In recent years, significant advances in the treatment of Clostridium difficile infection (CDI) have risen. We review the most relevant updated recommendations in the current standard of care of CDI and discuss emerging therapies, including antibiotic, alternative therapies (probiotics, toxin-binding resins, immunotherapy) and new data on fecal transplantation. Upcoming surgical options and other rescue therapies for severe refractory disease are also addressed.
Although oral metronidazole is a first-line therapy for non-severe CDI, emerging data have demonstrated its inferiority relatively to vancomycin, particularly in the setting of recurrent and/or severe infection. After a CDI recurrence for the first time, fidaxomicin has been shown to be associated with lower likelihood of CDI recurrence compared to vancomycin. Fecal transplantation is now strongly recommended for multiple recurrent CDI and may have a role in refractory disease. Oral, frozen stool capsules may simplify fecal transplantation in the future, with preliminary promising results. Diverting loop ileostomy combined with colonic lavage is a potential alternative to colectomy in severe complicated CDI. Potential alternative therapies requiring further investigation include toxin-binding resins and immunotherapy.
Nos últimos anos surgiram várias novidades no tratamento da infecção por Clostridium difficile. Os autores apresentam as principais recomendações actuais para o tratamento desta infecção e discutem as terapêuticas emergentes, nomeadamente a antibioterapia, terapêuticas alternativas (probióticos, resinas ligadoras de toxinas e imunoterapia) e novos dados sobre transplante fecal. São também abordadas a terapêutica cirúrgica e outras terapêuticas de resgate para a doença refractária.
Embora o metronidazol per os seja a terapêutica de primeira-linha na doença ligeira a moderada, dados recentes demonstram a sua inferioridade em relação à vancomicina, nomeadamente na infecção grave e/ou recorrente. Após um primeiro episódio de recidiva, a fidaxomixina mostrou associar-se a menor risco de novas recorrências comparativamente à vancomicina. O transplante fecal é actualmente recomendado na doença com múltiplas recidivas e poderá ter um papel na doença refractária Cápsulas de fezes congeladas, administradas por via oral, podem simplificar este procedimento, tendo apresentado resultados promissores. A ileostomia de derivação combinada com limpeza/descontaminação cólica é uma potencial alternativa à colectomia na doença grave complicada. Terapêuticas alternativas como as resinas ligadoras de toxinas e a imunoterapia estão ainda em investigação.
In the past decade, there has been an alarming increase in the incidence and severity of Clostridium difficile infection (CDI), including recurrent and refractory disease, with inherent increase in morbidity, mortality and costs.1 Significant advances in the treatment of CDI have recently risen.2,3 Recent data on antibiotic therapy, fecal transplantation, alternative non-antibiotic therapies (such as toxin-binding resins and immunotherapy) and surgery for severe disease, have expanded the potential fields of research and led to significant changes in the current standard of care.2,3 The most relevant clinical guidelines on CDI treatment issued in the last two years were from the American College of Gastroenterology (ACG) in 20132 and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in 2014.3 In the present review we discuss the most relevant updated guidelines and emerging promising treatments for CDI, namely:
- 1.
New data on antibiotic therapy: current factors that determine the antibiotic choice and new upcoming options.
- 2.
The rising promise of fecal transplantation: clinical indications, emerging techniques, clinical efficacy and safety.
- 3.
The current role of non-antibiotic alternative therapies: probiotics, toxin-binding resins and immunotherapy.
- 4.
New data on surgery for severe refractory disease and the potential role of other rescue therapies.
