Colonic inflammatory bowel diseases have a higher risk of developing colorectal cancer compared to the general population, which is why they require endoscopic screening techniques with specific follow-up intervals based on the different risk factors described on the literature.
This position paper analyzes the current scientific evidence for the different endoscopic techniques available today, how their implementation should be carried out in endoscopic units and describes in detail how their implementation should be carried out, in which patients and with what interval, and finally, what should be the response to finding dysplasia, proposing a specific follow-up algorithm.
Los pacientes con enfermedades inflamatorias intestinales de localización colónica tienen mayor riesgo de desarrollar cáncer colorrectal que la población general, por lo que precisan de técnicas endoscópicas de cribado con intervalos de seguimiento basados en los diferentes factores de riesgo descritos.
En el presente documento de posicionamiento analizamos la evidencia científica vigente para las diferentes técnicas endoscópicas disponibles en la actualidad, cómo debe realizarse su implementación en las unidades de endoscopia, y se describe con detalle cómo debe ser técnicamente su realización, a qué pacientes y con qué intervalo debe realizarse, y finalmente, cuál debe ser nuestra actitud ante el hallazgo de displasia, proponiendo un algoritmo de seguimiento específico.
“Practitioners strive to be precise and, to achieve this, they use their intelligence, training, knowledge and experience. Use of the random biopsy to detect dysplasia is the antithesis of this approach.” This is the idea published by Ray Soetikno et al.,1 gastroenterology and dysplasia experts, at the end of 2019 when reflecting on the SCENIC consensus published in 2015,2 an international and multidisciplinary consensus based on the GRADE methodology that resulted in a radical change to clinical practice guidelines for colorectal cancer (CRC) screening of our patients with inflammatory bowel disease (IBD).
We know that patients with long-standing IBD, especially ulcerative colitis (UC) but also colonic Crohn's disease (CD), are at a higher risk of developing CRC and therefore we must use the best endoscopic technique available to prevent and/or detect IBD early. It has been shown over recent decades that the use of such screening techniques in IBD patients is associated with a reduced risk of CRC, decreased deaths due to CRC and increased detection of CRC at earlier stages.3
The future of screening will be guided by molecular biomarkers that will be capable of distinguishing those patients with a high risk of CRC and even those patients who already have dysplasia and need specific screening. However, until then, we must use the scientific evidence currently available to us to respond to a series of questions that we will list and answer during this positioning document.
Is endoscopic surveillance effective in IBD patients?Patients with left-sided or extensive UC are at a higher risk of developing CRC than the general population, with a prevalence of 3.7% and an incidence rate of 2% at 10 years in classical studies with evident selection bias.4 More recent meta-analyses5,6 confirm that, although this incidence rate has decreased over recent decades, the risk of CRC is still estimated to be 1.21 times higher than that of the general population. With respect to patients with colonic CD, the data seem to be similar, although with less evidence.7
The effectiveness of colonoscopic surveillance in IBD patients has not been evaluated in randomised clinical trials (RCT). A Cochrane review published in 2006 concluded that “there is no clear evidence that surveillance colonoscopy prolongs survival in patients with extensive colitis”.8 However, it identified indirect evidence in retrospective case-control studies of a reduced risk of death in IBD patients with cancer diagnosed during surveillance.9–11 One retrospective population-based study observed a survival rate of 100% in CRC patients in the surveillance group compared to 74% in CRC patients in the non-surveillance group (p = 0.042).12
A more recent Cochrane review3 published in 2018, which included 7,199 patients evaluated in observational studies, concluded that CRC detection is significantly higher in the non-endoscopic surveillance group (3.2%; 135/4,256) compared to the surveillance group (1.8%; 53/2,895) (OR: 0.58; 95% CI: 0.42-0.80), p < 0.001. Also, endoscopic surveillance was associated with a lower rate of CRC-associated death (8.5% vs 22.3%) (OR: 0.36; 95% CI: 0.19-0.69; p = 0.002) and a higher rate of early-stage CRC detection (15.5% vs 7.7%) (OR: 5.40; 95% CI: 1.51-19.30; p = 0.009).
Based on these data, it can be concluded that surveillance in patients with long-standing IBD is effective since it detects a higher number of cases of CRC at earlier stages and reduces the rate of CRC-associated death.
What is the technique of choice for dysplasia screening in IBD patients?Classically, patients with long-standing IBD have been screened using conventional endoscopy and random biopsies, with a total of 34 biopsies from all segments of the colon required to detect dysplasia with a 90% probability, and double this number (64 biopsies) required to increase the confidence interval to 95%.13 It is estimated that, with this strategy, approximately one case of dysplasia can be detected per 1,505 biopsies taken.14 However, over recent years, dysplasia lesions that were not previously visible have been detected thanks to improvements and advances in endoscopic technology.15
There are currently several advanced endoscopy techniques in this specific setting: virtual chromoendoscopy (VCE) (narrow band imaging [NBI], i-scan, FICE), dye chromoendoscopy (DCE) and autofluorescence imaging (AFI), which over the years have been compared to white-light endoscopy (WLE) and high-definition white-light endoscopy (HDWLE). RCT have been performed comparing these techniques. The results of these trials have been included in four meta-analyses,16–19 although the results from the different trials are not comparable due to different selection criteria (one includes conference abstracts, another only RCT and another RCT and observational studies), which, when added to the high heterogeneity and low quality of some of the studies, makes it difficult to reach conclusions.
Virtual chromoendoscopy (VCE) compared with white-light endoscopy (WLE) and high-definition white-light endoscopy (HDWLE)VCE techniques use filters built into the endoscope without dyes. Blue is the light that is preferentially absorbed by haemoglobin. As such, the capillary network and the pit pattern of the mucosa can therefore be seen better, avoiding the use of dyes and allowing quicker procedures.20 On including the three RCTs comparing VCE (NBI, i-scan or FICE) (161 patients) with HDWLE (171 patients),21 no differences in dysplasia detection were found per patient (RR: 0.72; 95% CI: 0.45–1.15). However, there was a statistical difference in favour of HDWLE in the dysplastic lesion detection analysis (RR: 0.64; 95% CI: 0.48–0.90), with no heterogeneity detected in either of the two analyses (I2 = 0%) but with the quality of evidence decreasing due to inaccuracies in both.
