In recent years several meta-analyses regarding resting-state functional connectivity in patients with schizophrenia have been published. The authors have used different data analysis techniques: regional homogeneity, seed-based data analysis, independent component analysis, and amplitude of low frequencies. Hence, we aim to perform a meta-analysis to identify connectivity networks with different activation patterns between people diagnosed with schizophrenia and healthy controls using voxel-wise analysis. Method: We collected primary studies exploring whole brain connectivity by functional magnetic resonance imaging at rest in patients with schizophrenia compared with healthy controls. We identified 25 studies included high-quality studies that included 1285 patients with schizophrenia and 1279 healthy controls. Results: The results indicate hypoactivation in the right precentral gyrus and the left superior temporal gyrus of patients with schizophrenia compared with healthy controls. Conclusions: These regions have been linked with some clinical symptoms usually present in Plea with schizophrenia, such as auditory verbal hallucinations, formal thought disorder, and the comprehension and production of gestures.
Schizophrenia is a severe, complex, and heterogeneous mental illness that causes severe disturbances in behaviour and cognition. This disease is caused by genetic and environmental factors and usually emerges in adolescence or early adulthood (Owen et al., 2016). Various structural and functional neuroimaging studies have tried to relate the myriad clinical manifestations of this disease with different structural and functional brain anomalies (Van Den Heuvel & Fornito, 2014). A growing body of literature conceptualises schizophrenia as a disorder with a significant dysfunction of brain connectivity within and between different large-scale brain networks.
These connectivity disturbances, in turn, are closely related to the different symptomatic dimensions of schizophrenia (Dong et al., 2018; Friston & Frith, 1995; Li et al., 2019; Van der Heuvel & Fornito, 2014). For example, the severity of positive symptoms has been associated with anomalies in the connectivity strength between the frontal and temporal regions. In contrast, the severity of negative symptoms has been related to decreases in whole-brain functional connectivity (Skudlarski et al., 2010). Researchers have found alterations in the default mode network (DMN), which is related to self-referential thoughts. Some studies have shown that resting-state functional connectivity (rsFC) decreases in patients with schizophrenia, compared with healthy controls (HC), between the posterior cingulate cortex (PCC) and the medial prefrontal lobes, the lateral prefrontal cortex, the parietal cortex, and the insula (Bluhm et al., 2007; Moran et al., 2013). Nevertheless, other authors suggest a pattern of hyperconnectivity within the DMN in this illness (He et al., 2013; Woodward et al., 2011). In addition, the reduced connectivity within the DMN of patients with schizophrenia in the precuneus may correlate with the severity of the negative symptoms, specifically in the apathy domain (Forlim et al., 2020). Other researchers have shown rsFC anomalies in patients with schizophrenia in the cognitive control network (CCN) both at rest (Horne et al., 2021) and when performing cognitive control tasks (Fornito et al., 2011; Ray et al., 2017). As its name suggests, the alterations in this large-scale brain network are closely related to the neuropsychological deficits frequently found in schizophrenia (Ray et al., 2017). The salience network (also known as the core network [CN]) comprises the ACC, the anterior insula, and the dorsolateral prefrontal cortex (DLPFC) and plays an important role in processing the interoception and salience detection of external stimuli (Palaniyappan & Liddle, 2012). Some studies reported that this brain network presents abnormal rs-FC in schizophrenia (Menon et al., 2022; Palaniyappan & Liddle, 2012). Among other symptoms, its alteration may be related to reality distortions frequently found in these patients (Menon et al., 2022; Palaniyappan & Liddle, 2012).
Dysfunctions of the aforementioned brain connectivity networks are related to various groups of schizophrenia symptoms. However, abnormal functioning of other brain connectivity networks has been linked to somewhat more specific symptoms often found in patients with schizophrenia. Thus, alterations in the self-reference network that includes the ventromedial prefrontal cortex, the orbitofrontal cortex, the ACC, and the gyrus rectus could be the basis for the presence of delusions of reference in this disease (Lariviere et al., 2017). Abnormalities in brain areas of the auditory network that include the bilateral middle and superior temporal cortex, Heschl's gyrus, and the temporal pole have been related to the presence of auditory hallucinations (Woodruff et al., 1997). The language network comprises structures mainly in the left hemisphere, such as the inferior frontal gyrus, the frontal operculum, the superior temporal gyrus, the middle temporal gyrus, and the inferior parietal lobe (Friederichi & Gierhan, 2013). Structural and functional alterations within this network and in some other brain areas outside the language network have been associated with formal thought disorder in schizophrenia (Cavelti et al., 2018). Furthermore, alterations in the praxis network, including the superior frontal gyrus, the inferior frontal gyrus, the superior temporal gyrus, the parietal cortex, and the precentral gyrus, among other structures, could be related to the gesture alterations often observed in schizophrenia (Walther, Mittal, et al., 2020).