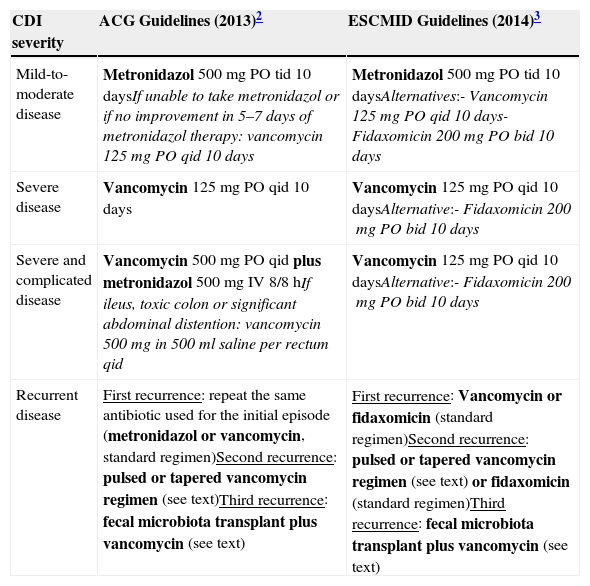
Both the ACG2 and the ESCMID guidelines3 concerning antibiotic treatment for CDI were based on disease severity and are summarized in Table 1. In the ESCMID guidelines,3 severe CDI is defined as an episode of CDI with one or more signs and symptoms of severe colitis or a complicated course of disease (resulting in need for intensive care unit admission, colectomy or death). Guideline parameters for severe CDI include white blood cell count of >15,000cell/μL, serum albumin <3g/dL, and/or a serum creatinine level >1.5 times the premorbid level.2,3Clostridium difficile infection in patients with greater age (≥65 years), serious comorbidity, or immunodeficiency may also be considered risk factors for severe course.3 However, the definition for severe CDI is not consensual and is variable among studies.2,3 For the purposes of the treatment decisions, determination of disease severity is left to clinician judgment and may include any or all of these criteria.2,3
| CDI severity | ACG Guidelines (2013)2 | ESCMID Guidelines (2014)3 |
|---|---|---|
| Mild-to-moderate disease | Metronidazol 500mg PO tid 10 daysIf unable to take metronidazol or if no improvement in 5–7 days of metronidazol therapy: vancomycin 125mg PO qid 10 days | Metronidazol 500mg PO tid 10 daysAlternatives:- Vancomycin 125mg PO qid 10 days- Fidaxomicin 200mg PO bid 10 days |
| Severe disease | Vancomycin 125mg PO qid 10 days | Vancomycin 125mg PO qid 10 daysAlternative:- Fidaxomicin 200mg PO bid 10 days |
| Severe and complicated disease | Vancomycin 500mg PO qid plus metronidazol 500mg IV 8/8hIf ileus, toxic colon or significant abdominal distention: vancomycin 500mg in 500ml saline per rectum qid | Vancomycin 125mg PO qid 10 daysAlternative:- Fidaxomicin 200mg PO bid 10 days |
| Recurrent disease | First recurrence: repeat the same antibiotic used for the initial episode (metronidazol or vancomycin, standard regimen)Second recurrence: pulsed or tapered vancomycin regimen (see text)Third recurrence: fecal microbiota transplant plus vancomycin (see text) | First recurrence: Vancomycin or fidaxomicin (standard regimen)Second recurrence: pulsed or tapered vancomycin regimen (see text) or fidaxomicin (standard regimen)Third recurrence: fecal microbiota transplant plus vancomycin (see text) |
The two first-line antibiotics most often used to treat CDI are metronidazol and vancomycin.2,3 Metronidazol has been shown to be effective in inducing a clinical response in mild-to-moderate CDI (90% vs. 98%, comparing to vancomycin),4 has lower cost and it is believed to carry lower vancomycin-resistant enterococci selection risk.2 It is usually given orally 500mg three times daily for 10 days. Since it has biliary excretion and increased exudation across the intestinal mucosa during CDI, it can also be administered intravenously (500mg every 8h) in patients in whom oral therapy is not feasible. There is increasing evidence of the emergency of metronidazol-resistant strains of C. difficile,5 but larger studies are needed to determine its clinical significance. In patients with mild-to-moderate CDI who fail to respond to metronidazol therapy within 5–7 days, it should be promptly changed to vancomycin.2
Several studies have suggested that vancomycin is superior to metronidazol in achieving clinical success for all patients (81.1% vs. 72.7%),6 particularly for those with severe CDI (76% vs. 97%).4 Clinical success occurred in 4%, 8.3% and 12.2% more patients treated with vancomycin compared to metronidazol for mild, moderate and severe CDI, respectively.6 Therefore, oral metronidazol is usually recommended for treatment of mild-to-moderate disease, whereas oral vancomycin is generally preferred for treatment of severe CDI. Vancomycin is usually given orally 125mg four times daily for 10 days. The use of high doses of vancomycin (500mg four times daily) has been included in the Infectious Diseases Society of America guidelines (2010)7 for the management of severe complicated CDI. However, doses of vancomycin higher than 125mg four times daily have not been shown to be more effective than the standard dose.8 Therefore, there is insufficient evidence to support the use of vancomycin doses greater than 125mg four times daily. Oral vancomycin is poorly absorbed from the gastrointestinal tract (resulting in fewer adverse effects) and luminal drug levels are high and remain high throughout the antibiotic course. On the other hand, because metronidazol is absorbed from the gastrointestinal tract, luminal levels are dependent on the drug exudation across the intestinal mucosa during CDI and decrease during treatment as mucosal inflammation improves and diarrhea resolves. Therefore, vancomycin pharmacokinetic properties are considered superior to those of metronidazol in severe CDI. Although there is a theoretical risk of promoting vancomycin-resistant enterococci with vancomycin, studies have shown no differences in the colonization and transmission rates of vancomycin-resistant enterococci between vancomycin and metronidazol.9,10 In patients with severe and complicated CDI, simultaneous use of metronidazol and vancomycin is recommended (metronidazol 500mg intravenous every 8h plus vancomycin 125mg orally four times a day).2 In patients with ileus, toxic colon or significant abdominal distention, vancomycin enema (500mg in 500ml saline per rectum four times a day) should be added.2
