Social anxiety severely impacts adolescents’ social interactions with others; however, the underlying neural mechanism has not been revealed. This study examined how adolescent's social anxiety level influences the interbrain synchrony within adolescent-parent dyads during emotional processing by using electroencephalograph (EEG) hyperscanning. A sample of 25 adolescent-parent dyads completed the picture processing task. Adolescents’ ages ranged from 10 to 14 years old. The results showed that (1) at parietal areas, greater gamma interbrain synchrony was observed in the high social anxiety adolescent-parent dyads (HSAs) than the low social anxiety adolescent-parent dyads (LSAs) in the positive conditions. However, greater gamma interbrain synchrony of the picture processing task was observed in the LSAs than the HSAs in the negative conditions. (2) Compared with the neutral condition, LSAs induced greater interbrain synchronization in the negative condition than in the neutral condition at central and parietal areas. However, HSAs induced greater interbrain synchronization in the positive condition than in the negative condition at parietal areas. (3) HSAs induced greater interbrain synchronization at parietal areas than in the central areas in positive conditions. The results provide neurological evidence that the way parent and adolescent process different emotions in the same emotional episode could be affected by the adolescent's anxiety level.
Social anxiety affects emotional and social interaction such as increased emotional avoidance, emotion suppression, self-focused attention, repetitive negative thinking (Golombek et al., 2020), etc. As a biomarker of social interaction, the electroencephalogram (EEG) interbrain synchrony indicated the moment-to-moment interaction between brains and reflected the connection within the dyad during the social interaction at the neural level (Dikker et al., 2017). Previous findings suggest that the increased changing and complex social environment that adolescents face results in social interactions with their parents more challenging compared to social interactions with other people (Golombek et al., 2020). Examining the relationship between social anxiety and the interbrain synchrony during adolescent-parent social interactions will improve our understanding of the biological bond of the adolescent-parent dyad, and create a possible assessment marker for the influence of social anxiety on social interactions in adolescence.
Social AnxietySocial anxiety is defined as an intense fear of the close connection and negative evaluation from other people during social interaction (Morrison & Heimberg, 2013). For instance, it is common that one may experience significant anxiety and fear while being observed and/or performing in front of others. High levels of fear and concern about social situations could lead to limitations of social functioning in one's daily life (Werner et al., 2011). Spence and colleagues (1999) found that compared to non-anxious children, children with social anxiety interacted less with other children, spoke to them less, and interacted with them for shorter periods of time.
An important feature of social anxiety is maladaptive emotion regulation and emotional processing which is normally in the form of situational avoidance, emotion suppression, negative rumination and poor emotion understanding (Werner et al., 2011). Especially during social interactions, social anxiety has been shown to be associated with poor interpersonal interpretation of other's emotional expression in a negative and personalized way, as well as with subjects over-examining themselves because of their self-focused attention (O'Toole et al., 2013). Previous research indicated that socially anxious individuals do not have a reduced ability to detect basic emotions (e.g., happiness and anger); instead, their poorer emotional understanding dampens their social and emotional interaction with others when it needs a more complex understanding of an emotional situation (O'Toole et al., 2013). Socially anxious individuals interpret other people's emotional expressions in a distorted and negative way, which affects their social interaction with others in a negative way (Beck & Emery, 1985). Moreover, previous studies suggested that socially anxious individual tend to have poorer emotion recognition accuracy and are more attentive to negative emotions, less attentive to positive social cues, and more likely to label neutral stimuli as negative. These specific emotionally perceptual biases hamper emotional processing (Golombek et al., 2020).
Adolescent's social anxiety and adolescent-parent interactionThe negative impacts of social anxiety on social and emotional lives are especially significant in the adolescent group (Golombek et al., 2020). Previous research indicated that high social anxiety adolescents show deficits in emotional expression and have a high level of negative emotional suppression compared to non-socially anxious adolescents (Golombek et al., 2020). Because of the fear of negative evaluation during social interactions with others, social anxiety is also related to the limited access to functional emotional regulation strategies and the engagement of dysfunctional emotional regulation (Hinrichsen & Clark, 2003). Interactive situations require interpersonal involvement. The high social anxiety individuals with high self-focused attention tend to be attentive to their emotional responses. When HSA individuals become aware of their internal emotional experience, it increases a threat for them, and triggers inhibition behaviors which may hamper social interactions (O'Toole et al., 2013).
Previous research showed that the increased frequency of social interaction and the pursuit of independence during adolescence make the negative impact of social anxiety more salient in social interactions than other age groups, especially with their parents (Golombek et al., 2020). The pattern and the quality of adolescent-parent interactions are influenced by adolescents’ anxiety level and the parenting style (Van Zalk et al., 2018). Further, some findings suggest that there is a bidirectional link between over-controlling parenting behaviors and adolescents’ social anxiety (Hudson & Rapee, 2001). Individuals’ social anxiety may predict emotionally controlling behaviors by mothers. Mothers’ emotionally controlling behaviors are also highly related to their children's social anxiety level within a dyad (Van Zalk et al., 2018). In addition, the relationship between children's anxiety and their mothers' negative emotions is stronger if the child showed more helplessness and dependence. The negative components of the adolescent-parent interaction (e.g., anxiety experience, negative emotions, and adolescents’ independence) lead to a strained atmosphere between dyads (Asbrand et al., 2017). Different types of parenting behavior may occur as a result of the quality of interaction between adolescent-parent dyads. When social situations were perceived as threatening, anxious adolescents made more avoidant responses; correspondingly, parents may become more involved during interactions to prevent the adolescent from facing potential anxiety (Hudson & Rapee, 2001). In this case, not only do parental behaviors potentially shape adolescents’ behavior, but adolescents’ behavioral characteristics may also play an important role in shaping their perceptions of parental behaviors (Van Zalk & Kerr, 2011). The reciprocity and bidirectional connections between adolescent-parent social and emotional interactions make adolescents’ behavior and emotional responses visible at the same time as their parents’ behavior and emotional responses (Asbrand et al., 2017).