Likewise, researchers have identified alterations in the connectivity between these cerebral networks. For example, Woodward et al. (2012) suggest that patients with schizophrenia present reduced segregation between the DMN and the CCN compared with HC. In the same study, however, the authors found no differences in the connectivity between the CN and other brain networks. In contrast, Huang et al. (2019) showed evidence of a hyperconnectivity of the CN and the prefrontal cortex and cerebellum, as well as a hypoconnectivity between the cortico-striatal-thalamic-cortical subcircuit and the CN in patients with schizophrenia.
It is clear that in schizophrenia, there are alterations within and between several large-scale rsFC brain networks. In addition, these alterations have been related more or less consistently to various symptoms of the illness. However, the anatomical locations of the connectivity effects and their nature (increases or decreases of connectivity) are less clear. In recent years, some meta-analyses have explored resting-state functional magnetic imaging (rs-fMRI) in patients with schizophrenia compared with HC to identify some regular patterns. For example, Xiao et al. (2017) showed evidence that patients with schizophrenia had increased connectivity, estimated with regional homogeneity (ReHo), in the right superior frontal and right superior temporal gyrus, as well as a decreased ReHo connectivity in the right fusiform gyrus, in the left superior temporal gyrus, the left postcentral gyrus, and the right precentral gyrus. In their meta-analysis, Dong et al. (2018) focused on seed-based studies, showing that patients with schizophrenia present hypoconnectivity in the DMN, the affective network (AN), the ventral attentional network (VAN), the thalamic network (TN), and the somatosensory network. They also showed hypoconnectivity between the VAN and the TN, the VAN and the DMN, the VAN and the CCN, the CCN and the TN, and the CCN and the DMN. They only found hyperconnectivity between AN and VAN. Li et al. (2019) focused on independent component analysis (ICA); their findings support hypoconnectivity in specific brain networks in patients with schizophrenia, specifically the auditory network, the core network, the self-referential network, the DMN, and the somatomotor network. Finally, Gong et al. (2020) showed that patients with schizophrenia present a decreased amplitude of low-frequency fluctuation (ALFF) in the bilateral postcentral gyrus, bilateral precuneus, left inferior parietal gyrus, and right occipital lobe. In addition, they found an increased ALFF in the right putamen, right inferior frontal gyrus, left inferior temporal gyrus and right anterior cingulate cortex.