Dye chromoendoscopy (DCE) compared with white-light endoscopy (WLE) and high-definition white-light endoscopy (HDWLE)The first RCT to prove the usefulness of DCE compared with WLE for dysplasia detection in patients with long-standing IBD was published in 2003 (Kiesslich et al.22). After this, five more clinical trials were included in a meta-analysis published in 201123 involving a total of 1,277 patients, which clearly shows the superiority of DCE for both the detection of all dysplastic lesions and the detection of flat dysplastic lesions. DCE increased the per-patient dysplasia detection rate by 2–3 times and the per-lesion dysplasia detection rate by 4–5 times.
In the Italian meta-analysis,17 DCE is significantly superior to HDWLE (five studies included) (OR: 4.218; 95% CI: 1.240–14.345). However, these results were not confirmed in a more recent publication including only the two RCTs published by that time.19 More recently, a new RCT has demonstrated the superiority of DCE to HDWLE,24 with similar real-world results obtained in the Spanish multicentre study (endoCAR group)25.
Dye chromoendoscopy (DCE) compared with virtual chromoendoscopy (VCE)A comparison of VCE and DCE requires a more exhaustive analysis than a simple statement that there are no differences in dysplasia detection, which is the conclusion reached by the different meta-analyses published. The first study to compare DCE and NBI was designed by the Hospital Clínic de Barcelona group and was a randomised, crossover (back-to-back) clinical trial26 involving 60 patients with long-standing IBD who underwent both DCE and NBI. There were no significant differences in the diagnosis of dysplastic lesions. However, DCE was significantly superior in detecting any type of lesion and it was estimated that, in absolute terms, NBI missed six dysplastic lesions per patient compared to two missed lesions with DCE. A subsequent Australian study using the same methodology confirmed these results.27 More recently, the Leuven28 and Birmingham29 groups have published the results of their clinical trials using a different methodology, randomising patients to either VCE or DCE. No differences were found between the two techniques used in the two studies for the detection of dysplasia. However, this parallel methodology does not allow dysplastic lesions missed in the same patient to be calculated.
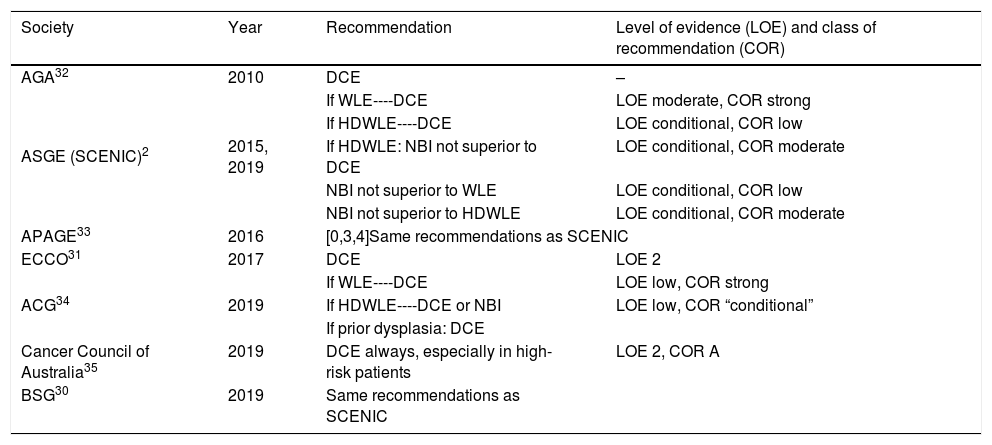
Based on this available evidence, we consider dye chromoendoscopy (DCE) to be the technique of choice for CRC screening in patients with long-standing IBD or at a high risk of CRC, consistent with most current national and international clinical and endoscopic guidelines (Table 1).2,30,35
CRC screening recommendations according to different guidelines.
| Society | Year | Recommendation | Level of evidence (LOE) and class of recommendation (COR) |
|---|---|---|---|
| AGA32 | 2010 | DCE | – |
| ASGE (SCENIC)2 | 2015, 2019 | If WLE----DCE | LOE moderate, COR strong |
| If HDWLE----DCE | LOE conditional, COR low | ||
| If HDWLE: NBI not superior to DCE | LOE conditional, COR moderate | ||
| NBI not superior to WLE | LOE conditional, COR low | ||
| NBI not superior to HDWLE | LOE conditional, COR moderate | ||
| APAGE33 | 2016 | [0,3,4]Same recommendations as SCENIC | |
| ECCO31 | 2017 | DCE | LOE 2 |
| ACG34 | 2019 | If WLE----DCE | LOE low, COR strong |
| If HDWLE----DCE or NBI | LOE low, COR “conditional” | ||
| If prior dysplasia: DCE | |||
| Cancer Council of Australia35 | 2019 | DCE always, especially in high-risk patients | LOE 2, COR A |
| BSG30 | 2019 | Same recommendations as SCENIC | |
ACG: American College of Gastroenterology; AGA: American Gastroenterology Association; APAGE: Asian Pacific Association of Gastroenterology; ASGE: American Society of Gastrointestinal Endoscopy; BSG: British Society of Gastroenterology; ECCO: European Crohn's and Colitis Organisation.
Choosing those IBD patients who are to receive endoscopic surveillance is one of the most important aspects of dysplasia and CRC screening. Despite recent improvements in endoscopy procedures, colonoscopy is still an invasive technique with a risk of complications that requires bowel preparation, which, in spite of huge improvements, is not pleasant for the patient. In addition, available resources must be optimised and therefore only those patients who will benefit, according to available scientific evidence, are offered screening.
There is currently a series of risk factors that helps determine which IBD patients should be offered dysplasia and CRC screening.
Disease durationAs has been explained above, although the risk of CRC among the UC population has fallen,4 it is still higher than the risk among the general population5,6. It is estimated that the cumulative incidence for the first 10 years after diagnosis of UC is <0.5%, increasing to 1% at 10 years, 3% at 20 years and 7% at 30 years.35,36 This same risk applies to colonic CD, doubling after 10 years.35
Extent of diseasePatients with extensive UC clearly have a higher risk of CRC, with a 19.2- and 14.8-fold (95% CI: 11.4–18.9) excess risk of CRC estimated in two classical European studies.37,38 With respect to the risk of CRC in patients with left-sided UC, the data are controversial, probably due to differences in the definition of extent in older studies. Nevertheless, although the risk of developing CRC in patients with left-sided UC described in the 1990s is not as common during the first two decades of disease, the incidence is the same in these studies in the fourth decade,37,39 and even at 15 years in more recent studies.7
Disease activityThe role of disease activity has been analysed in various studies,40 and although it is very likely that the risk of developing CRC is associated with the underlying inflammatory process, there are some controversial data.