Interbrain synchrony between adolescents and parentsDuring social interactions, social cues are received from others through their actions, emotional expression, and postures (Hari et al., 2015). When engaging in these social situations, people tend to make their behaviors, emotions, physiological activities, and neural activities synchronize with others spontaneously (Palumbo et al., 2017). In this case, to provide the adolescent-parent dyads access to each other's internal states, the synchronization may promote their emotional sharing and facilitate social understanding.
Interbrain synchrony between child and parent in biological rhythms and social signals is thought to be first experience of children's social interaction. Interbrain processes emerge within the caregiver-infant bond, which is one of the most important features of brain maturity (Djalovski et al., 2021). The moment-to-moment interaction between the dyad's brain may reflect the bidirectional stimulus-to-brain coupling. It may result from the inter-individual top-down modulation during the social and emotional interaction (Reindl et al., 2018). The interbrain synchrony between adolescent-parent dyads is also thought to shape the developing brain during this sensitive development period and has long-lasting effects on the adolescent's socio-emotional development (Feldman, 2015). It shows the quality and connection between adolescents and parents during the social interaction at the physiological and neural level.
Previous research indicated that the interbrain synchrony is a sensitive marker of social and emotional interactions, which may be driven by sharing attention, emotional sharing, and social understanding (Dikker et al., 2017). In general, interbrain synchrony can be divided into four types: interactive synchrony, induced synchrony, driven synchrony, and pseudo synchrony. Specifically, induced synchronization refers to the fact that two interacting individuals tend to be synchronized under the influence of common external stimuli with no real interaction (Burgess, 2013). For example, when two individuals view a movie together, they will show synchrony even if there is no information transmission and interaction process (Hasson et al., 2008). The phase resetting theory indicated that neural oscillation is a temporal sampling of environment. The prominent events (such as physical stimulation of external input) can cause ongoing neural responses to phase rearrange to match the time structure of these events and optimize their coding (Schroeder & Lakatos, 2009). According to the phase resetting theory, in the process of social interaction, each participant is sending out significant social signals (such as gaze, body posture, voice and other verbal or non-verbal cues). As the trigger of neural synchronization, these signals trigger the ongoing phase rearrangement of nerve oscillations by themselves and their partners, which leads the phase difference between the two trails of signals to remain constant or to appear covariant (Wang et al., 2021). Previous research found that child-parent dyads had an increased interbrain synchrony in prefrontal brain areas (e.g., dorsal lateral prefrontal cortex (DLPFC) and prefrontal cortex (PFC)) during social interactions (Reindl et al., 2018). There was significant interbrain synchrony between mother and child in the medial left cluster of the prefrontal cortex in a dyadic task of watching animation (Azhari et al., 2019). Also, during emotional stimulation and emotional processing (e.g., in positive and negative emotional induced situations), an increased interbrain synchrony was found among frontal, parietal, and occipital sites which indicated the exchange of emotional information within dyads (Costa et al., 2006). During maternal expression of positive and negative emotions, the interbrain synchrony between dyads increased, which may reveal the modulation of interpersonal neural synchrony by emotional stimulation (Santamaria et al., 2020).
Hyperscanning refers to the simultaneous recording of the neural activity of two or more individuals during interactions (Koike et al., 2015). Hyperscanning research requires a combination of devices from cognitive neuroscience, such as electroencephalogram (EEG), functional near-infrared spectroscopy (fNIRS), and functional magnetic resonance imaging (fMRI). Compared to other methods, EEG-based hyperscanning offers higher temporal resolution (on a time scale of milliseconds) and allows more accurate recording of the neural mechanisms underlying real-time social interactions. It also can be combined with event-related potentials (ERP) to analyze specific EEG components related to individual and social interactions (Zhang et al., 2019; Hu et al., 2018). The EEG-based hyperscanning technique is very promising and widely used in the study of social interaction to index interbrain synchrony (Reindl et al., 2018). For example, in the studies of decision-making, Zhang and colleagues (2019) used the prisoner's dilemma task and found that P3b synchronization was higher when subjects displayed cooperative beliefs. In addition, hyperscanning is also widely used in the study of verbal and non-verbal interactions (Leong et al., 2017). Jiang and colleagues (2012) used hyperscanning and found that the left inferior frontal gyrus synchronization was significantly enhanced only in face-to-face communications.
