with the presence of light blue crests (79% and 95%, respectively).51
When WLI was compared with NBI for the detection of IM, a multicentre RCT showed a higher percentage of detection of this type of GCPL in the NBI group (17.7% versus 7.7%).52 Sensitivity and specificity were 59.1% and 98.6% with WLI and 92.3% and 94.3% with NBI, respectively.
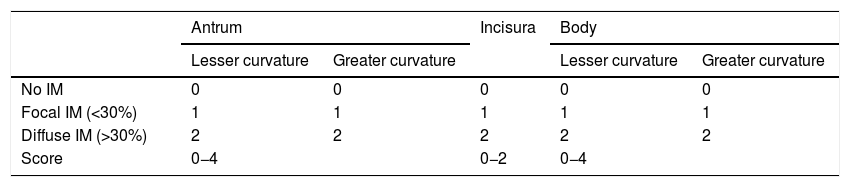
With the intention of making the diagnosis of IM with NBI more reproducible, an endoscopic classification has been proposed that scores its presence or absence in five different areas of the mucosa.53 The endoscopic grading of gastric intestinal metaplasia (EGGIM) scoring system gives a score between 0 (normal without areas of IM) and 10 (extensive IM in all areas of the gastric mucosa) (Table 2). The diagnostic accuracy of the extension of the IM went from 83% with WLI to 94% with NBI. The benefit of NBI was greater in OLGIM stages III and IV. This endoscopic EGGIM classification was very recently validated,54 and the optimal cut-off point to detect these OLGIM III/IV lesions was an EGGIM score >4. Furthermore, it could be appropriate to stratify the individual risk of developing GC.55
Regarding additional use of magnification with NBI (M-NBI) for the diagnosis of IM, diagnostic precision was similar when compared to studies in which magnification was not available according to a recent meta-analysis.56 In fact, the marker that was most suitable and easily available at all centres was the tubulovillous mucosal pattern without additional magnification (sensitivity 88% and specificity 97%).
Regarding the detection of IM with other technologies, such as BLI or LCI, in this same meta-analysis, suitable sensitivity and specificity were observed when BLI was used together with magnification (89% and 97%, respectively). However, as it was a single study, definitive conclusions could not be drawn. Regarding LCI without magnification, the overall sensitivity and specificity for detecting IM in the two studies included were 73% and 92%, respectively.57,58
Adding near focus to NBI improves identification of extensive gastric IM. Lage et al.59 found that the addition of NBI and near focus increased the precision of WLI from 60% to 73%. Sensitivity and specificity for endoscopic diagnosis of extensive IM were 92% and 96%, respectively.
Digital CE in EGC detection. With WLI without magnification, suspicious EGC lesions appear as areas with irregular margins, colouration or surfaces.
Two RCTs have compared second-generation NBI without magnification and WLI in the detection of GC. A study by Ang et al.52 did not show a higher incidence of GC in patients randomised to NBI compared to the WLI group in an Asian population of subjects over 50 years of age who underwent a screening or diagnostic gastroscopy (2.4% in the WLI group and 1% in the NBI group; P = .19). In another more recent RCT,60 in a highly selected population (high-risk patients and in an area with a high incidence of GC), the percentage of GC detection showed no significant between-group differences (WLI 1.9% versus NBI 2.3%; P = .41). The proportion of lesions not detected in the first examination also showed no statistically significant differences when the first examination was with WLI (27.5%) or with NBI (22.4%). Sensitivity and specificity for detecting EGC were 80% and 88%, respectively, with WLI, and 76.8% and 91% with NBI. Therefore, in this highly selected population of patients with a high pre-test probability of EGC, NBI without magnification did not show a clear improvement in the detection of ECG compared with WLI.
Expert opinionThe different published studies have consistently shown that the different CE techniques, conventional or digital, increase diagnostic precision to detect GCPLs when compared to WLI.
Although the quality of the available scientific evidence on the usefulness of conventional CE in the detection of GCPLs and EGC is high, most of the studies included are from Asia, their methodological quality is heterogeneous, the number of patients is relatively low, and the type of staining used is highly varied (methylene blue, indigo carmine or biCE).