Both metronidazol and vancomycin are broad-spectrum antibiotics which cause significant disruption of the commensal colonic microbiota. Oral fidaxomicin (200mg twice daily), recently approved for the treatment of CDI (2011), has a narrow antimicrobial spectrum, leading to less colonic microbiota disruption and is also less likely to induce vancomycin-resistant enterococci and Candida species.11,12 Fidaxomicin was not inferior to vancomycin for initial cure of CDI, but no data are available on the efficacy in severe complicated disease.13,14 In comparison with vancomycin, fidaxomicin has been associated with lower CDI recurrence rate (16.9–19.6% risk reduction).14 However, this superiority was only seen for CDI not caused by NAP1/BI/027 strains.14 Because its cost is significantly high, fidaxomicin is usually reserved for patients with severe CDI who fail to respond to vancomycin. Oral teicoplanin (100–200mg twice daily), another recently approved antibiotic for CDI treatment (2013), has demonstrated CDI cure rates similar to vancomycin.15 Like vancomycin, fidaxomicin and teicoplanin are minimally absorbed from the gastrointestinal tract (resulting in few adverse effects). However, further studies are needed to define the role of fidaxomicin and teicoplanin in the CDI treatment algorithm.15
Patients with inflammatory bowel disease (IBD) are newly recognized at an elevated risk for acquiring CDI and suffering adverse outcomes.2 In patients who have IBD with severe colitis, simultaneous initiation of empiric therapy directed against CDI (oral vancomycin, 125mg four times per day) and treatment of an IBD flare may be required while awaiting results from C. difficile testing.2 Ongoing immunosuppression medications can be maintained in patients with CDI, but escalation of the corticosteroid dose or initiation of anti-TNF therapy probably should be avoided for 72h after initiating therapy for CDI.2
The management of recurrent CDI is a therapeutic challenge since there is no consistently effective therapy. In the treatment of the first CDI recurrence, the overall clinical success of vancomycin was higher compared to metronidazol (83.3% vs. 67.6%, respectively).6 Therefore, in the 2014 ESCMID guidelines,3 the use of vancomycin (or fidaxomicin, standard regimen) has been recommended as the first-line therapy for first CDI recurrence (instead of metronidazol). The second recurrence should be treated with a pulsed or tapered vancomycin regimen (125mg four times daily for 10 days, followed by 125–500mg per day every 2–3 days for at least 3 weeks – pulsed regimen – or followed by a gradual reduction of the dose to 125mg per day – tapered regimen) or with fidaxomicin (standard regimen).2,3 Pulsed and tapered regimens seem to be more effective than conventional vancomycin regimen (recurrence rate 14% vs. 31% vs. 54%, respectively).16 If there are more than two CDI recurrences, fecal transplantation should be strongly considered, combined with oral antibiotic treatment (vancomycin 500mg orally four times daily, 4 days).3
New agents have been proposed for CDI treatment, such as tigecycline and rifaximin, but more studies are required to assess their efficacy.2,3 Intravenous tigecycline has been described as a rescue therapy for patients with severe CDI who fail to respond to vancomycin.17,18 Rifaximin is an oral, non-absorbed antibiotic that has shown decreased incidence of recurrent diarrhea, when given immediately after finishing standard anti-CDI antibiotics.19–21 However, a recent randomized control trial did not find a decrease in CDI recurrence with rifaximin22 and there is also concern about possible emergence of rifaximin resistance.23
Other potential new antibiotics with promising results in vitro and in animal models, but still requiring investigation in humans, are rifalazil (an absorbable antibiotic related to rifampin),24 ramoplanin (a non-absorbable antibiotic apparently effective on both vegetative and spore forms of C. difficile)25 and REP3123 (a novel inhibitor of methionyl tRNA synthetase, an enzyme which is required for bacterial growth and toxin formation, highly selective for gram positive bacteria).26
3The rising promise of fecal transplantation: from enemas to frozen capsulesClostridium difficile colonizes and infects the intestinal tract after the normal gut microbiome has been altered by antibiotic therapy.1 Thus, the rationale for fecal transplantation in CDI is the reestablishment of the normal bowel flora. In 1958, Eiseman et al.27 performed the first fecal microbiota transplantation (FMT) for pseudomembranous enterocolitis, with promising results.
