Previous studies have indicated that glucose metabolism and altered hippocampal structure and function play a pivotal role in cognitive deficits in schizophrenia (SZ). This study was designed to explore the inter-relationship between glucose metabolism, hippocampal subfield volume, and cognitive function in the antipsychotics-naive first episode (ANFE) SZ patients.
MethodsWe chose the fasting insulin, glucose, and insulin resistance (HOMA-IR) index as biomarkers of glucose metabolism. Cognitive function was assessed by the MATRICS Consensus Cognitive Battery (MCCB). The hippocampal subfield volume, glucose metabolism biomarkers, and cognitive function were evaluated in 43 ANFE SZ and 29 healthy controls (HCs).
ResultsCompared with HCs, SZ patients had higher fasting blood glucose and insulin levels and HOMA-IR (all p < 0.05). Correlation analysis revealed that category fluency performance was positively associated with fasting glucose level. Fasting insulin or HOMA-IR was positively associated with the hippocampal subfield volume in patients (all p<0.05). Moreover, the spatial span index score was associated with the volume of the right presubiculum, subiculum, and right hippocampal tail. In addition, multiple regression analysis found that the interaction effects of insulin × right fimbria or insulin × left fimbria were independent predictors of the MCCB total score.
ConclusionsOur findings suggest that abnormal glucose metabolism and cognitive decline occur in the early stage of SZ. The interaction between abnormal glucose metabolism and hippocampal subfields was associated with cognitive functions in SZ.
Schizophrenia (SZ) is a severe and debilitating disease with a prevalence of approximately 1% in the population (McGrath et al., 2008). Impairments in multiple domains of cognitive functioning usually occur prior to the first onset of this disease, such as working memory, executive function, attention, and processing (Harvey, 2009; Xiu et al., 2020). Cognitive impairment is a hallmark symptom of SZ that is closely associated with functional outcomes and persists throughout the course of the disease (Millan et al., 2012; Zhu et al., 2022). However, the underlying pathophysiology of cognitive impairment in SZ remains unclear.
Accumulating studies have shown that cognitive function may be affected by abnormal blood glucose, insulin resistance, and lipid disturbance (Zhong et al., 2012; Kim & Feldman, 2015; Ekblad et al., 2017). For example, a cohort study including 3695 subjects found that higher insulin resistance and fasting insulin levels at baseline were predictors of a steep decline in verbal fluency performance during 11 years of the follow-up period (Ekblad et al., 2017). Interestingly, abnormal glucose metabolism is a key risk factor for clinically significant cognitive impairment, not only in healthy subjects but also in SZ patients (Qiu & Fratiglioni, 2015; Bora et al., 2017, 2018). Nandeesha et al. found that plasma glucose was negatively associated with various cognitive domains, suggesting that abnormal glucose metabolism may increase the risk of cognitive impairment in SZ patients (Nandeesha, Keshri, Rajappa, & Menon, 2020). Recent meta-analyses have also revealed that comorbid metabolic syndrome is significantly correlated with cognitive impairment in SZ (Bora et al. 2017; Hagi et al., 2021). In addition, studies have shown that disrupted interactions between glucose homeostasis and brain dopamine signaling are a candidate mechanism to better understand the pathogenesis of SZ (Sauerzopf et al., 2021).
The hippocampus is a complex structural area of the brain that plays a critical role in memory (Small et al., 2011). SZ patients exhibit impaired declarative memory and abnormal hippocampal structure and function, even in their nonpsychotic relatives (Adriano et al., 2012; Haukvik et al., 2015; Ho et al., 2017). Reduced hippocampal volume may be a trait feature of SZ (Sasabayashi et al., 2021). A meta-analysis including 2028 SZ patients and 2540 control subjects shows that the greatest difference in brain structure between SZ patients and controls is hippocampal volume loss in patients (van Erp et al., 2016). The hippocampus is known to be composed of several inter-connected subfields (Tamminga et al., 2010; Adriano et al. 2012), which have been reported to be abnormal in SZ patients (Nakahara et al., 2018; Xiu et al., 2021). In addition, our previous study also reported that the abnormalities in hippocampal subfields were associated with the Hopkins Verbal Learning Test (Xiu et al. 2021).