Of all the digital CE systems, NBI is the most extensively studied for the detection of IM and has consistently shown increased sensitivity and specificity compared to WLI. The other currently available systems have not yet been extensively studied, although it is possible that they yield very similar results (especially BLI).
Finally, CE with second-generation NBI, without magnification, has not demonstrated improvement in the detection of EGC when compared to WLI in the hands of experts and areas of high incidence. It therefore seems that the most determining factor for the detection of EGC is suitable training of the endoscopist when it comes to locating abnormalities (such as irregularity, depressed areas or changes in colour) in the mucosa surface with WLI.
Histological diagnosis and evaluation of the extent of IM- 10
What biopsy protocol is recommended for the diagnosis and evaluation of the extent of IM?
It is recommended that two topographic areas (antrum, including incisura, and body) be biopsied using NBI to target the areas that raise the most suspicion of IM in biopsies.
Quality of evidence: low. Grade of recommendation: strong in favour.
Agreement: 4.9.
EvidenceThe histological study of the samples obtained by endoscopic biopsy is the gold standard for the diagnosis of GCPLs. Current evidence suggests that conventional or digital CE-guided biopsies combined with random mapping are the ideal method to detect GCPLs.50 In a study by Buxbaum et al.,61 100% of patients with IM and 95% of areas with IM were detected using this strategy. The explanation is that random biopsies detect some cases that are not identified with NBI and, in expert hands, targeted biopsies with NBI increase the diagnostic capacity of random mapping. The most widely accepted protocol is the updated Sydney protocol,62 which recommends obtaining at least five biopsies: two from the antrum (one from the lesser curvature and one from the major curvature), 3 cm from the pylorus; two from the body (one from the lesser curvature and one from the major curvature), about 4 cm from the incisura; and one from the incisura angularis. The biopsies must be immediately immersed in a suitable fixative solution and sent in at least two separate containers indicating the location/origin, one for the antrum/incisura and the other for the body. This biopsy protocol enables diagnosis of H. pylori and staging of patients with IM and atrophy. Various studies63,64 support the minimum protocol of five biopsies, but warn that with this number some premalignant lesions could be missed. In fact, some guidelines, such as those of the ASGE, recommend obtaining seven to twelve random biopsies.65
The usefulness of adding a biopsy of the incisura is also contested. Although this location has been considered an area of early onset of atrophy or metaplasia, with a higher prevalence of premalignant lesions and more reliable for the diagnosis of atrophy and IM than the antrum or the body,64,66 other studies have concluded that biopsies of the incisura add little information to those obtained from the body and the antrum. Among them, a retrospective review of more than 400,000 gastric biopsies concluded that the assessment of the incisura contributed minimal additional diagnostic information to four biopsies (two of the body and two of the antrum), which was the strategy that showed the highest diagnostic performance.67 Similarly, a review of gastric biopsies obtained according to the updated Sydney protocol in 272 patients from an area with low risk of GC found that the IM was located in isolation in the incisura in only 3.3% of cases.68 However, other authors69,70 have found that biopsies of the incisura better predict gastritis severity than biopsies of any other part of the gastric mucosa. Isajevs et al.70 observed that the most intense atrophy and IM were more common in the incisura than in the antrum or in the body, and that excluding the histological evaluation of the incisura led to an underestimation in staging of 18% using the OLGA staging system and 4% using the OLGIM staging system (this figure reached 30%–35% in advanced OLGIM/OLGA stages). Similarly, Varbanova et al.71 reported that 8% of cases with atrophy and 3% of with IM would not have been correctly identified if the incisura had not been biopsied, and the OLGA/OLGIM stage would have been significantly less advanced. In line with these studies, Kim et al.72 found that including incisura biopsies prevents understaging of gastritis, which does not occur when biopsies from the anterior or posterior aspect of the body or antrum are added. Finally, another study in patients at high risk of GC due to family history detected understaging in 15% and 30% of cases, if incisura biopsies were not included, compared to the use of the original OLGA/OLGIM systems.73 As regards random biopsies, it is recommended that flat mucosal areas be biopsied, since protruding areas with a regular surface and vascular pattern may represent islands of preserved mucosa that falsely suggest the existence of atrophy.74