In one case-control study of 68 patients with UC and CRC, it was demonstrated that higher colonoscopic (OR: 2.5; p = 0.001) and histological (OR: 5.1; p = 0.001) inflammation scores were associated with a higher risk of CRC.41 However, more recent studies, such as the one conducted by Mooiweer et al.42 in 2013 or the aforementioned study conducted in the Netherlands7, found no significant differences in CRC risk between subjects with mild, moderate or severe inflammation. Matsuoka et al.43 reported that the risk of CRC was associated only with active phase inflammation (RR: 0.04; 95% CI: 0.01–0.11) or mild colitis (RR: 5.80; 95% CI: 3.52–9.55) at 5-year follow-up.
Strictures and pseudopolypsIn 1990, the retrospective registry compiled by Lashner et al.44 described the presence of dysplasia and CRC in strictures that were principally located in the left colon in 27 patients. More recent data45 confirm this increased risk of cancer in patients with strictures (OR: 5.7; 95% CI: 1.7–18.9), with malignancy rates of up to 25% having been described.46 This study also identified appearance late in the course of the disease (61% probability of malignancy in strictures developing after more than 20 years vs 0% in those occurring before 10 years) and location proximal to the splenic flexure (86% probability of malignancy vs 47% in sigmoid and 10% in rectum) as risk factors.
The presence of post-inflammatory polyps or pseudopolyps is also associated with an increased risk of CRC, probably because of prior increased inflammation of the adjacent mucosa.9,45 It is also sometimes difficult to reach a differential diagnosis with adenomatous polyps. It is therefore recommended that the mucosa surrounding the polyp be carefully inspected and its presence requires that the endoscopic surveillance interval be reduced.31
Primary sclerosing cholangitis (PSC)PSC is a clearly established risk factor for developing CRC in patients with IBD, with CRC incidence rates of 4.9% vs 0% and 11% vs 0% in two published cohort studies.47,48 Furthermore, up to a nine-fold increased risk,49 with a clear increased risk particularly 10 to 20 years after diagnosis of PSC, has been published. Nevertheless, given the subclinical course of PSC, and sometimes UC, and the resulting significant delay in diagnosis, CRC screening in these patients is required from the very year of diagnosis.
Family history of CRCOnce again, on analysing this risk factor, there are some studies that have reported a significantly increased risk of CRC in patients with IBD and relatives with CRC42 and others that have found no statistical association.7,49
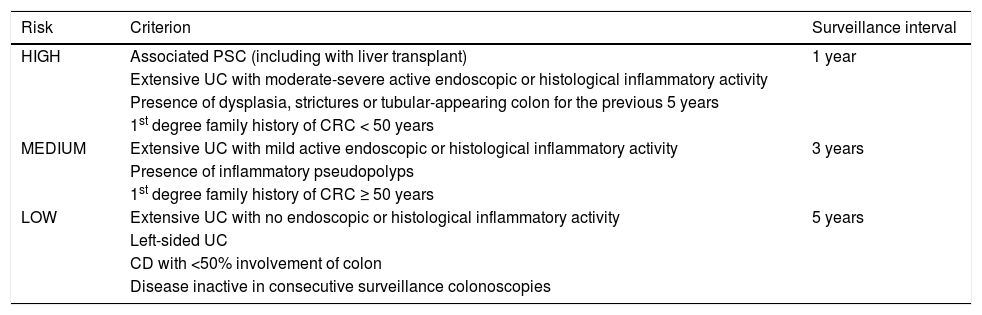
Based on this evidence, chromoendoscopy is indicated in all patients diagnosed with left-sided or extensive UC and in all patients diagnosed with CD involving at least 1/3 of the colon with more than eight years of disease. One exception to this rule is patients with associated PSC, in whom surveillance should commence at diagnosis. Outside the recommendations for endoscopic surveillance is UC involving only the rectum (proctitis [E1]) and CD in the ileum or with <1/3 of the colon involved, given that there appears to be no increased risk of CRC in these patient sub-groups. Table 2 summarises the recommended surveillance intervals.
Endoscopic surveillance according to risk factors.
| Risk | Criterion | Surveillance interval |
|---|---|---|
| HIGH | Associated PSC (including with liver transplant) | 1 year |
| Extensive UC with moderate-severe active endoscopic or histological inflammatory activity | ||
| Presence of dysplasia, strictures or tubular-appearing colon for the previous 5 years | ||
| 1st degree family history of CRC < 50 years | ||
| MEDIUM | Extensive UC with mild active endoscopic or histological inflammatory activity | 3 years |
| Presence of inflammatory pseudopolyps | ||
| 1st degree family history of CRC ≥ 50 years | ||
| LOW | Extensive UC with no endoscopic or histological inflammatory activity | 5 years |
| Left-sided UC | ||
| CD with <50% involvement of colon | ||
| Disease inactive in consecutive surveillance colonoscopies |
Implementation of any technique requires a series of steps to ensure optimal yield. In the case of DCE, this implementation procedure is not generally well known in our setting in clinical practice. The SCENIC consensus gives a series of recommendations regarding both the necessary equipment and the procedure.2 The authors of this review emphasise the fundamental role of systematic DCE training and the need for measures to evaluate the yield of endoscopic surveillance using DCE in IBD patients.
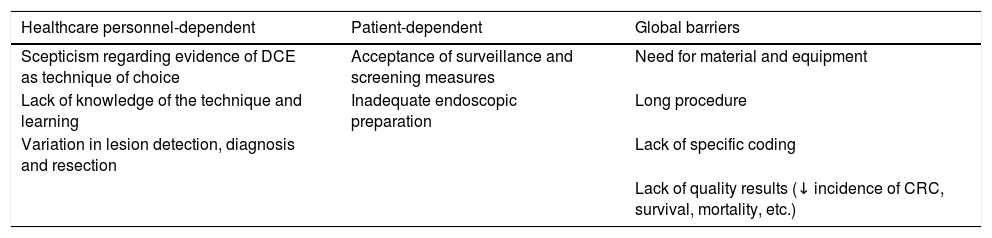
The implementation of DCE in different departments is established in a consensus document published in 2016 by international experts.50 As stated by these experts in their article, there is a series of barriers to implementation at different hospitals, which are summarised in Table 3.