Recent literature has shown that abnormal glucose metabolism is associated with a decrease in hippocampal volume (Mucellini et al., 2017). Overweight induced by high-sugar diets can lead to insulin resistance, which may impair hippocampal function (Jones et al., 2021; Kim et al., 2021). Kim et al. found that the influences of obesity on hippocampal volume and cognitive impairment were mediated by impaired glucose metabolism in elderly individuals without mental illness. Moreover, the smaller the hippocampal volume, the lower the delayed recall score for visual information, recognition memory, and verbal learning (Kim et al., 2021). Our recent study shows that elevated blood glucose levels interact with widespread disruption of white matter structure, and have an impact on cognitive decline as measured by the MATRICS Consensus Cognitive Battery (MCCB) in the early onset of SZ (Zhang et al., 2020). All these studies have indicated that there is a close relationship between abnormal glucose metabolism and reduced brain volume or cognitive decline in SZ patients. Considering the clinical implications of evaluating the relationship between glucose metabolism and cognitive decline in SZ patients, we were motivated to conduct a study investigating the inter-relationship between glucose metabolism, cognitive function, and hippocampal subfields in SZ patients. Our study is the first report to investigate the relationship between glucose metabolism and hippocampal subfields in SZ patients. We hypothesized that an increase in fasting blood glucose or insulin would be significantly correlated with cognitive deficits and reduced hippocampal subfield volume in SZ patients.
Materials and methodsParticipantsAccording to the criteria in previously published literature, forty-three antipsychotics-naive first episode (ANFE) SZ inpatients were recruited in Beijing Huilongguan hospital (Lieberman et al., 2003). According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria, patients were diagnosed as SZ by an experienced psychiatrist using the Structured Clinical Interview for DSM-IV (SCID). The inclusion criteria included: age 18∼45 years; disease course < 5 years; no substance and alcohol abuse or dependence; no previous use of antipsychotic drugs; no other type of psychotropic medication, beyond antipsychotics; and not receiving oral or injectable hypoglycemic medications. The mean duration of untreated psychosis in patients was 16 months (range 1–60 months).
A total of 29 age-, sex-. education-matched healthy controls (HC) were recruited from the Beijing region during the same period. SCID interviews were used to rule out those with mental disorders. In addition, we also obtained the medical history, physical examination, and laboratory test of each participant. The exclusion criteria included: major medical comorbidities; history of allergies; hypertension, diabetes; cerebrovascular diseases; pregnancy, or breastfeeding.
Assessment of cognitive functionTwo clinical psychologists were trained on how to use the MCCB in order to assess each participant's cognitive function within 72 h of blood collection (Green et al., 2004). It consists of 10 standardized cognitive metrics, with a global composite score. The MCCB was previously translated into Chinese and its clinical validity and test-retest reliability were established among healthy controls and SZ patients (Zou et al., 2009).
Laboratory test of patients and healthy controlsThe whole blood of patients and HCs were collected at 7 A.M. following overnight fasting. The samples were transferred to the laboratory for testing. The fasting concentrations of insulin and glucose were determined by the enzymatic photometric assay and chemiluminescence method. All assays were performed by a laboratory technician who was blinded to the status of the samples. The calculation formula of insulin resistance index (HOMA-IR) was: fasting blood glucose concentration × insulin / 22.5.
MRI data acquisition and preprocessingNone of the SZ patients took antipsychotic drugs during the scan within three days of admission. All participants were right-handed. A General Electric 3.0T MRI scanner together with SPGR (spoiled gradient echo) sequence for structural T1-weighted scans were used in this study. The parameters used were described in our previous study (Xiu et al. 2021) and included slice thickness = 1.2 mm, matrix size = 256 × 256 and 142 slices, repetition time = 6.2 ms, echo time = 2.8 ms, flip angle = 8◦, and field of view = 240 mm. Gross motion artifacts were excluded by inspecting each scan.
A development version of the FreeSurfer 5.3 software (http://surfer.nmr.mgh.harvard.edu) was used to perform subcortical segmentation (Fischl et al., 2002; Jovicich et al., 2006). We established a hippocampal subfield segmentation map according to high-resolution (0.13 mm) ex vivo MRI data of the postmortem medial temporal tissue at 7-tesla (Iglesias et al., 2015). This algorithm was reported to realistically specify the granular cell layer (GCL) within the dentate gyrus, as well as the molecular layer (ML) within the hypothenar and CA regions, which was more accurate than previously reported methods (Van Leemput et al., 2009). In this study, the hippocampal subfields included CA1, CA3, CA4, ML, fimbria, GCL, presubiculum, subiculum, and the hippocampal tail (Xiu et al. 2021). The quality control (QC) procedures were as follows. First, we excluded outliers in the hippocampal subfield that exceeded three standard deviations. Second, we visually inspected the hippocampus image to exclude poorly registered segmentations or apparently misallocated subfields. All images successfully passed QC.
