Colonoscopy is currently considered to be the gold standard for evaluation of colonic mucosa inflammation in patients with ulcerative colitis (UC), but the procedure is invasive and cannot be repeated frequently, especially in the paediatric population. The aim of this study was to assess the role of faecal calprotectin (FC) as a predictor of endoscopic disease activity in paediatric patients with UC in clinical remission.
Material and methodsSingle-centre prospective study. Clinical remission was defined as Paediatric Ulcerative Colitis Activity Index <10. Endoscopic findings were assessed according to the Mayo Endoscopic Subscore (MES). MES≤1 was defined as endoscopic remission. All participants provided fresh faecal samples for measurement of FC.
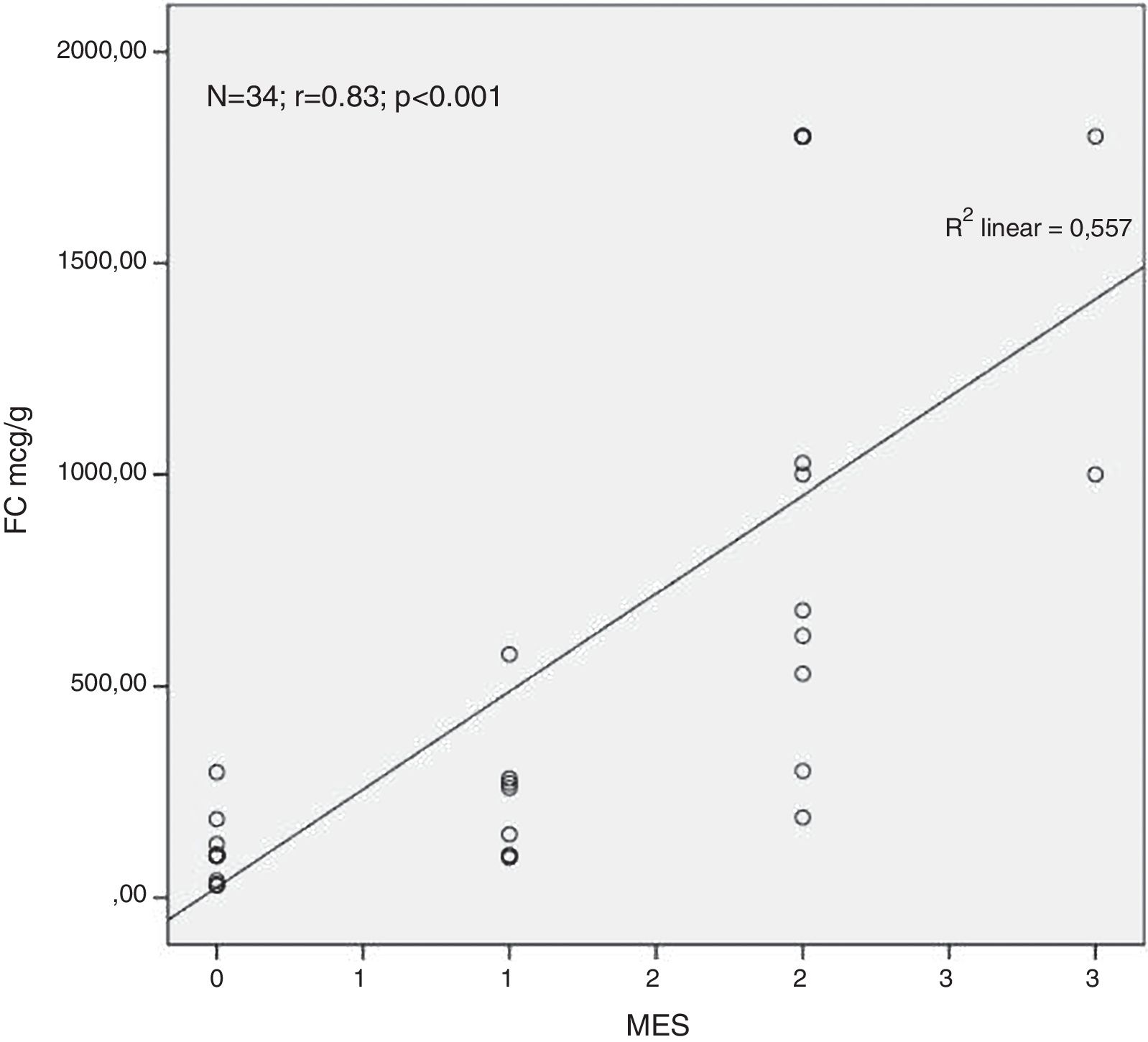
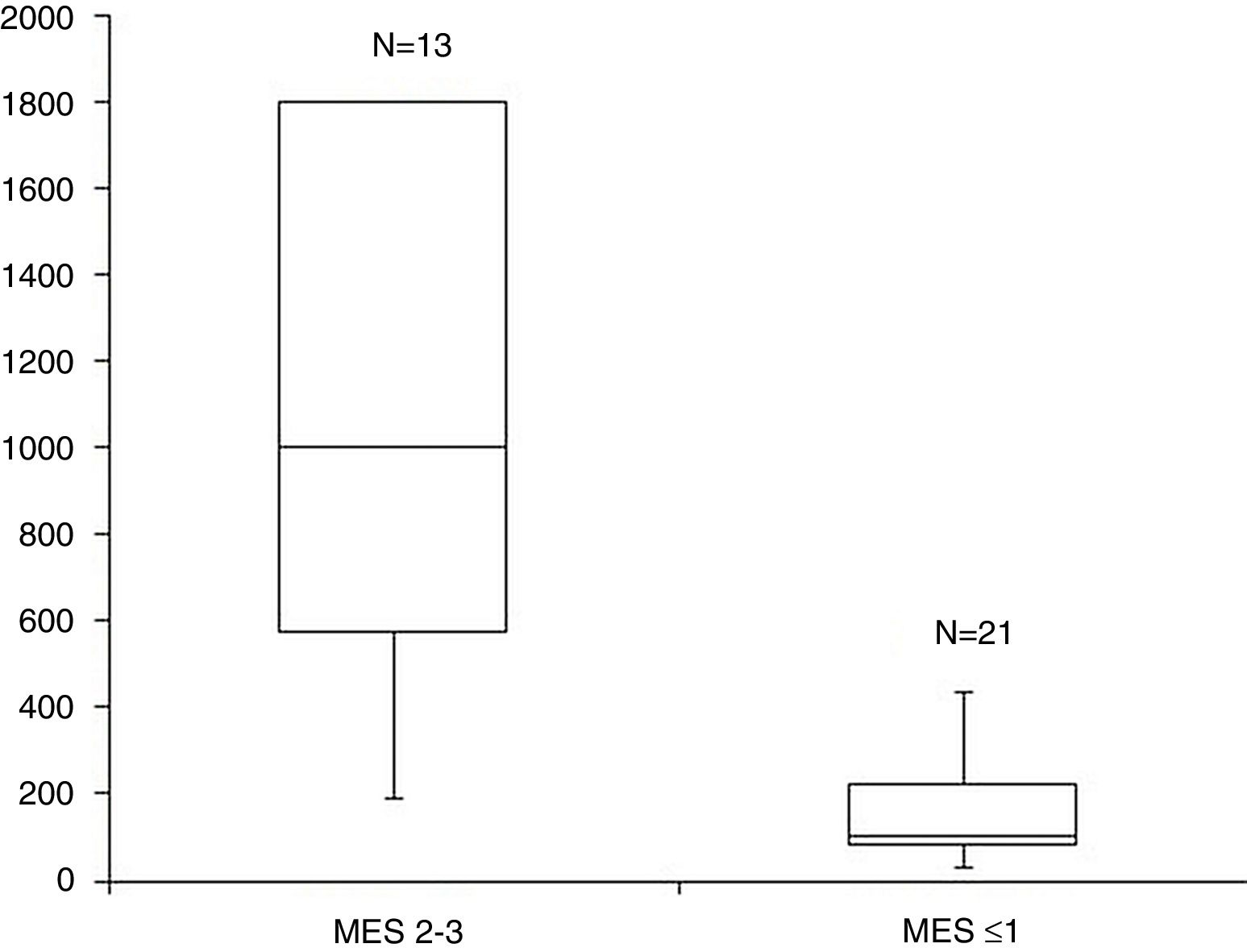