Expert opinionCurrently, there is a discrepancy between the different clinical guidelines when recommending a protocol for gastric biopsies. Although the original Sydney system did not include incisura biopsies, its subsequent update does recommend it, as does the fourth edition of the Maastricht Consensus.75 Implementation of the Sydney protocol including the incisura enables risk stratification based on the OLGIM system. The updated MAPS II guidelines continue to recommend at least four biopsies (two in the antrum and two in the body), but recognise that the histological evaluation of the incisura can be considered in order to increase the diagnosis of premalignant lesions, especially when targeted biopsies with CE cannot be taken.76
If suitable biopsies were not taken in the index or diagnostic gastroscopy, or if all biopsies were sent in the same container and the diagnosis of IM was made incidentally, it is recommended that the endoscopic examination be repeated using CE to obtain suitable histological mapping,76 at least in patients at high risk of GC. In patients at moderate risk of GC, the decision to repeat the gastroscopy should be made on a case-by-case basis depending on age, comorbidities, preferences and so on. The most widely accepted recommendation is to obtain at least five biopsies (two of the antrum, two of the body and one of the incisura) and send them in at least two containers (body and antrum/incisura), indicating whether the biopsies were random or targeted areas raising suspicion of IM.
Visible lesion- 11
How should a visible lesion be evaluated?
It is recommended that the horizontal margins of visible lesions and their risk of submucosal invasion be evaluated by experienced endoscopists using endoscopes with magnification and CE.
Quality of evidence: high. Grade of recommendation: strong in favour.
Agreement: 4.4.
It is recommended that visible lesions susceptible to endoscopic resection be biopsied (1−2 times) to confirm the neoplastic origin of the lesion
Quality of evidence: low. Grade of recommendation: strong in favour.
Agreement 4.8.
In visible lesions, it is proposed that endoscopic ultrasound be reserved only for those with signs raising suspicion of deep submucosal invasion.
Quality of evidence: low. Grade of recommendation: weak in favour.
Agreement: 4.5.
In visible lesions not eligible for endoscopic resection due to suspicion of deep invasion, it is recommended that (at least six) biopsies be taken for suitable histological assessment.
Quality of evidence: low. Grade of recommendation: strong in favour.
Agreement: 4.4.
Endoscopic excision is recommended as the treatment of choice for visible neoplastic gastric lesions with no evidence of deep submucosal invasion.
Quality of evidence: low. Grade of recommendation: strong in favour.
Agreement: 4.9.
EvidenceThe first step with a visible lesion is a suitable optical evaluation. Observational studies and RCTs have found that diagnostic precision in the evaluation of gastric lesions increases with learning systems and the experience of the endoscopist.16,77,78 The use of conventional CE has been shown to contribute to better delimitation of the lateral margins of the lesion, especially in cases of well-differentiated tumours.79–81 A recent RCT that enrolled 343 patients concluded that CE with indigo carmine without magnification and M-NBI have similar precision in delineating the lateral margins of the EGC (85.7% versus 88%, respectively; P = .63).82
The vast majority of published studies use magnification for the diagnosis and characterisation of dysplasia and EGC. The lesion is evaluated using, in general, the vessel plus surface classification (irregular microvascular and/or superficial pattern within a demarcation line). The available evidence on the use of digital CE magnification for the characterisation of visible lesions comes from observational studies, RCTs and several meta-analyses.83–85 Most of the studies have focused on M-NBI, although there are also studies with other digital CE systems.86–88 All of them have indicated that these systems, used with magnification, improve the ability to identify and delimit the horizontal margins with a diagnostic precision exceeding 85%–90% and identify some signs that suggest infiltration of the submucosa, such as dilation of blood vessels or the presence of vessels with different calibres.84,86,88–94 In a study published by Hu et al.,83 sensitivity and specificity were 86% and 96% with M-NBI, and 56% and 79% with WLI. The diagnostic performance of the M-NBI was negatively influenced by the depressed lesion morphology and size <10 mm. In a meta-analysis by Zhang et al.,84 sensitivity and specificity were 83% and 96% with M-NBI, and 48% and 67% with WLI. More recently, a meta-analysis published by Rodríguez-Carrasco et al.56 confirmed the trend towards the use of magnification for this indication, since it included only two studies (out of a total of 19) in which it was not used. Furthermore, this study showed that with M-NBI specificity increases (98% versus 94%) at the expense of a decrease in sensitivity (86% versus 94%) when compared to WLI.