Statistical analysisThe chi-square test and analysis of variance (ANOVA) for categorical and continuous variables were performed to compare differences between groups. ANCOVA was performed to examine whether there were differences in cognitive function or hippocampal subfields between HCs and patients. Since age, sex, years of education, and BMI were found to be associated with cognitive function, these variables were added to the ANCOVA model as covariates. For hippocampal subfields, we also adjusted for the intracranial volume (ICV).
We performed a Pearson correlation analysis between the hippocampal volume, cognitive function, and fasting blood glucose, insulin, or HOMA-IR in participants. Then, a multiple linear regression analysis was carried out to determine the independent factors for cognitive function in participants. In this model, the MCCB total score or its index scores were considered dependent factors, and the hippocampal subfield was an independent factor. Sex, onset age, education, and ICV were controlled for as covariates. Bonferroni correction was used to adjust for multiple tests.
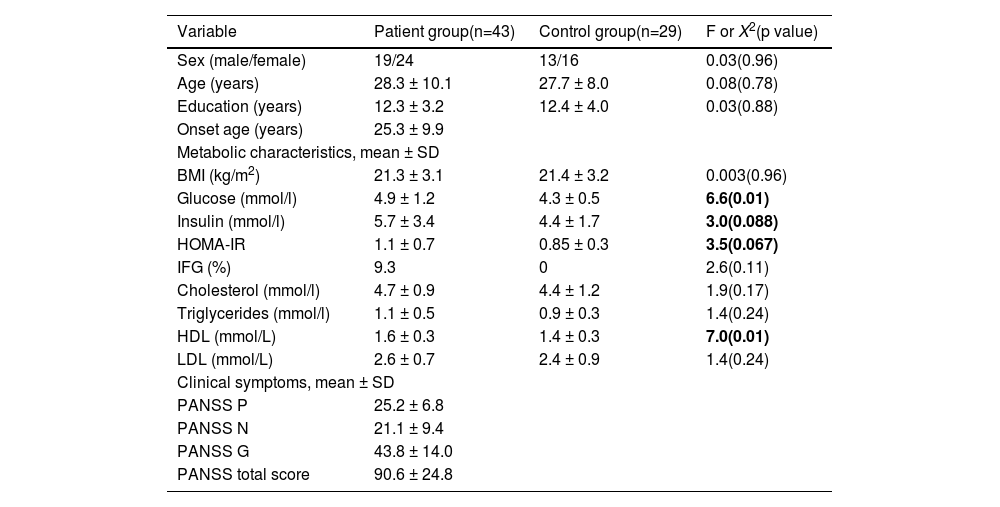
ResultsComparison of glucose metabolism between patients and controlsThe ANFE patients and controls were matched by sex, age, and education (all p > 0.05) (Table 1). No significant difference was found in the head motion and the signal to noise ratio (SNR) between patients and controls. Patients showed higher levels of high-density lipoprotein (HDL) (1.6 ± 0.3 vs 1.4 ± 0.3) and glucose (4.9 ± 1.2 vs 4.3 ± 0.5) (all p <0.05), and a trend towards significance in insulin (5.7 ± 3.4 vs 4.4 ± 1.7) and HOMA-IR (1.1 ± 0.7 vs 0.85±0.3). These significances remained significant after controlling for sex, age and education.
Demographic characteristics and clinical data in patient group and control group.
Note: P positive subscore, N negative subscore, G general psychopathology subscore, BMI body mass index, IFG Impaired Fasting Glucose.
BMI was not used as the inclusion criteria for patients and control subjects, so two recruited patients were overweight. Three patients were identified as hyperglycemic (glucose levels > 6.1 mmol/L) (7.7%) compared to 0 in controls.