Barriers for implementation of dye chromoendoscopy.
| Healthcare personnel-dependent | Patient-dependent | Global barriers |
|---|---|---|
| Scepticism regarding evidence of DCE as technique of choice | Acceptance of surveillance and screening measures | Need for material and equipment |
| Lack of knowledge of the technique and learning | Inadequate endoscopic preparation | Long procedure |
| Variation in lesion detection, diagnosis and resection | Lack of specific coding | |
| Lack of quality results (↓ incidence of CRC, survival, mortality, etc.) |
After understanding the main existing barriers and adhering to the recommendations of these international expert authors, the steps outlined below should be followed to implement DCE in our units:
- 1
Systematic training using atlases, videos, online learning, attending specific courses and workshops, using existing detailed algorithms to perform pancolonic DCE in IBD:20,51
- •
Learning and use of standardised terminology to characterise endoscopic and histological dysplastic lesions
- •
Uniformity in the reporting of dysplasia and CRC to allow comparison of results between surveillance programmes
- •
Awareness of surveillance intervals and indications for DCE
- 2
Ensuring that endoscopists are familiar with DCE protocols, in terms of both the necessary equipment and material:
- •
Type of endoscope and magnification (if possible)
- •
Dye and its concentration: indigo carmine/methylene blue
- •
Method of administration of the dye: use of dye-spray catheter
- •
Inspection method with the skill to discern lesions that are endoscopically resectable or, if not, to refer them for surgery
- •
Endoscopic resection techniques2,51
- 3
Practical learning with an endoscopist who is an expert in DCE, performing at least five procedures
- 4
Monitoring of quality by recording dysplasia detection data and surveillance intervals:
- •
Photodocumentation of lesions, which is important both as a quality issue and for shared decision-making, which can also have medical and/or legal implications. Images (photos or videos) must record reference points in the colon (ileocaecal valve, appendix, terminal ileum), the extent and severity of inflammation, if present, the presence of strictures and perianal disease. Images must also be obtained of each focal lesion before and after staining and, for resections, the submucosal injection technique used and the integrity of the resection must be documented. Tattoos applied to concerning lesions (e.g. large unresected lesions or lesions with resections performed at different times or incomplete resections) may also be documented
- •
Quality of endoscopic preparation
- •
Surveillance of dysplasia according to established algorithms
- •
Monitoring and review of quality measures in order to avoid unnecessary colectomies (proportion of procedures in which targeted biopsies are performed, proportion of patients in whom dysplasia is detected, or with flat lesions, proportion of complete resection of dysplastic lesions and interval cancer rate)
Finally, one important aspect of the implementation of DCE is an assessment of its profitability (cost-effectiveness). Although the cost of purchasing the high-quality, high-definition endoscopic equipment (endoscopes, processors, monitors, etc.) is significant, it is already available and widely used at most of our hospitals. The cost of the dye is variable and depends on the concentration and volume used, but it tends to be low and much cheaper, even when using a specially prepared formula instead of commercial preparations with the right dilution. Using the dye for infusion in the bottle or flushing pump of the endoscopic equipment may also shorten the procedure time and reduce costs by avoiding the cost of the catheter for some procedures. However, a dye-spray catheter is often going to be required to improve the visualisation of any lesions detected.
It is estimated that the mean time to perform a DCE is 11 min (range: 9–12 min) longer than the mean time taken to perform a standard colonoscopy when targeted or random biopsies are taken.52 However, random biopsies are not necessary in most procedures2 and this saves time, thereby also reducing the cost of the pathologist's tests.
Cost-effectiveness may improve if the interval cancer rate decreases. In this sense, one cost-effectiveness study53 comparing different surveillance strategies concluded that chromoendoscopy is more cost-effective and less expensive than white-light endoscopy with biopsies at all surveillance intervals.
The implementation of dye chromoendoscopy in our IBD units is relatively simple after studying the technique and performing five procedures with an expert endoscopist.
How should dye chromoendoscopy be performed? Preparation, technique and description of lesionsDye chromoendoscopy involves spraying a specific dye directly onto the gut mucosa to enhance the mucosal surface pattern, which improves the identification and characterisation of lesions.54 Dyes that have been validated for staining in this setting are indigo carmine (the most commonly used contrast stain) and methylene blue (contraindicated in patients with glucose-6-phosphate dehydrogenase deficiency). Although potential DNA damage in stained tissues has been described in animal models with methylene blue,55 this effect does not appear to have any relevant significance in actual clinical practice in humans.56
Dye chromoendoscopy must be performed according to the SURFACE methodology57 (Strict patient selection, Unmask the mucosal surface, Reduce peristaltic waves, Full length staining of the colon, Augmented detection with dyes, Crypt architecture analysis and endoscopic targeted biopsies) in the absence of macroscopic inflammatory activity: UCEIS 0-1 or SES-CD 0-1.
When inserting the endoscope, all residual stool must be lavaged and suctioned, sometimes using a mucolytic or anti-foaming agent (dimethicone or N-acetylcysteine). After reaching the caecum, uniform staining is started by spraying specially prepared indigo carmine 0.4% (prepared by adding 1 g of indigo carmine to 250 ml of sterile water) onto the mucosa using a dye-spray catheter, or by adding 1–4 g of indigo carmine to the flushing bottle (2 ampoules containing 5 ml of indigo carmine 0.8% in 250 ml of water, or 1 ampoule containing 10 ml of methylene blue 1% in 240 ml of water2 if specially prepared indigo carmine is not available). Higher concentrations of solution should be applied when lesions are detected (1 ampoule containing 5 ml of indigo carmine 0.8% in 25 ml of water or 1 ampoule containing 10 ml of methylene blue 1% in 40 ml of water2), administered by syringe into the endoscope channel for better characterisation of the lesions.