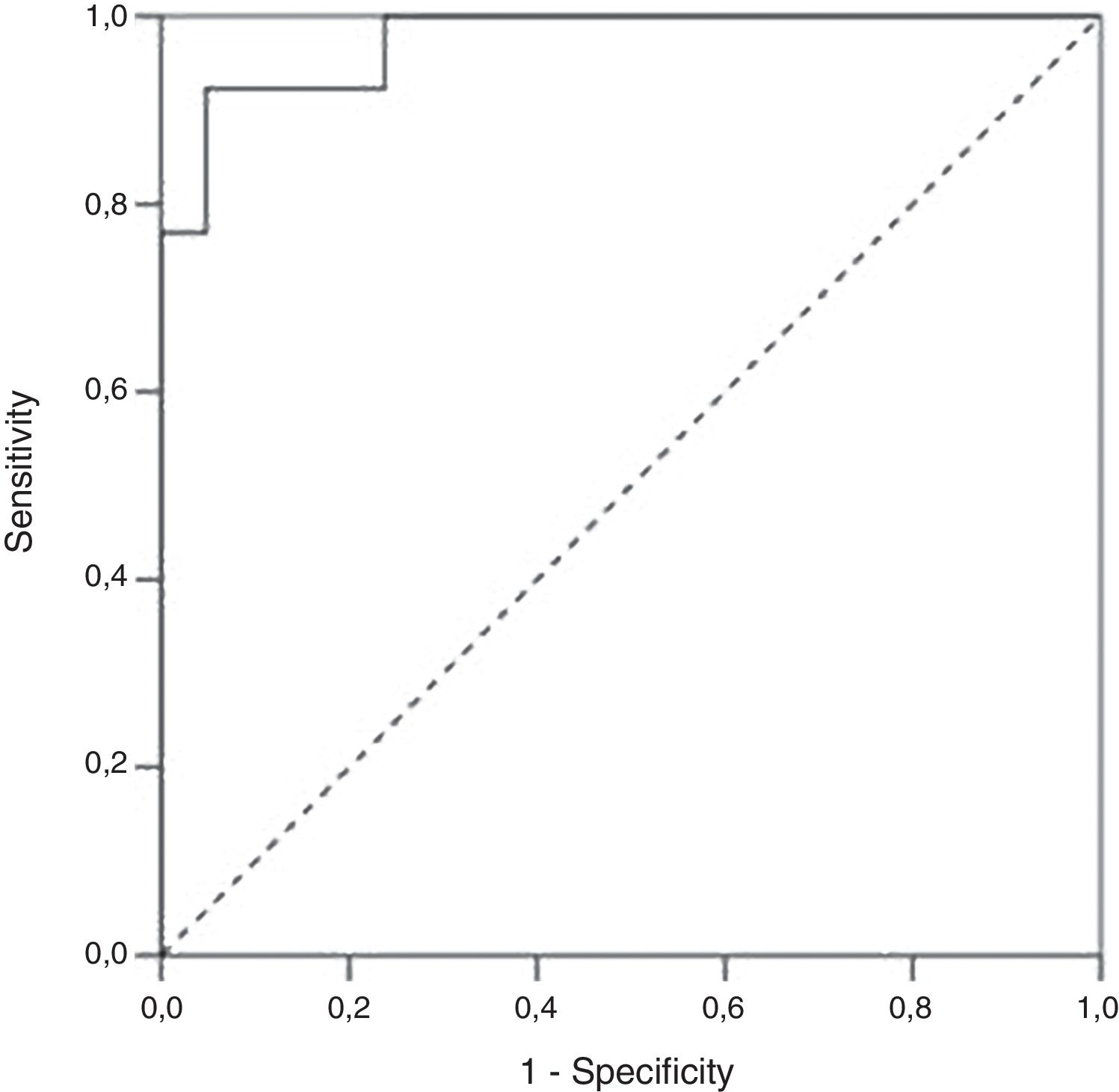
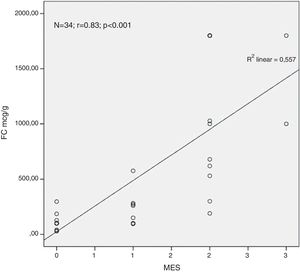
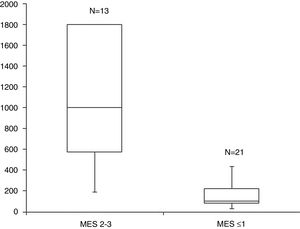
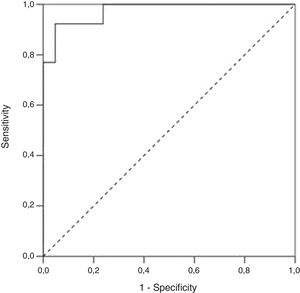
ResultsA total of 34 visits of 24 children with UC were included in the study. There was a strong positive correlation between FC levels and endoscopic disease activity (n=34, r=0.83, p<0.001). The median FC levels in the subgroup with endoscopic activity (MES 2–3) were significantly higher than the median FC levels in the subgroup without endoscopic activity (MES≤1) (1000μg/g, IQR 575–1800μg/g vs. 100μg/g, IQR 80–223μg/g, p<0.001). At a cut-off of 298.5μg/g, FC had 92.3% sensitivity, 95.2% specificity and an AUROC 0.974 (SE 0.023, 95% CI 0.93–1, p<0.001) to predict endoscopic activity.