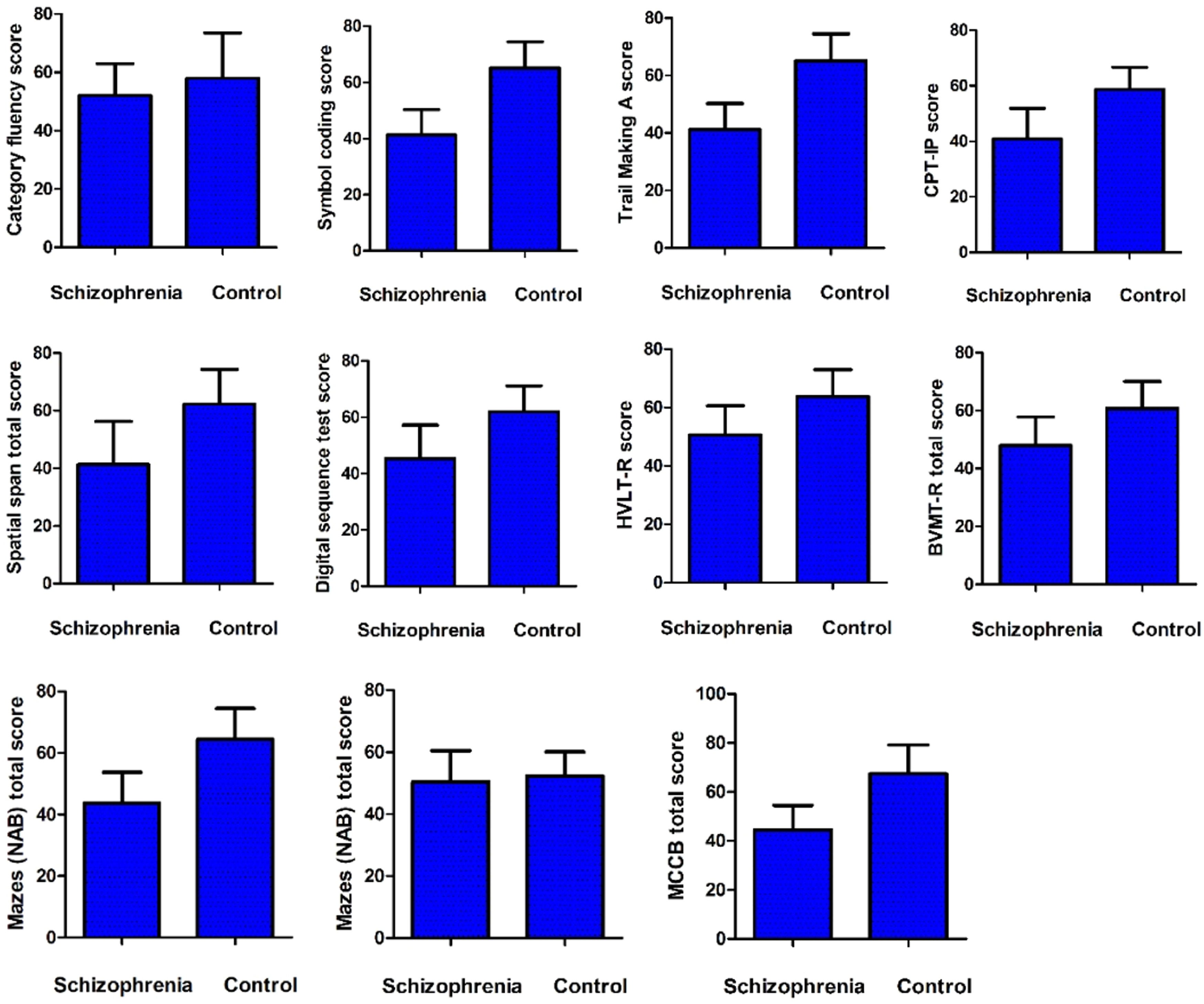
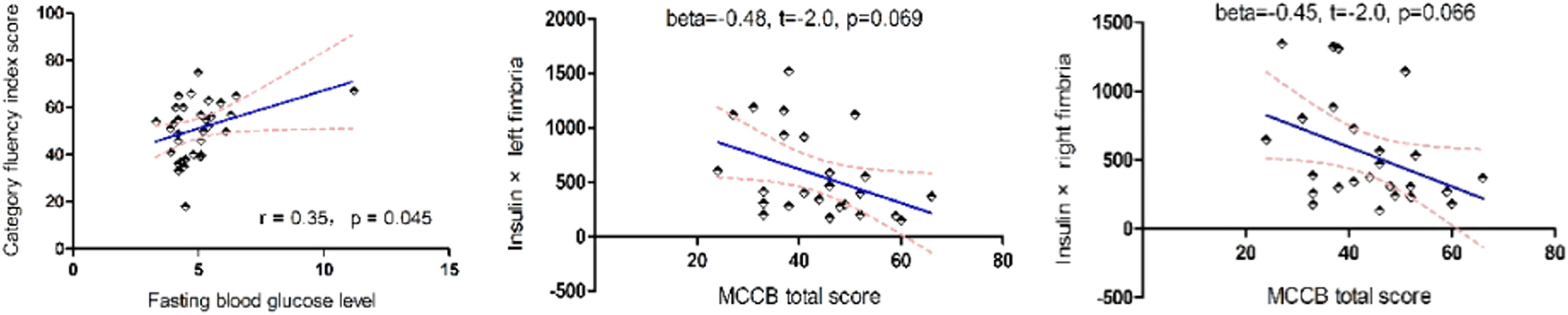
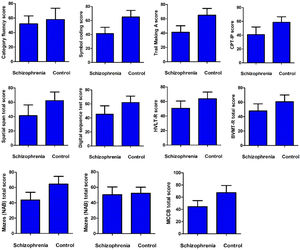
Cognitive function and glucose metabolismCompared with healthy controls, the MCCB total score and its index scores were significantly lower, except for category fluency index scores (p > 0.05) (Fig. 1). Correlation analysis showed a positive association between category fluency performance and fasting blood glucose level (r = 0.35, p = 0.045) or BMI (r = 0.35, p = 0.044) in ANFE patients (Fig. 2). There was no significant association between fasting insulin levels or HOMA-IR and cognitive function on MCCB (all p>0.05).
Comparison of cognition between first-episode patient and healthy control subject.
MCCB Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery, HVLT Hopkins Verbal Learning Test, BVMT Brief Visuospatial Memory Test, NAB Neuropsychological Assessment Battery, MSCEIT Mayer-Salovey-Caruso Emotional Intelligence Test, CPI-IP Continuous Performance Test: Identical Pairs.
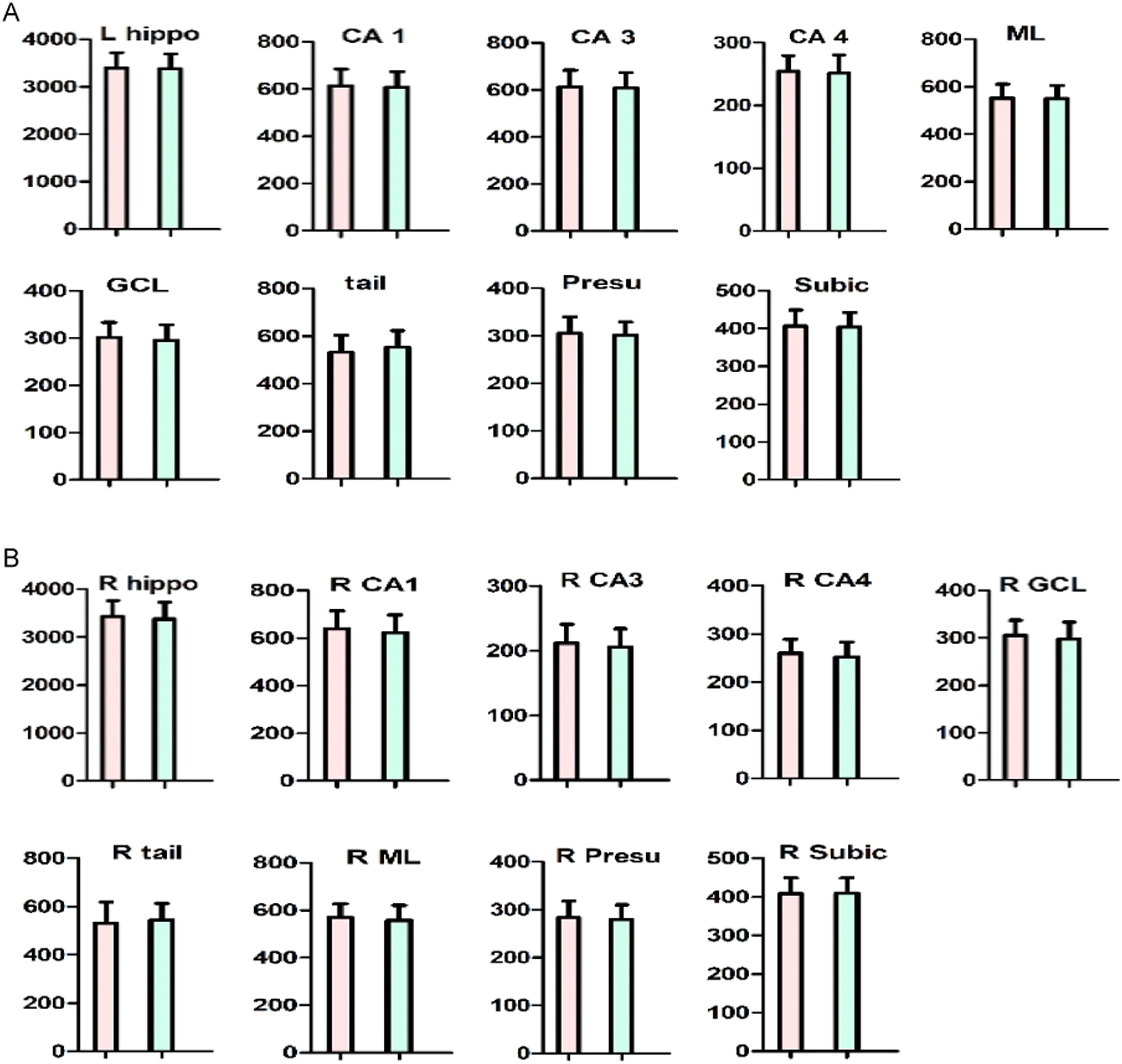
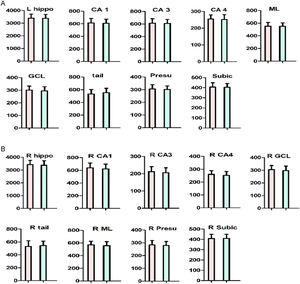