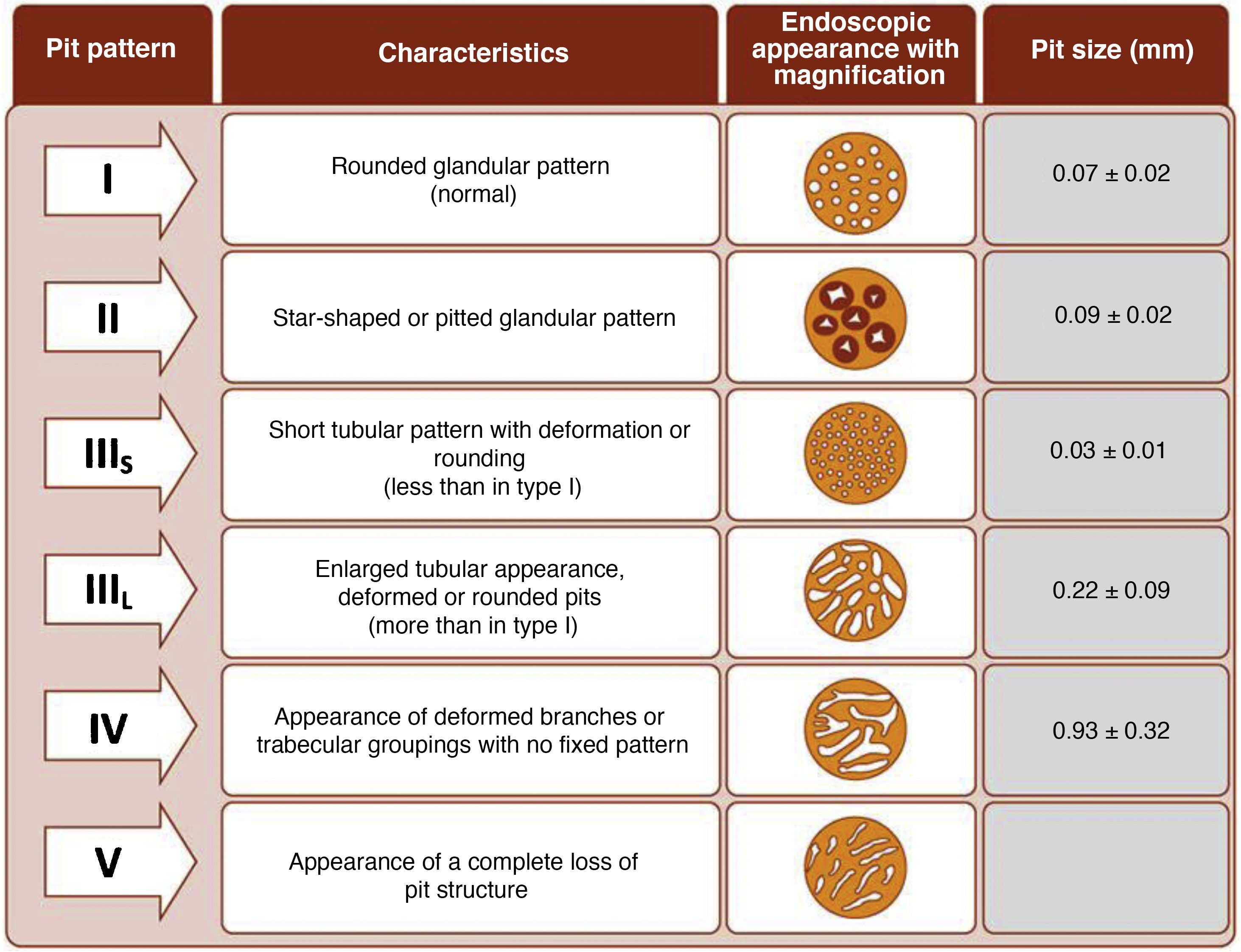
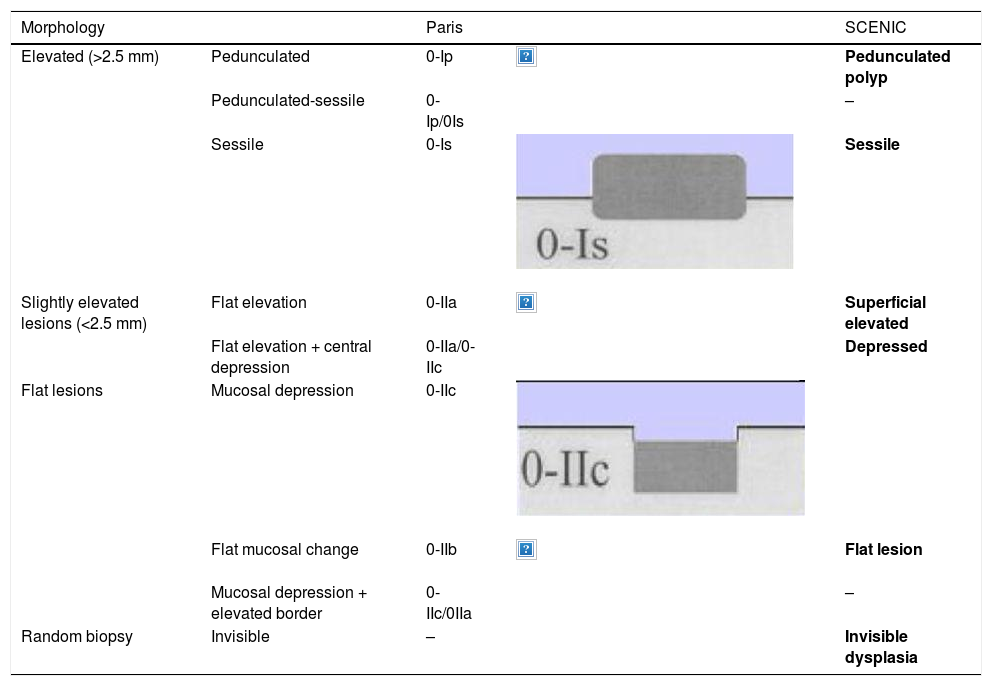
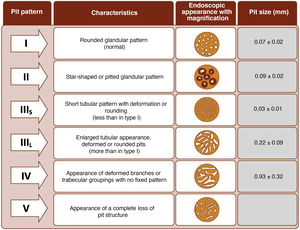
The endoscope is withdrawn using a rotational motion, focusing sequentially on 20–30 cm segments, with reinsertion and slow withdrawal of the endoscope and suctioning any excess dye to allow detailed visualisation of the mucosa. On detecting a lesion, the morphology of the lesion must be correctly described according to the modified Paris Classification criteria outlined in the SCENIC consensus (Table 4)2 and a description must be given of the pit pattern first described by Kudo et al.,58 differentiating non-neoplastic patterns (I and II) from suspected dysplasia (III to V) (Fig. 1). When describing lesions, terms such as dysplasia-associated lesion or mass (DALM) or adenoma-like should not be used. In addition to dysplastic lesions, hyperplastic serrated lesions or serrated adenomas are being observed more and more frequently in patients with IBD. Such lesions may also contribute to the development of CRC and are identified in up to 11% of patients, with 86% of lesions having a Kudo pit pattern type II.59
Lesion morphology Paris classification and equivalence.