DiscussionFC is an accurate surrogate marker of endoscopic activity in children with clinically quiescent UC.
Actualmente, la colonoscopia es considerada como el gold standard para la evaluación de la inflamación de la mucosa colónica en pacientes con colitis ulcerosa (CU), pero este procedimiento es invasivo y no se puede repetir frecuentemente, especialmente en la población pediátrica. El objetivo de este estudio es evaluar el papel de la calprotectina fecal (CF) como predictor de la actividad endoscópica de la enfermedad en pacientes pediátricos con CU en remisión clínica.
Material y métodosEstudio prospectivo monocéntrico. La remisión clínica se definió según el Índice de Actividad Pediátrico de Colitis Ulcerosa (Paediatric Ulcerative Colitis Activity Index) <10. Los hallazgos endoscópicos fueron evaluados según el Subscore Endoscópico de Mayo (SEM). SEM≤1 se definió como remisión endoscópica. En todos los participantes se obtuvo una muestra de heces para medición de la CF.
ResultadosUn total de 34 visitas de 24 niños con CU se incluyeron en el estudio. Hubo una fuerte correlación positiva entre la CF y la actividad endoscópica de la enfermedad (n=34, r=0,83, p <0,001). La mediana de los niveles de CF en el subgrupo con actividad endoscópica (SEM 2–3) fue significativamente superior a la mediana de los niveles de CF en el subgrupo sin actividad endoscópica (MES≤1) (1.000 μg/g, IQR 575 μg/g–1.800 μg/g vs. 100 μg/g; IQR 80 μg/g–223 μg/g; p <0,001). Al nivel de corte de 298,5 μg/g la CF obtuvo una sensibilidad del 92,3%, una especificidad del 95,2% y un área bajo la curva de 0,974 (SE 0,023; IC del 95%, 0,93-1; p <0,001) para predecir actividad endoscópica.