There was no significant difference in hippocampal subfields between ANFE patients and controls (all p>0.05) (Fig. 3). Also, there was no significant sex-by-group interaction on all hippocampal subfields (p>0.05). The age range of subjects in this study was 18∼45 years, although all subjects were within 5 years of a psychotic episode, which is a wide range. We compared cognitive function, hippocampal subfields, and other variables between younger (≤30 years) and older (>30 years) subjects. The results revealed no significant differences in cognitive function, glucose metabolism parameters, or all hippocampal subfields (all p>0.05) except for left parasubiculum volume (F = 7.8, p = 0.008), which did not pass Bonferroni correction (adjusted p>0.05).
Comparison of hippocampus subfields between first-episode patient and healthy control subject.
A. The left hippocampus fields. B. The right hippocampus fields.
The red bar indicates schizophrenia. The blue bar indicates control subjects. The Y-axis refers to hippocampus subfield volumes.
Insulin was positively correlated with subfield volumes of left fimbria (r = 0.42, p = 0.009), right subiculum (r = 0.36, p = 0.025), and right fimbria (r = 0.42, p = 0.008). HOMA-IR was positively associated with subfield volumes of left presubiculum (r = 0.35, p = 0.032), left parasubiculum (r = 0.45, p = 0.005), left fimbria (r = 0.35, p = 0.035) and right subiculum (r = 0.33, p = 0.049). There was no significant association between fasting blood glucose, HDL and hippocampal subfields in patients (all p>0.05).
After controlling for age, education, sex, and ICV, there was a significant association between the trial-making test (TMT) index score and right hippocampal fissure subfield volume (r=−0.42, p = 0.021) in patients. In addition, there was a significant association between Hopkins Verbal Learning Test-Revised (HVLT-R) total score and subfield volumes of left CA3 (r=−0.38, p = 0.036), left hippocampal fissure (r=−0.42, p = 0.021) and right hippocampal fissure (r=−0.39, p = 0.033). However, none of these results showed significant differences after the Bonferroni correction.