| Morphology | Paris | SCENIC | ||
|---|---|---|---|---|
| Elevated (>2.5 mm) | Pedunculated | 0-Ip | Pedunculated polyp | |
| Pedunculated-sessile | 0-Ip/0Is | – | ||
| Sessile | 0-Is | Sessile | ||
| Slightly elevated lesions (<2.5 mm) | Flat elevation | 0-IIa | Superficial elevated | |
| Flat elevation + central depression | 0-IIa/0-IIc | Depressed | ||
| Flat lesions | Mucosal depression | 0-IIc | ||
| Flat mucosal change | 0-IIb | Flat lesion | ||
| Mucosal depression + elevated border | 0-IIc/0IIa | – | ||
| Random biopsy | Invisible | – | Invisible dysplasia | |
After describing the lesion, its removal must be assessed. This is possible when the lesion's margins are well defined and there are no suspected invasive components. En bloc resection should be attempted whenever possible by performing a polypectomy, endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD).60 If referral to a reference centre is required or the lesion is highly suspicious for dysplasia, the lesion should be tattooed.
Peri-lesional biopsies are becoming more and more controversial given their low diagnostic yield in the detection of dysplasia and their questionable clinical impact, with rates of 0.7–5% described but with no association to the subsequent development of high-grade dysplasia or cancer.61–63 In the two Spanish chromoendoscopy studies (EndoCAR25 and Grupo de Enfermedad Inflamatoria Intestinal de Castilla y León [Castile and Leon Inflammatory Bowel Disease Group, GEICYL]64), peri-lesional biopsies did not identify dysplasia.
With regard to the systemic taking of random biopsies, over recent years numerous studies have demonstrated the low profitability and limited success of this strategy. As a result, targeted biopsies are the surveillance method of choice over random biopsies.65,66 Most dysplasia is visible in UC67,68 and detection rates from random biopsies performed using chromoendoscopy are very low.2 ECCO31, BSG30, ACG34, APAGE33 and Cancer Council of Australia35 guidelines therefore recommend targeted biopsies over random biopsies when performing chromoendoscopy. The prospective, multicentre, GETAID study69 confirms the low yield of dysplasia identification by random biopsies (1.2% per colonoscopy), similar to the yield demonstrated in other studies using chromoendoscopy (1.2-6.2%). Dysplasia by random biopsies was associated with a personal history of CRC, a tubular-appearing colon or the presence of PSC and therefore multiple random biopsies are perhaps recommendable in these high-risk patients. If, during the procedure, no lesions are identified and no endoscopic activity is observed, random biopsies (two per segment) may be considered to determine histological activity and, based on the results, a recommendation will be given as to whether more endoscopic surveillance is required.2
It is common to observe numerous and sometimes large lesions with Kudo pit pattern type I and II in the rectum and sigmoid. Given the high negative predictive value (NPV) of the Kudo classification,25,64,70,71 which has proven highly accurate for ruling out dysplasia, and the fact that most dysplastic lesions are located in the proximal colon,25,29,64 resection of Kudo type I lesions and potentially Kudo type II lesions in the sigmoid and rectum may not be necessary. This could avoid endoscopic complications in patients who are often asymptomatic and save time during procedures. In the GEICYL study, lesions located in the rectum and sigmoid were protective against dysplasia.64 Although most lesions with Kudo pit pattern type II correspond to hyperplastic lesions, they may, in some cases, correspond to serrated lesions with possible potential for malignancy. In the Iacucci et al. study,59 80% of serrated adenomas were located in the proximal colon and were flat lesions with an irregular spiral vascular pattern. Over the coming years, new studies will be required to evaluate the natural history of these lesions in IBD.
Finally, a good chromoendoscopy report must contain all the information described above:
- -
Assessment of mucosal scarring (UCEIS/SES-CD)
- -
Assessment of colon cleanliness using an objective scale (e.g. Boston scale using segment scores)
- -
Presence and/or absence of pseudopolyps
- -
Morphology of lesions according to the modified Paris classification Description of borders and presence and/or absence of ulceration
- -
Pit pattern of lesions according to the Kudo classification
- -
Photodocumentation of lesions and/or video
- -
Resectability of lesions, with a description of the type of removal
The use of indigo carmine or methylene blue allows the morphology and pit pattern to be described with a high negative predictive value for ruling out dysplasia. Lesions should be removed, without peri-lesional biopsies, and the need for random biopsies should only be considered with a personal history of CRC, a tubular-appearing colon or associated PSC.
What is the significance of dysplasia at this time and what should be done if dysplasia is detected?Dysplasia or 'intraepithelial neoplasia' is the neoplastic alteration of intestinal epithelial cells confined within the basement membrane, without invasion of the lamina propria. The appearance of a series of molecular and histological changes within the context of associated chronic inflammation conditions the development of CRC, which is generally associated with the following sequence: inflammation, dysplasia (indefinite, low or high grade) and carcinoma. Dysplasia is the marker related to an increased risk of malignancy in more than 90% of cases and is therefore part of endoscopic surveillance strategies in IBD.31 It is important to note that this sequence has certain limitations since 10–15% of cancers develop with no history of dysplasia or directly from a low-grade dysplasia (LGD) without the presence of high-grade dysplasia (HGD). The presence of indefinite dysplasia highlights the problems faced when identifying dysplasia, with a reported variable progression to HGD and CRC of 13–28%.72
However, finding dysplasia during patient surveillance (incidental dysplasia) has a lower associated risk than diagnosis during the initial colonoscopy (prevalent dysplasia) where the risk of HGD and CRC is higher (29% vs 16%). Recurrent dysplasia (LGD in two consecutive colonoscopies) has a huge impact on the need for colectomy, as does a finding of multifocal dysplasia (especially if one is HGD).