DiscusiónLa CF es un marcador indirecto preciso para actividad endoscópica en niños con CU clínicamente quiescente.
Ulcerative colitis (UC) is one of the two major types of inflammatory bowel disease (IBD). It is a chronic disorder with a fluctuating course, characterized by flares and remissions. The determination of the disease activity is essential to tailoring therapy and predicting the disease course. Colonoscopy is currently considered to be the gold standard for evaluation of intestinal mucosa inflammation of patients with UC, but the procedure is invasive and cannot be repeated frequently, especially in the paediatric population.1,2
Paediatric Ulcerative Colitis Activity Index (PUCAI) is a validated tool for evaluating disease activity in children with UC but it is based on subjective patient complaints and therefore cannot be very precise. Laboratory tests such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cell count and platelet count have been used for the determination of disease activity of UC, but none of them are specific for gut inflammation.3
Faecal markers include a biologically heterogeneous group of substances that either leak from or are actively released by the inflamed mucosa. In the last years, they developed an alternative approach to the assessment of the presence of intestinal inflammation. Faecal calprotectin (FC) is the most studied faecal marker.4 It is a small 36kDa calcium and zinc-binding protein, represents 60% of the cytosolic protein in the granulocytes. It is stable in faeces for up to 7days at room temperature and is resistant to degradation. The amount of calprotectin in faeces is proportional to the amount of neutrophil migration from the inflamed bowel wall into the intestinal lumen.5 Elevated FC levels are associated with the presence of active intestinal inflammation.6 Therefore FC is used as a reliable non-invasive marker for predicting disease activity in adult patients with UC.7,8 Data in children are still scarce.
The aim of this study was to assess the relationship between FC levels and endoscopic disease activity in paediatric patients with UC and to evaluate the role of FC as a predictor of endoscopic activity in children with clinically quiescent UC.
Material and methodsPatients and study designSingle centre prospective study including paediatric patients with previously confirmed diagnosis UC referred to our department for endoscopic evaluation after medical treatment and achievement of clinical remission.
All study participants had been diagnosed in accordance with the Porto criteria for the diagnosis of IBD in children and adolescents.9
Disease extent and severity had been assessed according to the Paris Classification – paediatric modification of the Montreal Classification for IBD.10 The Paris Classification defines four subtypes of UC based on disease extent: E1–ulcerative proctitis, E2–left-sided UC (distal to splenic flexure), E3–extensive UC (hepatic flexure distally) and E4–pancolitis (proximal to hepatic flexure).
Disease activity was determined using the validated instrument for measuring clinical disease activity in children and adolescents – Paediatric Ulcerative Colitis Activity Index (PUCAI). PUCAI is a numerical score based on evaluation of abdominal pain, number of stools per day, stool consistency, amount of blood in stools, nocturnal stooling and activity level. It ranges from 0 to 85 points and defines: inactive disease (<10 points), mild disease (10–34 points), moderate disease (35–64 points) and severe disease (65–85 points).11
The inclusion criteria for study enrolment were confirmed diagnosis of UC, clinical follow-up of at least 6 months, clinical remission defined as PUCAI<10 points, complete follow-up colonoscopy and faecal samples provided 1–3 days prior colonoscopy. The exclusion criteria were a new-onset UC or a disease relapse or an incomplete clinical remission (PUCAI≥10), an incomplete colonoscopy (cecum not reached), faecal samples provided after the colonoscopy, an accompanying intestinal infection (positive stool culture) and a regular intake of aspirin and/or other nonsteroidal anti-inflammatory drugs.
After calculating the PUCAI score at study baseline all eligible patients were instructed to give a stool sample for measurement of FC. Children provided the samples 1–3 days prior to colonoscopy (prior to bowel cleansing). All samples were processed and analysed according to the manufacturers’ instructions. FC levels were determined by a rapid quantitative test based on lateral flow immunochromatography (Quantum Blue® Calprotectin/Quantum Blue® Calprotectin High Range, Bühlmann Laboratories AG). FC values above the upper limit of the measurement ranges were registered as 1800μg/g and FC values below the lower limit were accordingly registered as 30μg/g.