The present studyEmotional and social interactions between adolescents and parents are crucial for adolescents’ development, especially for adolescents with social anxiety. However, little is known about the association of the levels of adolescents’ social anxiety with the interpersonal neural connectivity between adolescents and parents. Some previous studies also used self-report tests to assess an individual's social anxiety level. However, social desirability, cognitive processes, and survey conditions may result in biases in self-report testing of social anxiety (Bauhoff, 2014). Hyperscanning can reflect the interbrain synchrony, which is a sensitive biological marker of social and emotional interactions (Reindl et al., 2018). Moreover, previous research demonstrated that shared emotional stimulations could induce interbrain synchrony between a child and a parent because of the formation of parallel attuned emotional responses (Azhari et al., 2019). The interbrain synchrony between socially anxious adolescents and their parents may reflect their social and affective functions in a psychophysiological perspective. The combination of hyperscanning test results and self-report results provides both subjective and objective evidence for social anxiety. Thus, in the present study, the hyperscanning approach was adopted to examine how positive and negative emotional stimulations modulate the induced interbrain synchrony between adolescents and parents with different levels of social anxiety.
According to the phase resetting theory, induced synchrony was found within dyads when sharing emotional stimulation (Azhari et al., 2019). Because of the negative impacts of social anxiety on social and emotional interactions (Asbrand et al., 2017), we hypothesized that the high social anxiety adolescent and parent dyads would have lower levels of interbrain synchrony during emotional processing. Next, a previous study suggested that there were significant differences in the hyper-connectivity (e.g., in the gamma frequency band) between positive and negative emotional processing (Kang et al., 2012). Thus, we hypothesized that the level of adolescents’ social anxiety associated with the interbrain synchrony between adolescents and parents would be different in the positive and negative emotional conditions. To be specific, greater interbrain synchrony of the picture processing task would be observed in the parent and high social anxiety adolescent dyads than the parent and low social anxiety adolescent dyads in the positive conditions. However, greater interbrain synchrony of the picture-processing task would be observed in the LSAs than the HSAs in the negative conditions.
MethodsSampleParticipants were recruited via flyers that invited healthy volunteers to participate in a study of parenting and emotion. Interested families were invited to visit the university laboratory to take part in the study. Due to poor EEG data quality and technical error, two adolescent-parent dyads were excluded from the study, after which 25 adolescent-parent dyads remained. The 10-item Social Anxiety Scale for Children (Greca et al., 1998) was used to assess adolescents’ level of social anxiety level on a 3-point Likert scale (0= never, 1= sometimes, 2 = always; e.g., “I worry about doing something new in front of others.”). We averaged across the 10 items (including reverse scored items) for the adolescents’ social anxiety score (α = .88); LSAs (MLSAs=2.25, SD=.97) and HSAs (MHSAs=9.21, SD=4.63) differed significantly on the overall adolescents’ social anxiety score (p<.001).
According to the grouping method of the previous studies (McGrath et al., 2016), we used a median split for adolescents’ social anxiety score to define LSA and HSA groups. Accordingly, there were 12 low social anxiety adolescent (LSA) and parent dyads (seven male and five female; between 10 and 14 years old, Mage=11.75, SD=1.48; five mothers, Mage=40.20, SD=3.49; seven fathers, Mage=47.43, SD=4.54). There were 13 high social anxiety adolescent (HSA) and parent dyads (nine male and four female; between 10 and 14 years old, Mage=12.08, SD=1.12; seven mothers, Mage=42.49, SD=5.78; six fathers, Mage=42.67, SD=3.50) in the present study. The sample size in this study was also in line with typical hyperscanning EEG studies (Mu et al., 2016).
All the adolescents came from urban communities in Shenzhen city in China. In the sample, 40.00% of the adolescents were only children, whereas the others had one or more siblings. Approximately 92.00% of fathers and 72.00% of mothers had received a college education. Chi-square tests were conducted to examine the differences in sociodemographic characteristics between the HSAs and LSAs. Results showed that there was no significant difference in the parents’ level of education between the HSAs and LSAs (ps > .05). However, there was a significant difference in the characteristics of only children between the HSAs and LSAs (p =.009). To rule out the possible impact of family structure (e.g., only child vs. siblings), repeated measures ANOVAs were used and family structure was set as the covariate. Results showed that the characteristics of only children had no significant effect on the results, however (ps>.05).
All of the participants were right-handed and had normal or corrected-to-normal vision. No participant had a history of neurological or psychiatric disorder, as determined by self- and/or parent report. The research protocol was approved by the local Institutional Review Board. Informed consent was obtained from the participants and their parents before the study, and the adolescent-parent dyads were fully debriefed after the experiment.
InstrumentNinety pictures were selected from the Chinese Affective Picture System (CAPS; Bai et al., 2005); 30 negative (valence: M=2.68, SD=.38; arousal: M=5.41, SD=.38), 30 positive (valence: M=6.96, SD=.48; arousal: M=5.59, SD=.38) and 30 neutral (valence: M=5.51, SD=.24; arousal: M=3.79, SD=.45). Results of the F-tests showed that three types of pictures significantly differed in terms of valence, F(2, 87) = 991.75, p < .001, ηp2= .96. The positive pictures were more pleasant than the negative and neutral pictures (ps < .001), and neutral pictures were more pleasant than negative pictures (p < .001). Results of the F-tests showed that the three types of pictures significantly differed in terms of arousal ratings, F(2, 87) = 179.33, p < .001, ηp2 = .81. The negative and positive pictures were more arousing than the neutral pictures (ps < .001). The pictures were age-appropriate for adolescents. The negative picture set included unpleasing social situations and frightening animals, and the neutral pictures depicted subjects such as household objects.