Recurrence is a main problem in CDI. Although most patients respond to metronidazol or vancomycin, about 20% have a recurrence.1 After the first recurrence, rates are even higher, with some studies reporting an incidence of subsequent recurrences up to 65%.16,28 Efficacy of FMT in recurrent CDI had already been demonstrated in several case reports and small series.29–31 A quick and sustained improvement in symptoms and endoscopic findings usually occurs.31 In 2013, van Nood et al.32 published the first randomized controlled trial (RCT) demonstrating that FMT is effective (mostly in patients with multiple relapses), safe, logistically manageable and superior to vancomycin for recurrent CDI. Both the American (ACG, 2013)2 and the European (ESCMID, 2014)3 guidelines recommend FMT in multiple recurrent CDI (more than two CDI recurrences), even if non-severe. In both guidelines,2,3 FMT is not recommended for severe refractory CDI, but recent data from case reports and small series have shown favorable results.33–38 The ACG states that FMT should be considered in patients with severe CDI without response to antibiotics within two days and also in patients with non-severe CDI refractory to antibiotics (namely vancomycin) within one week.2
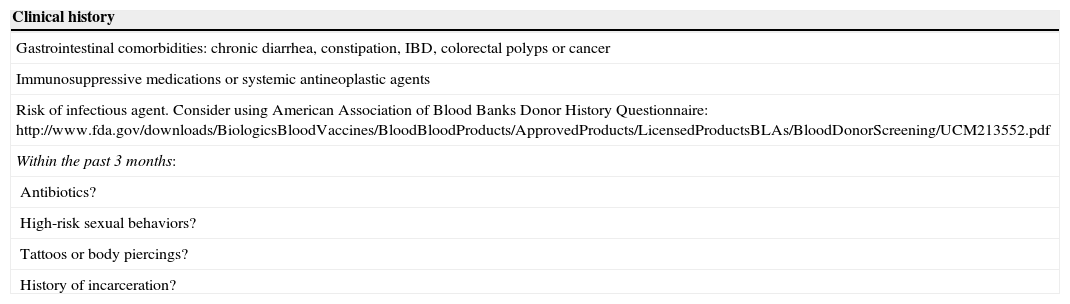
A FMT protocol is not yet established. Because FMT carries a potential risk of transmission of infectious diseases, donors’ history should be known and rigorous laboratory tests should be performed. Table 2 summarizes absolute contra-indications in donor selection for FMT, based on clinical history and laboratory screening tests.39 Recipients are also tested for HIV, hepatitis A, B and C and syphilis.39
Donor selection for FMT: screening for absolute contra-indications, based on clinical history and laboratory tests39.
| Clinical history |
|---|
| Gastrointestinal comorbidities: chronic diarrhea, constipation, IBD, colorectal polyps or cancer |
| Immunosuppressive medications or systemic antineoplastic agents |
| Risk of infectious agent. Consider using American Association of Blood Banks Donor History Questionnaire: http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/BloodDonorScreening/UCM213552.pdf |
| Within the past 3 months: |
| Antibiotics? |
| High-risk sexual behaviors? |
| Tattoos or body piercings? |
| History of incarceration? |
| Serologic tests | Stool tests |
|---|---|
| Clostridium difficile toxin | |
| HbsAg | Stool culture |
| Anti-HBc IgG/IgM | Stool ova and parasites |
| Anti-HAV IgM | Giardia antigen |
| Anti-HCV | Hp antigen |
| Anti-HIV types 1 and 2 | Cryptosporidium antigen |
| VDRL | Isospora (acid fast stain) |
| Rotavirus antigen |
Abbreviations: Hp, Helicobacter pylori; IBD, inflammatory bowel disease.