All study participants underwent a follow-up endoscopic examination for the assessment of endoscopic activity after medical treatment. The endoscopists were blinded to the FC results. The endoscopic activity was assessed by the Mayo endoscopic subscore (MES), ranging from 0 to 3: 0=inactive disease; 1=mild disease; 2=moderate disease and 3=severe disease.12 MES≤1 was accepted for an endoscopic remission.
StatisticsData are presented as medians and interquartile ranges (IQR). The Mann–Whitney test was used to compare the FC levels between the groups defined by an endoscopic disease activity. The association between an endoscopic disease activity and FC concentration was assessed by the determination of Spearman's rank correlation coefficient (r). A receiver operating curve (ROC) analysis was used to determine optimal cut off value for calprotectin to predict the endoscopic activity. A p-value <0.05 was considered statistically significant.
EthicsAll children were included in the study after their own verbal consent and after the written informed consent from their parents.
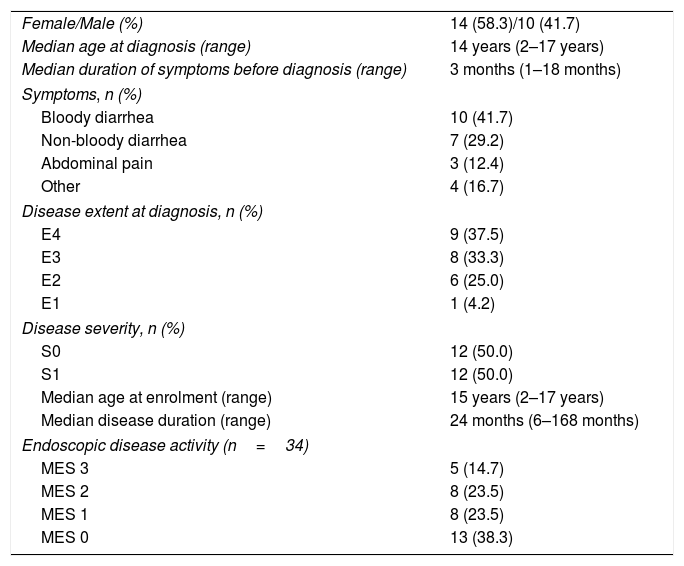
ResultsDemographic and phenotypic characteristicsA total of 34 visits of 24 children with UC, >6 months disease duration and confirmed clinical remission were included in the final analysis. Median follow-up was 24months (range 6–168 months). Median age at study enrolment was 15years (range 2–17years) and 58.3% of study participants were girls. Despite of the achievement of a clinical remission in 38.2% of the cases the patients demonstrated findings of an active endoscopic disease.
At diagnosis 9 (37.5%) of our children had pancolitis, 8 (33.3%) of them had extensive UC, 6 (25.0%) – left-sided UC and 1 girl had ulcerative proctitis. Clinical course was determined as S0 (never severe) in 12 (50%) patients and S1 (ever severe) in 12 (50%) patients. Demographic and phenotypic characteristics of the study population are summarized in Table 1.
Demographic and clinical characteristics of study population.
| Female/Male (%) | 14 (58.3)/10 (41.7) |
| Median age at diagnosis (range) | 14 years (2–17 years) |
| Median duration of symptoms before diagnosis (range) | 3 months (1–18 months) |
| Symptoms, n (%) | |
| Bloody diarrhea | 10 (41.7) |
| Non-bloody diarrhea | 7 (29.2) |
| Abdominal pain | 3 (12.4) |
| Other | 4 (16.7) |
| Disease extent at diagnosis, n (%) | |
| E4 | 9 (37.5) |
| E3 | 8 (33.3) |
| E2 | 6 (25.0) |
| E1 | 1 (4.2) |
| Disease severity, n (%) | |
| S0 | 12 (50.0) |
| S1 | 12 (50.0) |
| Median age at enrolment (range) | 15 years (2–17 years) |
| Median disease duration (range) | 24 months (6–168 months) |
| Endoscopic disease activity (n=34) | |
| MES 3 | 5 (14.7) |
| MES 2 | 8 (23.5) |
| MES 1 | 8 (23.5) |
| MES 0 | 13 (38.3) |
Analysing the relationship between the concentration of FC in the faeces and the endoscopic finding we found a very strong positive correlation between the FC levels and the endoscopic disease activity assessed by MES (n=34, r=0.83, p<0.001) (Fig. 1).