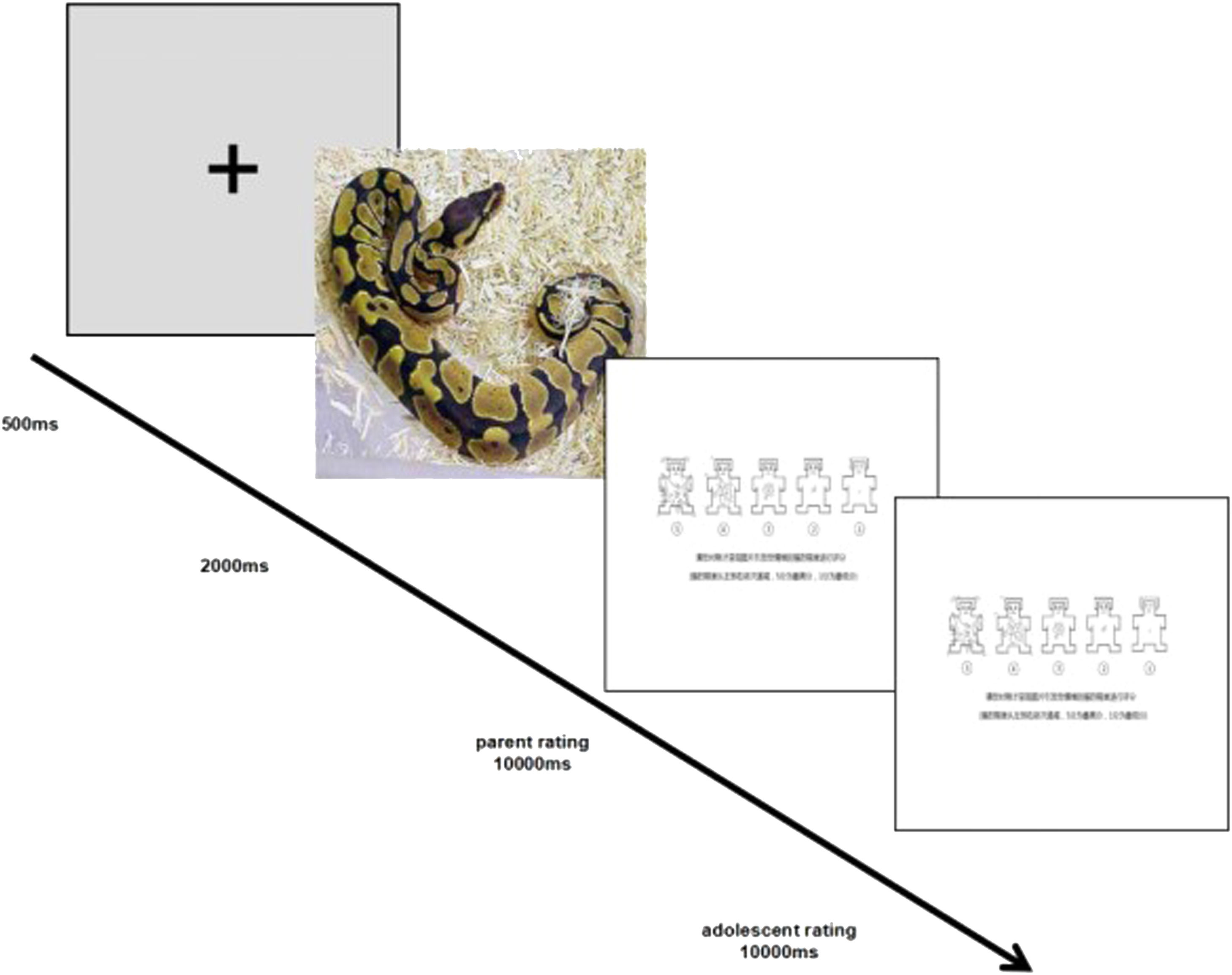
DesignWe employed a 2 Group (HSA vs. LSA) × 3 Valence (positive, negative, and neutral) mixed design. After completing the demographic information, the adolescent-parent dyads were asked to rate the picture processing task. The picture processing task has been shown to successfully assess participants’ neural responses to different emotional stimuli in both adolescent and adult samples in prior research (Hajcak & Dennis, 2009). Electroencephalograph (EEG) sensors were attached to adolescent-parent dyads and they were introduced to the procedures of the task before the experiment. The adolescent-parent dyads first viewed a practice series of pictures (nine pictures) displayed on the screen together. After a research assistant confirmed that the adolescent-parent dyads fully understood the procedure, the test experiment began. At the beginning of each trial, a fixation was presented in the middle of the screen for 500ms. Immediately following the fixation, a stimulus picture was presented for 2,000ms. The adolescent-parent dyads viewed the picture stimuli together. Then, the parents were asked to rate each picture on the arousal scales of the self-assessment manikin (SAM, Lang et al., 2005) on the computer by keyboards, ranging from low intensity/arousal (1) to high intensity/arousal (5). At last, the adolescents were asked to rate each picture on the arousal scales of the self-assessment manikin (SAM, Lang et al., 2005) on the computer by keyboards, ranging from low intensity/arousal (1) to high intensity/arousal (5). Participants were told to rate arousal based on the strength of their feelings in response to the picture (i.e., How strongly did you feel after viewing the picture?). The arousal ratings were not shown on the screen. The adolescent-parent dyads could not see each other's ratings.