Different routes of administration can be used in FMT: nasogastric or nasoenteric tube, gastroduodenoscopy, enema or colonoscopy. In upper delivery, smaller volumes and slower infusion rates minimize the risk of aspiration. Larger volumes seem to be associated with higher cure rates.40 In the prospective RCT from van Nood et al.32, 500ml of suspended stool were administered during 20–30min through a nasoenteric tube, with high cure rates (81 and 94% after 1 and 2 infusions, respectively). This route can have, at least theoretically, the advantage of having no risk of perforation in severe/fulminant cases. Enemas are inexpensive and easy to use, which allows self-administration. However, enemas only reach the splenic flexure.41 In the colonoscopic approach, the entire colon (and even terminal ileum) can be infused with stool. This technique seems to have the higher cure rates in non-controlled trials; however, only two studies compared it directly with other administration route and one of them found no statistically significant difference in clinical outcome.41 Larger head-to-head trials are needed. Three other concerns need to be cleared: the role of bowel cleansing (to reduce the density of C. difficile organisms), loperamide and simultaneous/sequential antibiotic regimen in improving or detracting the results.32,40–42
The amount of stool and the diluent are not standardized. A non-bacteriostatic saline solution is more often used, but tap water, milk and other diluents can be used without consistent differences. Usually, 50–60g of stool is suspended in 250–300ml of diluent. Both fresh and frozen stool apparently have excellent results.40,41,43
FMT appears to be safe. Reported adverse effects are mainly mild and transient gastrointestinal symptoms. No major complications have been reported.40,41 The procedure seems to be safe even in immunocompromised patients.44
These encouraging results were repeatedly reported in the last few decades. At the moment, FMT is doubtless a safe and highly effective therapy. Even though there is only one long-term follow-up study,31 it probably has lower long-term recurrence rates than most of the antibiotic therapies.41 However this technique is not widely used, probably because of three main reasons: (1) Lack of data from RCTs; (2) It is esthetically unappealing; and (3) Processing of donor material is logistically challenging and a standard protocol is not defined.45
CDI is now a growing emergent infection, so it is urgent to make larger trials and to know more about this highly effective therapy. The spread of stool donors bank will probably facilitate the procedure.41 A recent prospective study using frozen FMT capsules for recurrent and severe CDI showed promising results: resolution of diarrhea was achieved in 70% of the patients after a single capsule-based FMT and the remaining patients were re-treated, resulting in an overall 90% rate of clinical resolution of diarrhea.46 In the future, the infusion of a combination of selected bacteria instead of stool, namely by frozen capsules, can turn this treatment more attractive to patients and physicians.46
4Alternative (non-antibiotic) therapies: is there a role for probiotics, toxin binding resins or immunotherapy?4.1ProbioticsThe clinical role of probiotics for CDI prevention and treatment is still uncertain. Regarding the use of probiotics in the prevention of CDI in adults and children receiving antibiotics, a 2012 meta-analysis47 and a 2013 Cochrane analysis48 concluded that there is moderate-quality evidence that probiotic prophylaxis is safe and reduces CDI occurrence. However, in a subsequent multicenter RCT (including 2941 elderly adults with antibiotic exposure), probiotics (a preparation of Lactobacillus acidophilus and Bifidobacterium bifidum) were ineffective in CDI prevention.49 The strain, dose and duration of probiotic use in the several studies varied widely and further data are needed regarding probiotics role in CDI prevention.