Regarding digital CE without magnification, no studies having evaluated its precision in establishing lateral margins and the risk of deep submucosal invasion were found.
With WLI, observational studies have indicated that the presence of ulceration, irregular surfaces, size >3 cm, lack of distension with insufflation, marked redness, presence of excavation, convergence or abrupt disruption of the gastric folds and the presence of significantly raised borders are signs that suggest deep submucosal invasion.95–99 Endoscopic ultrasound is useful in locoregional staging of gastric neoplasms, although its precision in superficial neoplasms in determining the degree of submucosal invasion is moderate, according to a meta-analysis using Cochrane methodology (T1a versus T1b; sensitivity 87%; specificity 75%).100 Endoscopic ultrasound can both underestimate and overestimate the degree of invasion of the gastric wall; it can also yield false positives and negatives in lymph node staging.101 For this reason, both the ESGE and the Japan Gastroenterological Endoscopy Society (JGES) do not recommend its routine use, although it could be useful in decision-making in borderline lesions with risk factors for submucosal invasion.102,103
The optimal number of biopsies of a gastric lesion remains a matter of debate. The current trend in potentially resectable lesions is to perform fewer biopsies in order to prevent the appearance of fibrosis which may make endoscopic excision more difficult.2,102 An Asian RCT found that four biopsies prior to submucosal endoscopic dissection achieve better outcomes in terms of diagnostic efficiency.104 An observational study in patients with advanced GC concluded that three to four biopsies are sufficient for a suitable histological diagnosis.105 However, other authors have not found a clear benefit in increasing the number of biopsies and therefore advocate a more restrictive policy of limiting biopsies to the most suspicious area after a careful inspection with CE.106 To ensure that the sample is sufficient for molecular studies, the British guidelines on UGE quality recommend at least six biopsies, ideally from different areas of the lesion.4,107
Expert opinionThe first step with a visible lesion is a thorough optical inspection in order to predict the histology, delimit the horizontal margins and estimate the risk of deep submucosal invasion. This assessment should be performed by endoscopists with experience in diagnosis using high definition endoscopes with digital CE and magnification.
Several meta-analyses have demonstrated the usefulness of M-NBI to diagnose and characterise EGC. In our setting, the availability of gastroscopes with integrated magnification, especially at centres that perform advanced resection techniques for EGC, is supported by scientific evidence.
Biopsies of visible lesions are always necessary, either to confirm the neoplastic and non-inflammatory origin of lesions amenable to endoscopic treatment, or to make an accurate histological diagnosis when the lesion infiltrates the deep submucosa and endoscopic treatment is ruled out. In cases in which malignancy is strongly suspected due to the presence of the characteristics described (irregular microvascular and/or superficial pattern within a demarcation line), and in negative biopsies for dysplasia or cancer, a new endoscopic and histological evaluation of the lesion is necessary.
- 12
Which endoscopic resection technique is recommended?
Endoscopic submucosal dissection is recommended as the resection technique of choice.
Quality of evidence: low. Grade of recommendation: weak in favour.
Agreement: 4.3.
Endoscopic mucosal resection may be a valid alternative for small lesions (<10 mm) with a low risk of submucosal invasion.
Quality of evidence: low. Grade of recommendation: weak in favour.
Agreement: 4.6.