For healthy controls, there were significant correlations between the Symbol Coding (SC) index score and subfield volumes of the right hippocampal tail (r= −0.52, p = 0.013) and left hippocampal fissure (r= −0.56, p = 0.006), after controlling for sex, age, education, and ICV. Moreover, a significant association was observed between left CA3 and Mazes index score. In addition, the Category Fluency (CF) index score was associated with subfield volumes of left CA1 (r=−0.48, p = 0.024), left CA3 (r=−0.43, p = 0.048), and left hippocampal fissure (r=−0.58, p = 0.005).
Inter-relationship between abnormal glucose metabolism, hippocampal subfields, and cognition in patients and controlsLinear regression analysis revealed that the insulin × right fimbria or insulin × left fimbria interaction was an independent predictor for the MCCB total score only in ANFE patients (beta=−0.48, t=−2.0, p = 0.069 for left fimbria; beta=−0.45, t=−2.0, p = 0.066 for right fimbria) (Fig. 3). However, in control subjects, there was no correlation between the interactive effects of glucose or insulin and hippocampal subfields on the MCCB total score or its subscales (all p > 0.05).
DiscussionThis study is the first to evaluate glucose metabolism abnormalities and hippocampal subfields in SZ. It had several findings. 1) The fasting blood glucose level of ANFE patients was significantly positively correlated with category fluency performance, and higher fasting glucose levels were associated with better performance on the category fluency test. 2) The fasting insulin level was positively correlated with the left fimbria, right fimbria, and right subiculum of the hippocampal subfields, while HOMA-IR was positively associated with the left presubiculum, left parasubiculum, left fimbria, and right subiculum in SZ patients. 3) Multiple hippocampal subfields were correlated with category fluency performance or spatial span total score. 4) The interactive effect between abnormal insulin levels and hippocampal subfield volume destruction affected the cognitive function in ANFE patients.
We found that ANFE patients displayed a significant decline in several domains of cognitive function assessed by MCCB, which is consistent with the majority of previous studies (Zhang et al., 2019; Tang et al., 2020; Xiu et al. 2021), indicating widespread cognitive impairment in SZ patients. We also found elevated levels of fasting insulin, glucose, and HOMA-IR in ANFE patients, suggesting that glucose metabolism may be impaired during the early disease course of SZ. It is worth noting that we found a significant association between fasting blood glucose levels and category fluency performance in patients, which is in line with a previous study (Zhang et al. 2020). The integrity of brain-encoded information associated with semantic memory can be tested by category fluency, in which people are asked to name all members of a particular category (e.g., clothes, furniture, or animals) (Bokat & Goldberg 2003). Category fluency has been reported to be associated with speed, executive, and semantic impairments in SZ (Egeland et al., 2018). In this study, we found that higher category fluency scores were associated with better semantic memory, suggesting that higher fasting glucose levels in SZ patients were associated with better performance on the category fluency test and semantic memory.
However, the exact mechanisms underlying the relationship between disturbed glucose metabolism and cognitive function in SZ patients remain unclear. In particular, the relationship between glucose and cognitive function in SZ was contrary to our expectations. We proposed the following potential mechanisms to explain this relationship. It is well known that glucose homeostasis and downstream signaling pathways are involved in synaptic plasticity. Glucose provides energy for the biosynthesis of neurotransmitters involved in brain plasticity directly related to learning and memory (Suzuki et al., 2011). Craft et al. found that intranasal insulin therapy can improve the memory of patients with Alzheimer's disease (AD) and mild cognitive impairment (Craft et al., 2012). Moreover, patients with a first episode of acute psychosis experience significant psychosocial stress (Zhang et al., 2005). It is known that high hypothalamic-pituitary-adrenal (HPA) axis activation accompanied by an increase in circulating cortisol may increase glucose levels (Łopuszańska et al., 2014). Furthermore, acute exposure to stress and increased cortisol concentrations, learning, and memory willed be modulated and adapted to avoid re-exposure to adverse events, although the evidence is mixed (Sousa et al., 2021). It is speculated that acute stress may lead to increased glucose levels and worsen cognitive function, particularly memory, in SZ patients.