There is insufficient evidence to support administration of probiotics as an adjunct to antibiotics, in the treatment of CDI.2,3,50 There are several reports describing bacteremia or fungemia attributed to probiotics administration in immunocompromised and critically ill patients.51 Use of probiotics may be reasonable in patients with non-severe recurrent disease, as long as there are no significant comorbidities.50 There is some evidence that probiotics may be beneficial (reducing re-infection rate) in recurrent CDI, but not in the initial episode of CDI.52 There is no data supporting a role for probiotics in severe CDI.50
4.2Toxin binding resins (cholestyramine, colestipol and tolevamer)In vitro and in vivo studies demonstrated that the ability of cholestyramine and colestipol to bind to C. difficile toxins might support their use in the treatment of CD.53 However, cholestyramin and colestipol can also bind to vancomycin, which can decrease its activity.53 Tovelamer is a recently developed soluble high-molecular anionic polymer that binds specifically to toxins A and B.54
- a.
Primary treatment in non-severe CDI: Colestipol has not shown to be effective as primary treatment for CDI.55,56 Tovelamer, has been used in some RCTs as primary treatment for initial non-severe CDI, but has shown to be less effective than vancomycin or metronidazole.3,54,57
- b.
Adjunctive treatment in non-severe CDI: One study has analyzed the association of vancomycin and colestipol (tapered dose schedule) for relapsing infection, with positive results.58 However, the limited number of patients is an important drawback of this study.58
- c.
Severe disease: To our knowledge, there are no available studies concerning the applicability of toxin binding resins in severe CDI.
According to the ACG2 and the ESCMID3 guidelines, there is not enough evidence supporting the use of toxin-binding resins and polymers in the treatment of CDI.
4.3Immunotherapy (intravenous immunoglobulin; anti-toxin A and B monoclonal antibodies)The immune response to C. difficile colonization, namely anti-toxin A IgG serum levels, is a key factor for the severity and duration of CDI. One study has demonstrated that, after C. difficile colonization, asymptomatic carriers have higher anti-toxin A IgG levels when compared to patients that develop diarrhoea.59,60 These results have been used to support the use of intravenous immunoglobulin (IVIG) in the treatment of CDI.
- a.
Treatment of recurrent CDI: Some case reports have demonstrated that, in patients with recurrent disease and low levels of anti-Toxin A IgG, the use of IVIG (200–400mg/kg) may be beneficial in the improvement of the clinical status.61–63 The use of adjunctive treatment with monoclonal antibodies against C. difficile toxins A and B has shown promising results, reducing the recurrence rate from 25% to 7%.64
- b.