EvidenceResults from a meta-analysis show that underestimation of dysplasia in biopsies is common, and up to a third of lesions are diagnosed with high-grade dysplasia or invasive carcinoma after excision.108 For this reason, the guidelines recommend endoscopic resection of visible lesions with either the endoscopic mucosal resection (EMR) or the endoscopic submucosal dissection (ESD) technique. EMR and ESD have not been compared in RCTs. Numerous observational studies synthesised in four meta-analyses agree that ESD achieves higher percentages of en bloc resection and R0 resection and a lower percentage of recurrence. By contrast, ESD is associated with a longer procedure time and an increased risk of perforation, with no significant differences in the risk of bleeding after the procedure or in overall survival.109–112 Although most of the studies are from Asia, some European prospective series have already yielded comparable results.113,114 In lesions <10 mm with a low risk of deep invasion, the advantages of ESD over EMR have not been firmly established; therefore, currently, the clinical practice guidelines of the ESGE and the JGES accept EMR as a valid alternative.102,103
No RCTs have compared endoscopic excision of early GC versus surgical treatment. The available evidence comes from observational studies, mostly single-centre and retrospective. In 2015, a meta-analysis was published that analysed 11 retrospective cohorts with 2654 patients. Endoscopic treatment was associated with lower morbidity; no statistically significant differences were detected in terms of periprocedural mortality, local recurrence or five-year survival.115 In 2019, three meta-analyses were published that found that ESD is associated with lower morbidity, with no significant differences in terms of overall survival, although endoscopic treatment was associated with greater local recurrence.116–118 However, with suitable surveillance, the vast majority of recurrences can be treated with curative intent.119
Expert opinionEndoscopic treatment achieves similar cancer cure outcomes to surgery with less morbidity. It is essential to take into account the selection bias in the interpretation of the results in favour of EMR versus ESD in terms of safety, since lesions treated with EMR tend to be smaller and less complex. Furthermore, most perforations during ESD can be treated endoscopically and do not require surgical treatment. Finally, ESD is a complex technique with a long learning curve, meaning that its implementation requires suitable training according to the recommendations of the main scientific associations.6,103,120,121 According to the ESGE, after a training period that involves observing cases handled by experts and acting as an assistant to those experts in clinical practice, a process of guided learning using an animal model should be pursued. Finally, procedures presenting little difficulty will be performed, and supervised, in clinical practice. To maintain a sufficient level of experience, this association recommends performing >25 ESDs per year, regardless of the organ in which the technique is applied.122
ConclusionsThis position statement on the quality of UGE for the detection and surveillance of GCPLs is the result of the work of a group of expert gastroenterologists, endoscopists and pathologists who have attempted to offer their point of view based on current knowledge. Although the evidence in many of the questions posed is low, it was possible to provide a series of recommendations that will improve UGE quality, consequently, improve the capacity for the diagnosis of GCPLs in our setting, as well as standardise the management and follow-up thereof.
FundingThis position statement received no external funding.
Glòria Fernández Esparrach has received funding from the Instituto de Salud Carlos III [Carlos III Health Institute] through the project PI17/00894 (co-funded by the European Regional Development Fund “A way to make Europe”/“Investing in your future”).
Joaquín Cubiella has received funding from the Instituto de Salud Carlos III through the project PI17/00837 (co-funded by the European Regional Development Fund “A way to make Europe”/“Investing in your future”).
Conflicts of interestGlòria Fernández-Esparrach has received consulting fees from CDx Diagnostics and fees for organising courses from Olympus and Norgine.
José Carlos Marín-Gabriel has received speaker fees from Olympus, Norgine, and Casen Recordati.
Xavier Calvet has received research grants from Abott, MSD and Vifor; consulting fees from Abott, MSD, Takeda, Pfizer, Janssen and VIFOR; and speaker fees from Abbott, MSD, Janssen, Pfizer, Takeda and Allergan.
The other authors declare no conflicts of interest.
Please cite this article as: Fernández-Esparrach G, Marín-Gabriel JC, Díez Redondo P, Núñez H, Rodríguez de Santiago E, Rosón P, et al. Documento de posicionamiento de la AEG, la SEED y la SEAP sobre calidad de la endoscopia digestiva alta para la detección y vigilancia de las lesiones precursoras de cáncer gástrico. Gastroenterol Hepatol. 2021;44:448–464.