The experiment consist of a practice block, and then an experiment block. Nine pictures were used for the practice block. 90 pictures were used for the experiment block and were each displayed twice in a random order for a total of 180 trials. The nine pictures that appeared in the practice block were not used in the experimental block. E-Prime software was used to present all stimuli against a black background on a 21-inch monitor, with a viewing distance of approximately 80cm. The experimental session took 30-35 min for each dyad (see Figure 1). The adolescent-parent dyads were fully debriefed after the experiment was complete.
Dual-EEG recording and data analysisWe used two 32-channel portable EEG systems (BrainAmp, Brainproducts GmbH, Germany) to simultaneously and continuously record the EEG signals of the adolescent-parent dyads at a sampling rate of 500 Hz in an electrically shielded room. The adolescent-parent dyads were seated side by side during the picture processing task. Electrode impedance was kept under 30 kohms for all recordings. EEG signals were referenced offline to the averaged mastoid references. EEG signals were treated with band-pass filtering (1 - 40 Hz). Independent component analysis (ICA) was performed for ocular artifact reduction using EEGLAB. The artifact scored epochs were eliminated from all subsequent analyses. Onsets were set as the points where the emotional stimuli were presented. The baseline was 1000ms before the appearance of the emotional picture, and the analysis was segmented 2000ms after the appearance of the picture. Thus, the total duration of a single trial was 3000ms. Finally, the effective trials with different valence were superimposed and averaged. EEG data were transformed by the Short Time Fourier Transform (STFT) methods. STFT is the Fourier transform sequence of windowed signal, which is used to analyze the frequency variation of non-stationary signals. For the case where the frequency component of the signal varies with time, the time-localized frequency information is provided (Wang et al., 2020; Nagarajan et al., 2020). The time resolution is 3ms. Frequency range is 1-40Hz. Frequency resolution was set to 1Hz, and the window size was set to 0.2s (Tables 1 and 2).
Average Arousal Ratings between HSAs and LSAs in Different Conditions
Note. LSAs = parent and low social anxiety adolescent dyads, HSAs = parent and high social anxiety adolescent dyads.
Gamma Interbrain Phase-locking-value (PLV) between High and Low Social Anxiety Adolescent-Parent Dyads in Different Conditions at Fz, Cz and Pz
Note. LSAs = low social anxiety adolescent-parent dyads, HSAs = high social anxiety adolescent-parent dyads.
In previous studies, inter-brain synchrony has been measured by the phase synchronization of EEG signals (Santamaria et al., 2020). Thus, we used an interbrain phase-locking-value (PLV) index to estimate the interbrain phase synchrony between adolescent-parent dyads in the positive, negative and neutral condition. This interbrain phase synchrony index has been developed to measure whether the signals from the two interacting individuals are phase locked across time (Lachaux et al., 1999). In line with previous hyperscanning research (Lachaux et al., 1999), the interbrain PLV at a given time t and frequency f was calculated as the absolute value of the sum of the phase φ differences of two electrodes (j, k) from two individuals of a dyad across N epochs. The averaged interbrain PLV in gamma frequency bands (31-40Hz) after stimuli onset 300ms was calculated for further statistical analysis. The gamma frequency band has been considered to be highly related to emotional interaction (Mu et al., 2017). According to the EEG literature, electrodes in the midline were typically used to present the relevant brain areas. Thus, the interbrain PLVs in the present study were calculated at the representative electrode of the central areas (Cz), frontal areas (Fz) and parietal areas (Pz) (De Blasio et al., 2013; Behzadnia et al., 2017).
The interbrain PLV was assessed using a 2 Group (HSA vs. LSA) × 3 Valence (positive vs. negative vs. neutral) × 3 (electrode: Cz vs. Fz vs. Pz) ANOVAs, with valence and electrode as within-subject variables and group as a between-subject variable. The interbrain PLV was statistically evaluated using SPSS 20.0. The significance level was set at p < .05, and Greenhouse-Geisser correction was applied to p values associated with multiple-df comparisons. Partial eta squared was reported as a measure of effect size.
To rule out the possible impact of relevant variables of the adolescent-parent emotional interactions on our findings, demographic variables, adolescents’ level of depression, adolescents’ level of anxiety, adolescents’ level of social support, parents’ level of depression, parents’ level of anxiety, and the level of parent involvement between the high and low social anxiety adolescent-parent dyads were examined. Repeated measures ANOVAs were used and these relevant variables were set as the covariates. The results of the repeated measures ANOVA indicated that there were no significant main effects of demographic variables and other examined variables on our Gamma Interbrain PLV between the high and low adolescents’ social anxiety dyad groups (see Supplementary Material 2). Therefore, demographic variables and other examined variables were not included in subsequent analyses.