Treatment of severe disease: In severe disease, the applicability of immunotherapy is less clear. One retrospective study has failed to prove a benefit in the use of adjunctive therapy with IVIG when compared with the recommended antibiotherapy alone.65 The potential role of anti-toxins monoclonal antibodies in severe CDI is not defined.
According to the ACG2 and the ESCMID3 guidelines, there is not enough evidence supporting the use of immunotherapy in the treatment or prevention of CDI. However, IVIG may be helpful in patients with hypogammaglobulinemia, which is particularly common following solid organ transplants.2,66
A series of studies concerning the use of toxin-based vaccines for C. difficile are underway, namely using inactivated toxin A and B. Recently, some studies using genetically engineered recombinant fragments of these toxins have been developed in order to minimize the limitations and potential risks of using inactivated toxins.67,68
5New data on surgery for severe refractory diseaseMost patients with CDI infection respond well to oral antibiotherapy. However, approximately 3–10% of them progress to fulminant colitis, with systemic toxicity and organ failure.69 For those who fail to respond to medical therapy and progress to complicated disease, surgical intervention is required.3 Although post-operative mortality remains high, ranging from 35% to 80%,70 recent data suggest that for fulminant CDI, colectomy is associated with better prognosis than antibiotic therapy when no further improvement is seen.71 The severity of this disease led to the recent development of a risk scoring system, to rapidly identify patients who might benefit from surgical intervention.72
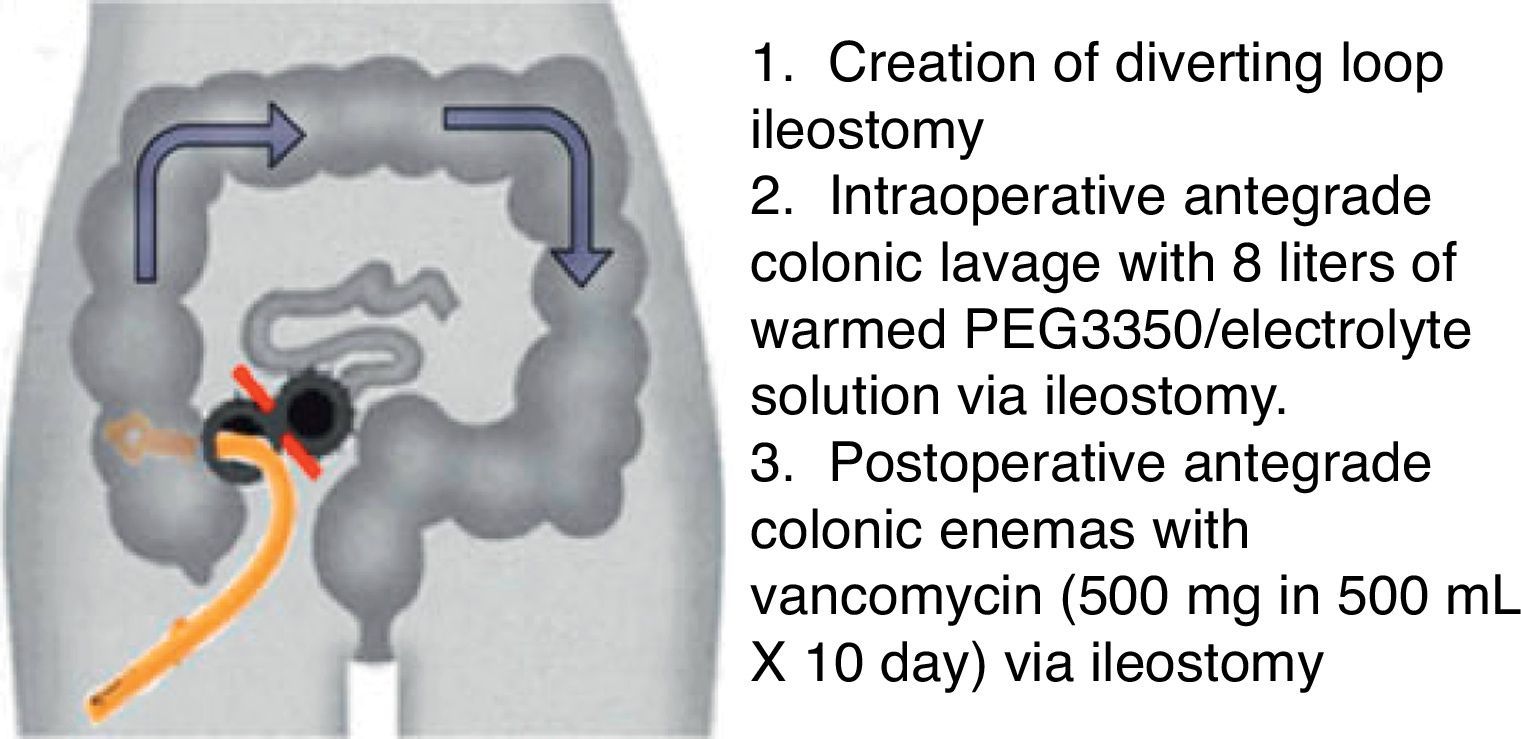
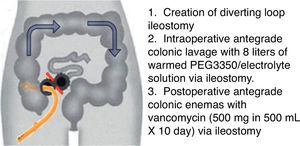
The conventional surgical management of severe, complicated CDI has been subtotal colectomy with end-ileostomy.73,74 Recently, a colon-preserving approach has been described as an alternative to subtotal colectomy (Fig. 1).75 The procedure starts with the creation of a diverting loop ileostomy. Then, an intraoperative colonic lavage in an anterograde way through the ileostomy, with 8l of warmed polyehyleneglycol 3350 or balanced eletrolyte solution is performed. Finally, in the postoperative period, a catheter is placed in the efferent limb of the ileostomy to deliver vancomycin flushes (500mg in 500ml, 10 days). When comparing with historical controls that underwent colectomy in the same institution, this approach was associated with reduced morbidity and 30-days mortality (19 vs. 50%, respectively). Moreover, the majority of surgeries were performed laparoscopically (83%). Preservation of the colon was achieved in 39 of 42 patients (93%).75 Although promising, this study included only 42 patients, and further prospective RCTs are necessary to validate this technique.