The second finding was that increased fasting insulin and higher HOMA-IR were significantly correlated with hippocampal subfield volumes, particularly presubiculum, parasubiculum, and fimbria in patients with SZ. In the brain, insulin receptors are widely expressed in the hippocampus, which functions to control metabolism and energy homeostasis (Zhao & Alkon 2001). Moreover, most of its energy is consumed to initiate action potentials and postsynaptic potentials (Howarth et al., 2012). In mice, alterations of insulin/IR signaling in the hippocampus may impair hippocampal plasticity (Soto et al., 2019). Interestingly, insulin may exert either trophic or harmful effects on the hippocampus depending on the timing and the duration of insulin stimulation. Over-nutrition changes the levels of glucose and insulin in the hippocampus and alters synaptic functions in the dentate gyrus (Lee et al., 2002; Mainardi et al., 2015). Notably, the average levels of fasting insulin and mean HOMA-IR were within the range of normal blood levels in ANFE patients, which can explain why we found positive associations between fasting insulin levels and hippocampus subfield volumes. Altogether, these studies support the idea that insulin-related signal regulates hippocampal structure and functions, actively involved in plasticity processes in the hippocampus.
Finally, despite being a weak effect, we did find that the interaction of insulin and hippocampus fimbria was an independent predictor of category fluency performance in ANFE patients only. This finding was consistent with a previous study that reported a close relationship between fasting insulin and the hippocampus in diabetic patients. Glucose metabolic homeostasis is now widely considered to have an impact on the activation of hippocampal plasticity, as it is a process with high energy demands, and its alterations have profound influences on cognitive function (Mainardi et al. 2015). A previous review regarding the effects of the environment on brain plasticity processes found that the combination of physical exercises and social activities dramatically influenced the neuroplasticity of the hippocampus in adulthood. Notably, this effect was associated with an improvement in glucose tolerance (Mainardi et al., 2010). Therefore, the current study provides further clinical evidence to understand the impact of the interaction between glucose metabolism molecules and hippocampal synaptic plasticity on cognitive function at the onset of SZ patients.
This study had several limitations. In the current scenario, measuring blood glucose and insulin levels is not sufficient to examine glucose metabolism and insulin resistance, while performing an oral glucose tolerance test along with measuring insulin levels at different time points may provide more realistic. It was a cross-section study and we did not conduct a second MRI scan after treatment with antipsychotics. Therefore, we cannot draw a definite conclusion about glucose metabolic abnormalities and hippocampal synaptic plasticity on the SZ-associated cognitive impairment. Also, the sample size is not large, which may cause a possible bias in the statistical analyses. Lastly, most of the findings in this study were correlational, meaning that causation could not be attributed to either of the correlated variables. This central design feature of the study was also a limitation.
In conclusion, we found a comprehensive picture of the inter-relationships between glucose metabolism, hippocampal subfields, and cognitive function in ANFE SZ. In addition to providing new evidence for the role of glucose metabolism interacting with hippocampal subfield volumes in the cognitive decline of SZ patients, we provide further evidence for the neurodevelopmental or neurodegenerative hypothesis of early stage of SZ from the perspective of a single risk factor and etiology, leading to a complex understanding of the mechanisms involved in the onset of SZ. Our findings, if replicated in future prospective, longitudinal cohort studies, will have many important clinical implications for the treatment of SZ to elucidate the causal relationship between abnormal glucose metabolism, reduced hippocampal subfields, and cognitive impairment. It also provides evidence of metabolic disturbances for the prediction of cognitive impairment in the early stages of this disease by translational methods.
FundingThis study was supported by grants from the Science and Technology Program of Guangzhou (202206060005, 202201010093, SL2022A03J01489), Guangdong Basic and Applied Basic Research Foundation Outstanding Youth Project (2021B1515020064), Medical Science and Technology Research Foundation of Guangdong (A2023224), the Health Science and Technology Program of Guangzhou (20231A010036), Scientific research project of traditional Chinese medicine of Guangdong (20211306), Guangzhou Municipal Key Discipline in Medicine (2021–2023), Guangzhou High-level Clinical Key Specialty, and Guangzhou Research-oriented Hospital. The authors report no biomedical financial interests or potential conflicts of interest.
Ethics approval and consent to participateThis study was approved by the Ethics Committee of Beijing Huilongguan Hospital. Each participant provided written informed consent.
Consent for publicationAll authors have read and approved the content and agree to submit it for consideration for publication in the journal.
Availability of data and materialNot applicable.
We would like to thank the participants in the study and their families.