The microscopic classification most commonly used at present in Europe is the Vienna classification, which stratifies dysplasia into 5 categories: negative for dysplasia, indefinite for dysplasia, LGD, HGD and invasive neoplasia. The greatest diagnostic challenges are associated with differentiating between regenerative changes and LGD, and between HGD and early invasive carcinoma, and therefore a second assessment by an expert pathologist is crucial before making any decisions regarding patient management.14,31
In routine practice, the macroscopic classification of dysplasia should be used, which since 2015 has followed guidelines and definitions adopted at the International Consensus (SCENIC) meeting with descriptive phrases from the Paris classification adapted to its potential endoscopic resectability (Table 4).2,73
Polypoid lesion (type I)Lesion protruding from the mucosa into the lumen >2.5 mm (reference: 2.5 mm = height of the closed cups of a biopsy forceps)
- •
Pedunculated polypoid lesion (Paris type Ip)
- •
Sessile polypoid lesion (Paris type Is)
These are generally endoscopically resectable and, irrespective of the grade of dysplasia, should be treated by complete endoscopic resection (confirmed by histological studies and with the tissue surrounding the resected lesion being free from dysplasia: staining or magnification techniques) and subsequent surveillance, preferably using chromoendoscopy (DCE) with a high-definition endoscope. It is important to ensure, especially in HGD lesions, that there are no other dysplastic lesions in the rest of the colon.2,31,73,74
The estimated incidence of CRC in these patients after endoscopic resection of polypoid lesions with dysplasia is approximately 0.5/100 patient-years, similar to the incidence after polypectomy in patients with no associated colitis.75 Later studies show cancer progression rates of 0–4.5% at 2 years and 0–13.6% at 4 years.76 In many of these studies, there is no differentiation between lesions with LGD or with HGD.
Sessile nonpolypoid lesion (type II)- •
Lesions elevated or protruding <2.5 mm (type IIa)
- •
Flat or non-elevated lesions (type IIb)
- •
Depressed lesions (type IIc), which sometimes occur with ulceration; more difficult or not endoscopically resectable
Combined lesions are common: for example, elevated sessile or nonpolypoid lesions accompanied by a flat lesion (Is + IIb/IIa + IIb).
Management of nonpolypoid lesionsIn all cases, and especially in combined lesions, it is recommended to assess the presence of ulceration in the lesion and accurately define the borders of the lesion (staining/magnification techniques) in order to differentiate lesions from the surrounding mucosa and perform complete resection.74 Visualisation of these lesions, especially flat or depressed lesions (IIb and IIc), is enhanced by chromoendoscopy (DCE) techniques with high-definition endoscopes (HDWLE). It is important to consider the low dysplasia diagnosis rates, which are around 15%,74 with the majority of lesions found being hyperplastic or regenerative changes. Resection of such lesions may harbour unnecessary risks. In recent publications, pit pattern interpretation (Kudo classification) using chromoendoscopy to differentiate between dysplastic lesions and hyperplastic lesions has shown sensitivity of around 93–100% with specificity of 88–97%,74,77 with negative and positive predictive values of 88% and 46%, respectively. The negative predictive value for DCE and NBI was comparable, with doubts as to whether these results could be extrapolated if colonoscopy without magnification were performed by untrained endoscopists. The Spanish IBD group (GEICYL)64 has recently published the results of its population-based study of dysplasia incidence rates using DCE. Although good correlation between lesions with Kudo pattern ≥ III and dysplasia was not shown, Kudo type I and II lesions were correctly identified, with a high negative predictive value (92%), even by non-expert endoscopists.
The SCENIC consensus statement does not consider the association between lesion type and the probability of predicting dysplastic or invasive lesions, but later publications have indicated a higher frequency of HGD in nonpolypoid lesions with description of Kudo patterns (III–V) on studying lesions with endoscopic magnification.73,74
An international expert panel has recently validated a new classification, The Frankfurt Advanced CE IBD LEsions system (FACILE), to improve prediction of dysplasia in lesions detected. This classification includes assessment of the surface pattern (rounded, regular or irregular pit pattern, unstructured) and the vascular pattern (not visible, regular or irregular). The four predictors of dysplasia include the nonpolypoid morphology of the lesion, irregular surface pattern, irregular vascular pattern and signs of inflammation within the lesion.78
Data on progression of nonpolypoid lesions to CRC are limited and originally come from observational studies and case series in which there is no clear differentiation between LGD and HGD.76 In these studies, there is a wide variation (40–100%) in the rates of full resection of the lesion with margins that are clear of dysplasia (R0), conditioning the prognosis of surveillance. The most recent surveillance studies include R0 resections <70% given the presence of large lesions with a high rate of fibrosis.79,80 This results in high heterogeneity and inconsistency when conducting meta-analyses of published studies.76
Overall, lower rates of progression to advanced lesions are currently observed than in earlier studies after the introduction of DCE and high-definition endoscopy in 2012. Studies reporting R0 > 70–80% show low rates of progression to HGD and CRC (even 0%), although with short follow-up periods (2 years).76 Taking a consecutive, multicentre study involving 69 patients and using DCE and high-definition endoscopy, conducted in 2000 and 2014, as an example, a rate of progression to advanced lesions following resection of LGD of 1.34 per 100 patient-years is observed with a mean follow-up of 3.3 years.81 A recent Belgian82 retrospective follow-up study involving 410 patients for more than 20 years reports a lower risk of advanced lesions (CRC and HGD) in comparison with earlier studies,83 with a risk of progression of LGD lesions in the event of resection of 2.3% at 1 year of follow-up (0.5% for CRC) and 13.8% (8.5% for CRC) at 10 years, with the risk of CRC after prior resection of HGD lesions being 9.1% at 1 year and 24.3% at 10 years. In total, 19% of patients undergoing colectomy for dysplasia had CRC in their surgical specimen (five with an associated stricture) and 42% of patients undergoing colectomy for HGD had cancer in their surgical specimen. The presence of metachronous lesions, PSC, colon strictures and a history of nonpolypoid dysplastic lesions >1 cm was associated with a RR of 15 for subsequent development of CRC.82,83
Invisible lesionInvisible dysplasia is dysplasia detected in random biopsies. It has already been mentioned that these lesions are, in fact, generally visible. In the literature, there are eight studies with a small number of patients, limited follow-up and heterogeneous cohorts that include patients who are at a high risk of CRC (PSC and multifocal dysplasia). These studies report progression rates for this type of dysplasia of 2.3–13% at 1 year and 4.6–44% at 2 years,76 with CRC progression rates of 0–28% with a mean follow-up of 2 years. The detection of LGD lesions using DCE or high-definition endoscopes has improved, with only 12% of lesions observed to have invisible LGD compared to 88% of invisible lesions before.81 In terms of surveillance, progression rates are also lower, with figures of 2.29/100 patient-years and CRC progression rates of only 3.8% with a mean follow-up of 5 years.