Diverting loop ileostomy and colonic lavage: operative strategy. Reproduced with permission from: Neal MD, Alverdy JC, Hall DE, et al. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann Surg 2011; 254(3):423–7. Copyright© 2011 Lippincott Williams & Wilkins.
The increase in the mortality rate of CDI over the past decade has stimulated the search for new therapies for severe refractory disease. Other non-surgical rescue therapies have been anecdotally reported. Tigecycline is an agent with in vitro activity against C. difficile.7 Intravenous tigecycline in addiction to the standard therapeutic regimen has shown to be effective in severe refractory CDI in some case reports.17,18,76 However, these findings were not confirmed in a recent retrospective case-control study.77 Therefore, further prospective clinical trials are warranted to evaluate tigecycline's clinical use in severe refractory disease. Immunotherapy (aiming the neutralization of C. difficile toxins) was also evaluated as a rescue therapy in CDI. The potential benefit of adjunctive treatment with IVIG in severe refractory CDI is documented in isolated case reports.62,78 A review of these cases suggests that the earlier the administration of IVIG, the greater the likelihood of attaining clinical benefit. Nevertheless, a retrospective analysis comparing 18 patients who received IVIG from 18 matched control cases failed to demonstrate significant difference in clinical outcomes.65 It has been recently demonstrated that the administration of monoclonal antibodies against toxins A and B, in addition to antibiotics, significantly reduced the recurrence of CDI.64 Since C. difficile toxins reach the systemic circulation, a potential intervention in severe CDI might be the administration of these monoclonal antibodies.15 However, further data are needed before adopting anti-toxins monoclonal antibodies in the treatment of severe CDI. Finally, although FMT has already demonstrated clinical benefits for treatment of recurrent CDI, published evidence in severe refractory disease is limited. However, as previously mentioned, clinical outcomes from published case reports make FMT a promising option in refractory disease, requiring further investigation.33–38
6Infection control and prevention of CDIPrevention and control of CDI in hospitalized patients require early detection and isolation with contact precautions (in a private room or in a room with another patient with documented CDI), careful attention to hand hygiene, gloves use, and effective environmental cleaning, using an Environmental Protective Agency-registered disinfectant with C. difficile sporicidal label claim.2,7 Single-use disposable equipment should be used whenever possible and medical equipment that must be shared between patients should be cleaned and disinfected with a sporicidal agent between uses.2,7 Reducing unnecessary antibiotic use can reduce CDI rates and antibiotic stewardship programs, as the Antimicrobial Use and Resistance module of the National Healthcare Safety Network (NHSN), are recommended to reduce the risk of CDI.2,79
7ConclusionIncreasing evidence points to higher efficacy of vancomycin over metronidazol for all patients with CDI.4,6,57 Such superiority is most evident and clinically relevant in the setting of severe disease and in recurrent CDI.4,6,57 Thus, current guidelines limit metronidazol use for patients with a first episode of mild-to-moderate CDI, which has the advantage of low cost and is assumed to be associated with lower vancomycin-resistant enterococci selection risk.3 Fidaxomicin has shown promising results in a recent RCT,14 but its high cost continues to limit its widespread use.13,14 In the treatment of severe, complicated CDI, diverting loop ileostomy and colonic lavage (Fig. 1) may be a potential alternative to colectomy, with outstanding results in a recent uncontrolled study, requiring further validation.75 Currently there is not enough evidence supporting the use of probiotics, toxin-binding resins or immunotherapy in the treatment or prevention of CDI.2,3 However, in a recent RCT,64 the use of adjunctive treatment with monoclonal antibodies against C. difficile toxins has shown to be effective, significantly reducing the recurrence rate. Anti-toxins monoclonal antibodies may also be a potential intervention in severe CDI, but their role is still not defined.15 The results of ongoing studies evaluating the use of toxin-based vaccines for CDI are awaited with great interest.67,68
Fecal transplantation is a rising promise in the treatment of CDI. The first RCT demonstrating FMT's efficacy was recently published,32 more than 50 years after its original report.27 At the moment, FMT is undoubtedly a safe and highly effective therapy for CDI, even in immunocompromised patients.29,44 In the future, the spread of fecal microbiota donor's banks and the standardization and simplification of the FMT procedure, as the use of frozen microbiota capsules, may expand its acceptance and spectrum of indications in CDI.41,45,46
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.