The median FC levels in the subgroup with endoscopic activity (MES=2–3) were significantly higher than the median FC levels in the subgroup without endoscopic activity (MES≤1) (1000μg/g, IQR 575–1800μg/g vs. 100μg/g, IQR 80–223μg/g, p<0.001) (Fig. 2).
We performed a Receiver Operating Characteristic (ROC) curve analysis to determine the optimal FC cut-off value for distinguishing patients with endoscopic activity (MES=2–3) vs. patients with endoscopic remission (MES≤1). At a cut-off 298.5μg/g FC had 92.3% sensitivity, 95.2% specificity and an AUROC 0.97 (SE 0.023, 95% CI 0.93–1, p<0.001) to predict endoscopic activity (Fig. 3).
DiscussionIn recent years mucosal healing (MH) has become an important therapeutic goal in IBD management, associated with better prognosis and long term outcome.13,14 Currently for objective evaluation of intestinal mucosal surface are used either repetitive endoscopic examinations or reliable surrogate markers. Endoscopic assessment is especially difficult in paediatric population. Children often experience problems with fasting and bowel preparation. The procedure is invasive and requires general anesthesia and hospitalization or day-care admission.4 As alternative, FC has been suggested as a valuable non-invasive marker of intestinal inflammation.6,15 FC concentrations correlate well with endoscopic and histological IBD activity and this correlation could be demonstrated in adults and children for both UC and CD.4,6,16-18 Our findings are consistent with these data. We found a very strong positive correlation between the FC levels and the MES in our UC patients (n=34, r=0.83, p<0.001). So we demonstrated that FC measurement could be used in the everyday practice for monitoring of disease activity in paediatric patients with UC. This will reduce the number of the follow-up colonoscopies.
Similar to other authors we found that patients with endoscopic activity (MES=2–3) had significantly higher FC concentration than patients without endoscopic activity (MES≤1).19,20,7,21
In our study the best cut-off level for FC to predict endoscopic activity was 298.5 and our results are in agreement with the published data. Szczepański et al. studied 46 patients with IBD (24 – UC and 22 – CD) and reported that FC cut-off value 233μg/g was able to predict endoscopic activity with sensitivity 1 and specificity 0.79. The area under curve (AUC) was 0.95.22 Previously Lobaton Ortega et al. found in 88 UC patients that FC cut-off value of 250μg/g had 94% sensitivity and 80% specificity to predict endoscopic activity.23 Bojic et al. reported 88% sensitivity and 100% specificity for FC cut-off value 250μg/g in predicting endoscopically active disease, and 78, 90%, in predicting histologically active disease.24 Other authors proposed different cut-off values for FC ranging from 120μg/g to 300μg/g as being able to predict disease relapse with good accuracy.21,25
Most studies conducted to assess the utility of FC to predict endoscopic activity are in adults and use ELISA for FC determination. In this study, we provide a FC cut-off value for predicting endoscopic activity in paediatric patients with UC. Another advantage of the study is that we use a point-of-care testing providing immediate results and thus allowing better clinical management.
ConclusionAlthough no FC cut-off level to differentiate mucosal healing from inflammation has been validated yet it is a valuable non-invasive option for monitoring of the disease activity in paediatric patients with UC. Routinely measuring FC can detect a subclinical inflammation and thus identify patients at high risk for a disease relapse.
Conflict of interestNo potential conflict of interest was reported by the authors.