ResultsBehavioral ResultsTo analyze the differences in the average arousal rates, we employed a 2 group (HSA vs. LSA) × 2 dyad (parent vs. adolescent) × 3 valence (positive vs. negative vs. neutral) repeated measures ANOVA. The main effect of valence was significant, F(2, 92) = 126.30, p < .001, ηp2 = .73. The arousal ratings of the positive and negative pictures were higher than the neutral pictures (ps <.001). The arousal ratings of the negative pictures were higher than the positive and negative pictures (ps <.001). The main effect of dyad was not significant, F(1, 46) = .25, p = .620, ηp2 = .01. The main effect of group was not significant, F(1, 46) = .04, p = .848, ηp2 = .00. The interaction of dyad and valence was significant, F(2, 92) = 3.91, p = .034, ηp2 = .08. The arousal ratings of the positive and negative pictures were higher than the neutral pictures in both parent and adolescent (ps <.001). The arousal ratings of the negative pictures of both parent and adolescent were higher than the positive and negative pictures (ps <.05). The interaction of group and valence was not significant, F (2, 92) = .18, p = .775, ηp2 = .00. The interaction of dyad and group was not significant, F(1, 46) = .00, p = .950, ηp2 = .00. The interaction of group, dyad, and valence was not significant, F (2, 92) = .07, p = .887, ηp2 = .00.
The adolescents’ and parents’ arousal levels were calculated by Pearson's correlation coefficients and independent t-tests were conducted to examine the differences between the HSAs’ and LSAs’ arousal rating. Results showed that there was no significant difference in arousal ratings (p = .552) between the HSAs and LSAs.
Neural resultsTo examine the differences in the interbrain synchrony between HSAs and LSAs when processing positive, negative and neutral emotional stimuli, we calculated the interbrain phase-locking-value (PLV) which has been developed to measure whether the signals from the two interacting individuals are perfectly phase locked across time. Results of the repeated measure ANOVA on the interbrain phase synchrony in the gamma band showed that the main effect of group was not significant, F(1, 23) = .12, p = .737, ηp2 = .00. The main effect of valence was not significant, F(2, 46) = 1.79, p = .179, ηp2 = .07. The main effect of electrode was also not significant, F(2, 46) = .13, p = .883, ηp2 = .01. The interaction of group and valence was not significant, F(2, 46) = 1.95, p = .153, ηp2 = .08. The interaction of group and electrode was not significant, F(2, 46) = 1.63, p = .207, ηp2 = .07. The interaction of valence and electrode was also not significant, F(4, 92) = 1.97, p = .106, ηp2 = .08.
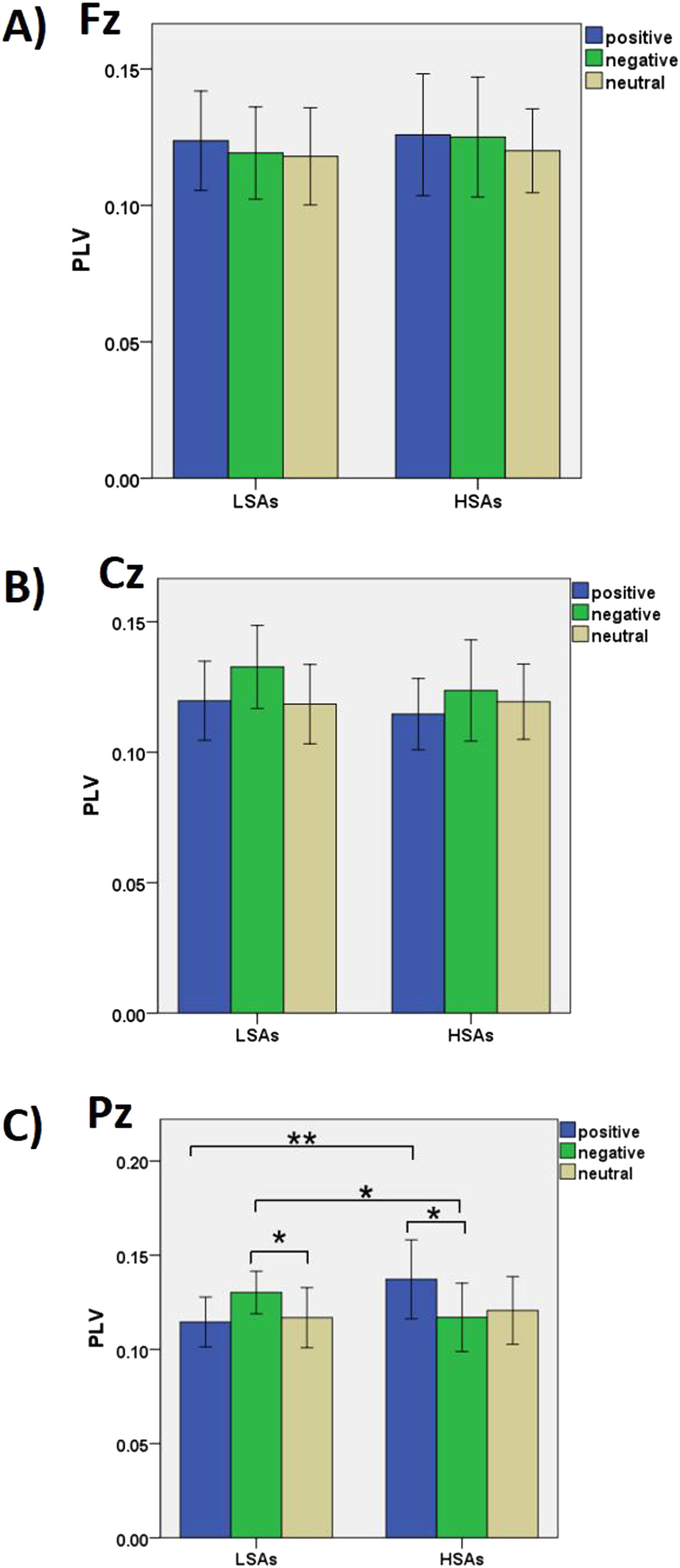
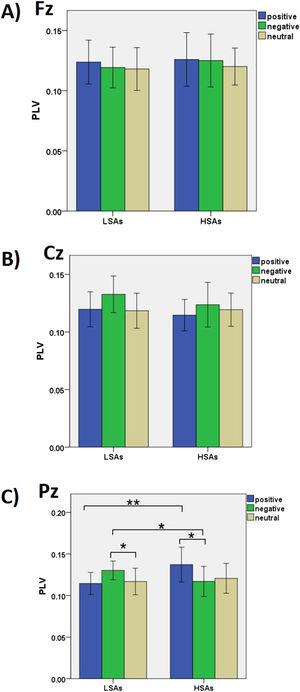
The interaction of a 2 group × 3 valence × 3 electrode was significant, F(4, 92) = 3.80, p = .007, ηp2 = .14 (see Figure 2). Post hoc analysis revealed that at Pz, greater gamma interbrain synchrony of the picture processing task was observed in the HSAs than the LSAs in the positive conditions (p = .004), but greater gamma interbrain synchrony of the picture processing task was observed in the LSAs than in the HSAs in the negative conditions (p = .041). There was no significant difference in interbrain synchronization between LSAs and HSAs at other sites (ps>.05). Compared with the neutral condition, LSAs induced greater interbrain synchronization in the negative condition at Cz (p = .024) and Pz (p = .035). However, HSAs induced greater interbrain synchronization in the positive condition compared to the negative condition at Pz (p = .017). There was no significant difference in interbrain synchronization between different conditions at other sites in the LSAs and HSAs (ps>.05). In addition, HSAs induced greater interbrain synchronization at Pz compared with Cz in positive conditions (p = .008).