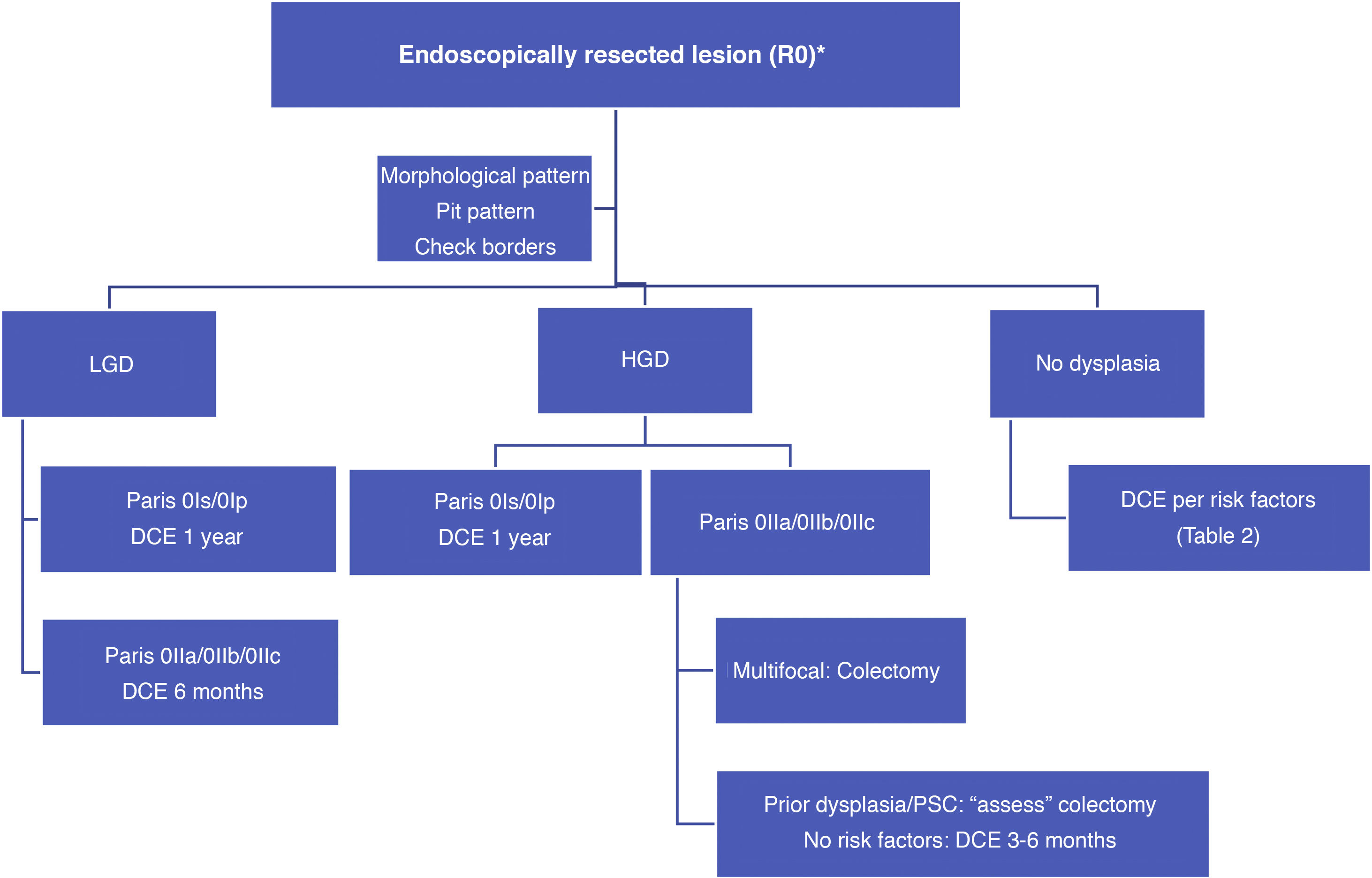
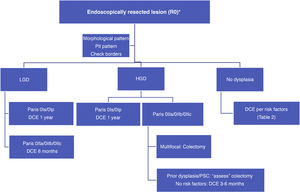
With such limited data, we suggest a surveillance algorithm whenever dysplasia is found and the lesion is fully resected (Fig. 2). If resection is not possible but dysplasia is present, surgery must necessarily be indicated.
ConclusionsDysplasia screening in high-risk IBD patients should preferably be performed using dye chromoendoscopy, with surveillance intervals established on the basis of known clinical, endoscopic and histological risk factors.
The natural history of dysplasia detected using high-definition endoscopes and chromoendoscopy techniques is unknown. Only time and multicentre registries will gradually help us to make decisions for our IBD patients in the medium term.
Conflicts of interestDr B. Sicilia: scientific advice, support for research and/or training activities with Tillots Pharma, Kern Pharma, AbbVie, Janssen, Pfizer and Takeda
Dr L. Arias: scientific advice, support for research and/or training activities with AbbVie, FAES Pharma, Kern Pharma, Ferring and MSD
Dr R. Vicente: scientific advice, support for research and/or training activities with AbbVie, Janssen, MSD, Pfizer, Takeda, Shire, Dr Falk, FAES Pharma and Ferring
Dr Y. Zabana: scientific advice, support for research and/or training activities with AbbVie, Adacyte, Almirall, Amgen, Dr Falk, FAES Pharma, Ferring, Janssen, MSD, Otsuka, Pfizer, Shire, Takeda and Tillots
Dr M. Mañosa: scientific advice, support for research and/or training activities with AbbVie, FAES Pharma, Ferring, Janssen, MSD, Pfizer and Takeda
B. Beltrán: consultant and advisor for Takeda, Pfizer, MSD and Fresenius
Dr M. Barreiro-de Acosta: scientific advice, support for research and/or training activities with MSD, AbbVie, Janssen, Kern Pharma, Celltrion, Takeda, Gilead, Pfizer, Ferring, FAES Farma, Shire Pharmaceuticals, Dr Falk Pharma, Chiesi, Gebro Pharma, Adacyte and Vifor Pharma.
Please cite this article as: Sicilia B, Vicente R, Arias L, Echarri A, Zabana Y, Mañosa M, et al. Recomendaciones del Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) sobre cribado de displasia en pacientes con enfermedad inflamatoria intestinal. Gastroenterol Hepatol. 2021;44:435–447.