DiscussionSocial anxiety has been characterized as the experience of fear or anxiety during social interactions in which the individual is exposed to possible negative evaluation by others (Morrison & Heimberg, 2013). Previous studies have described the negative impact of social anxiety on adolescents in the socio-emotional domains, especially the hindrance of social and emotional interactions with their close person (e.g., parents) (Golombek et al., 2020). However, to our knowledge, little neuroscientific evidence has described how adolescents’ social anxiety is related to interbrain synchronous activation during emotional interaction between adolescent-parent dyads at this particular age. The present study examined the differences in the interbrain synchrony during positive and negative emotional processing between adolescent-parent dyads with different adolescent social anxiety levels and provided psychophysiological evidence for this relationship. By identifying the relationship between adolescent social anxiety and interbrain synchrony during emotional processing, we have the insight to clarify the neural underpinnings of social anxiety that influence interpersonal interactions.
Interbrain synchrony reflects the quality of connection during socio-emotional interactions and the biological base of the emotional bond between the adolescent-parent dyads (Reindl et al., 2018). When viewing the emotional stimuli together, the shared emotional stimulation is proved to induce interbrain synchrony between parent and child, implying that each is inferring the mental state of the other (Azhari et al., 2019). Social anxiety influences the quality of interaction at a neural level and leads to the modulation of the interbrain synchrony. The results of the present study were consistent with the hypothesis that adolescents’ social anxiety is related to interbrain synchrony in the adolescent-parent dyads during the processing of positive and negative emotions. Greater gamma interbrain synchrony of the picture processing task was observed in the HSAs than in the LSAs in the positive conditions at the parietal areas. However, smaller gamma interbrain synchrony of the picture processing task was observed in the HSAs than in the LSAs in the negative conditions at the central and parietal areas. Previous research has found that high social anxiety individuals (both adolescents and parents) are more attentive to negative emotional stimuli and more likely to experience threats in social situations (Gonzalez et al., 2012). When they encounter social contacts and social interaction, high social anxiety adolescents tend to make more avoidant responses. Adopting the strategy of intentionally avoiding negative information may represent a regulatory tactic that high social anxiety adolescents choose in order to reduce the social threat. It might reflect a form of psychological escape (Hudson et al., 2008). In this paradigm the negative experience of adolescents during emotional interactions leads parents to be intrusively controlling, criticizing, or less warm toward their children in hopes of avoiding embarrassing situations (Van Zalk & Kerr, 2011). In this case, the emotional and neural synchrony within dyads might be reduced during the negative emotional stimulation because of the adolescents’ avoidant behaviors and the parents’ corresponding responses. It is worth noting that the joint activity (passively viewing emotional stimuli together) in the present study occurred with no face-to-face communication between dyads. The greater neural synchrony in the LSAs in the negative conditions indicates that non-anxiety adolescents were able to instinctively attune their mental state and align it with that of their parent with minimal feedback from behavioral cues when experiencing negative emotions with their parent (Azhari et al., 2019). Adolescents with low social anxiety seem to have good interpersonal coordination with their parents based on strong affective and social function.
Prior research showed that, compared to parents with non-anxious children, parents with anxious children show more disruptive and intrusive behaviors when their children present negative emotions (Hudson &Rapee, 2001). On the contrary, compared to parents with non-anxious children, parents with anxious children show less intrusive behaviors and are warmer toward their children when their children present positive emotions (Hudson et al., 2008). When children encounter a positive emotional event, their parents are prompted to be less intrusively controlling, pay less attention toward their children, and get involved with their children's positive experience (Hudson et al., 2008). In this case, the emotional and neural synchrony within dyads might be increased during the positive emotional stimulation because of the children and adolescents’ positive emotional status and the parents’ corresponding responses to their children.
Interbrain synchrony within dyads reflects the level of biobehavioral synchrony, which implies the coordination of neural and behavioral processes during social contact (Dikker et al., 2017). It measures the interpersonal and emotional synchronization and the coordination of brain activity between individuals (Dikker et al., 2017). In the current study, we found group differences in the gamma band interbrain synchrony indexed by the PLV when the dyads processed positive, negative and neutral emotional stimuli. This is consistent with the previous research suggesting that gamma band activity and its synchronization in human EEG are related to higher cognitive processes (e.g., memory, language, emotion, and social interactions) (Matsumoto et al., 2006). Also, there has been evidence for the functional importance of gamma oscillations in the amygdala when processing emotions. The gamma interbrain synchrony in the amygdala was prominent when positive and negative emotions engaged (Matsumoto et al., 2006). As a high frequency activity, the gamma interbrain synchrony may be a better predictor of the mental adjustment to emotional processing that the dyads perform rather than a predictor of the regulation of motor responses (Mu et al., 2017). Our finding provides a complementary perspective on the well-documented statement that social anxiety hampers social and emotional interactions on a behavioral level (Golombek et al., 2020).
In the present study, we also found that the gamma interbrain synchronizations between HSAs and LSAs were significantly different in parietal areas and central areas across different emotional conditions. Previous research suggests that the parietal and central areas mainly function in the involvement of emotional activation (Benzagmout et al., 2019). Additionally, we found that gamma activity in the parietal areas was modulated in response to emotional stimuli (Luo et al., 2009). Compared with neutral conditions, positive and negative emotions showed higher gamma activation in the parietal areas (Luo et al., 2009). Our results are consistent with the notion that high social anxiety individuals have specific emotionally perceptual biases that influence emotional processing (Gonzalez et al., 2012). In this case, the gamma activity differences in the parietal areas in our results may reflect the differences in bottom-up emotional processes between HSAs and LSAs (Kang et al., 2012). The different attentional and perceptional processing of emotional stimuli between HSAs and LSAs may lead to the differences in their gamma interbrain synchronizations.
Findings from the current study deepen our understanding of the relationship between adolescents’ social anxiety and the adolescent-parent bond. Particularly, it implicates that having high social anxiety may make socio-emotional interaction more difficult in negative emotional situations, especially in adolescence. Also, the high interbrain synchrony of the HSA dyads in the positive emotional situations suggests that cultivating positive emotion as a dyad is beneficial for adolescents’ interpersonal relationship, and help their socio-emotional development. In this case, cultivating positive emotion sharing during adolescence may have value for family relationships and family connections, especially for adolescents who are at risk of social anxiety problems. Family communication related programs could facilitate a smooth transfer from family to society. Moreover, as a reliable index of socio-emotional interaction, the values of interbrain synchrony were again verified in the current study.
One of the main limitations of the study was the relatively small sample size; while the sample size is in line with typical hyperscanning EEG studies (Mu et al., 2016), for the generalizability of the study, a larger sample would be needed to be able to explore the relationship between adolescent social anxiety and parent-child interactions. In addition, although median splitting was used to separate between groups in previous studies, this grouping method differs from a contrasted threshold grouping method. Therefore, the generalizability of our results is limited. It is important for future studies to use a contrasted threshold measure to separate between groups. Another limitation is that due to the nuances of adolescent-parent relationships and the evolutionary approaches to human emotional bonding, we only focused on adolescent-parent dyads. Future research could explore other social relationship dyads, such as peer dyads and student-teacher dyads. Furthermore, to examine the developmental trajectory of adolescents’ social anxiety and its relation to brain development and maturity, we could use a longitudinal design in the future. Lastly, although we found there was no significant main effect of parents’ level of depression and anxiety on our Gamma Interbrain PLV between the HSA and LSA adolescents (see Supplementary Material 2), we did not measure the parents' social anxiety level. A parent's social anxiety level may influence adolescent-parent interactions just as much as an adolescent's social anxiety level. Thus, different combinations of the dyads (e.g., parent anxious - adolescent anxious / parent non-anxious - adolescent anxious / parent non-anxious - adolescent non-anxious) could be studied in the future.
The findings of the current study contribute to the literature on the influence of adolescent social anxiety on socio-emotional interaction in adolescence. The current findings indicate that the impacts of adolescent social anxiety during socio-emotional interaction could be related to significantly higher gamma interbrain synchrony in the positive emotional situations and lower gamma interbrain synchrony in the negative emotional situations. As an important neural indicator of socio-emotional interaction, the present study warrants further investigation of socio-emotional interaction in this special age group.