We aim to perform a meta-analysis of functional connectivity in schizophrenia, focusing on the studies that performed whole-brain connectivity analyses in this disorder. The published meta-analyses in this field have not included the impact of the different analysis techniques (ICA, ReHo, ALFF, fractional amplitude of low-frequency fluctuation [fALFF], and other techniques) on the estimated effect sizes. Therefore, we analyse the possible relationships between the estimated effect size and these potential mediator variables. We also study the possible effect of other mediator variables on estimating the effect sizes: sample size; age; sex; and clinical characteristics of the illness, such as the Positive and Negative Syndrome Scale (PANSS) scores and the duration of schizophrenia.
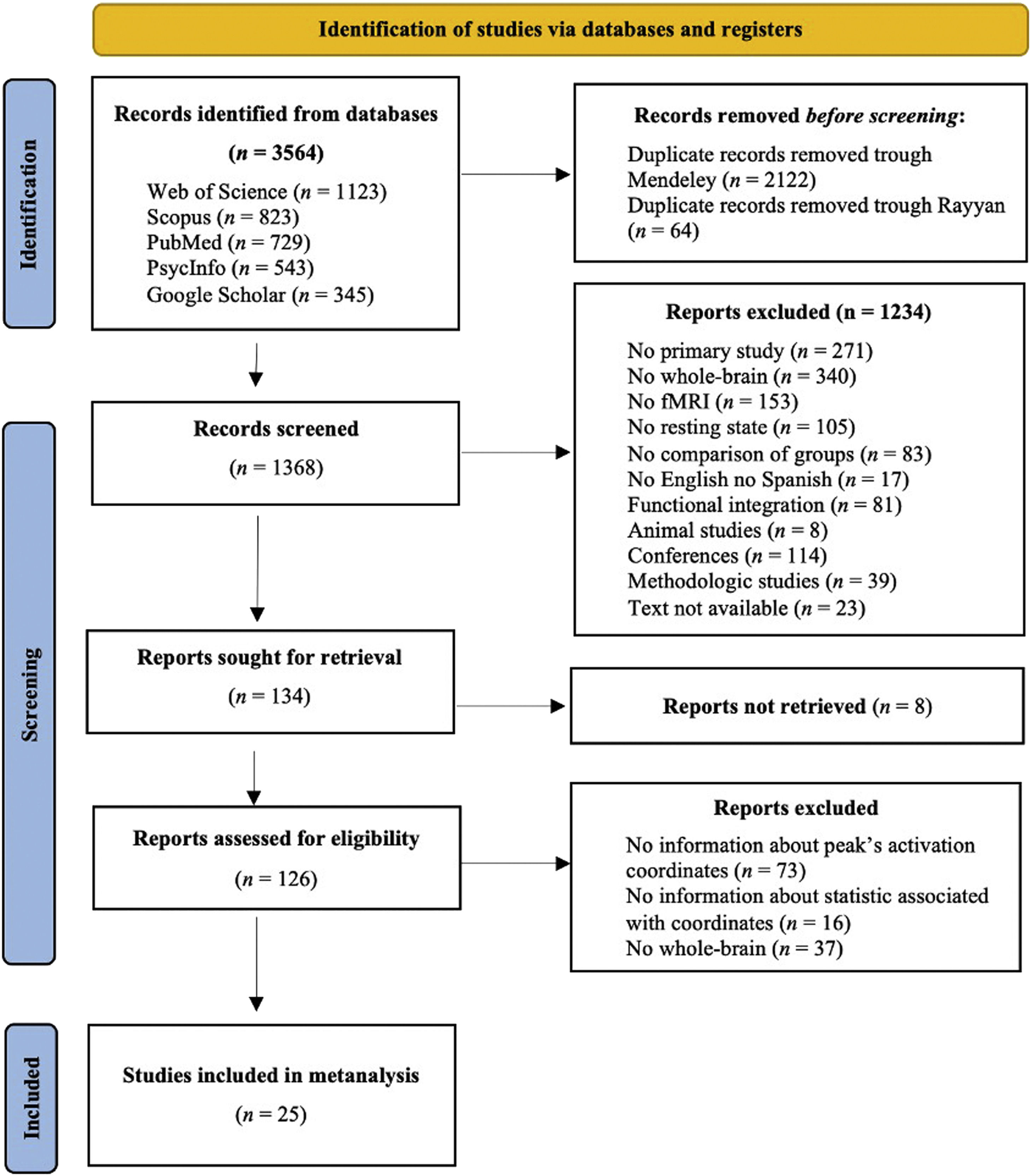
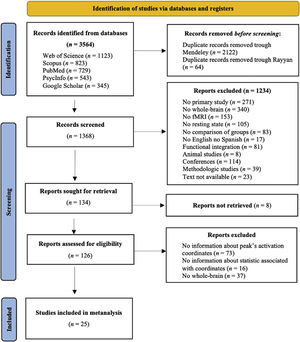
MethodsStudy selectionData sourcesTwo independent investigators performed a bibliographic search using the following databases: PubMed, Web of Science, PsycInfo, Google Scholar, and Scopus. Some examples of the Boolean algorithm with the keywords used are presented in the Appendix A. The search included all the studies published until 28 February 2021. We conducted this meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. The inclusion criteria were: (a) published in English or Spanish; (b) full-text was available; (c) primary study in the human population; (d) included a patient group diagnosed with schizophrenia following the Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV) criteria or the structured clinical interview for DSM-IV (SCID); (e) compared the brain activation between schizophrenia patients and healthy people; (f) used rs-fMRI; and (g) reported Montreal Neurological Institute (MNI) or Talairach coordinates of the whole brain contrast comparing people with schizophrenia to HC. The exclusion criteria were: (a) systematic reviews or meta-analyses; (b) methodological studies; (c) patients with schizophrenia with other psychiatric or neurological disorders; and (d) studies focused on dynamic connectivity or that used graph analysis or any other technique that does not identify coordinates. Fig. 1 shows the PRISMA flow chart. The search yielded a total of 3763 studies (Web of Science [n = 1123], Scopus [n = 823], PubMed [n = 729], PsycInfo [n = 543], and Google Scholar [n = 345]). Subsequently, we removed 2106 duplicate papers through Mendeley and 64 duplicate papers via Rayyan. In addition, we excluded 1234 studies after title/abstract screening because they did not meet the inclusion criteria. Later, 159 articles were sought to read the full text, but 8 studies could not be found. Thus, after the full-text screening, we excluded 73 studies because they did not indicate the peak's activation coordinates, 16 because they did not report on the statistics associated with the coordinates, and 37 because they did not analyse whole brain. Finally, we included 25 studies meeting the inclusion criteria; they aren’t marked in the refernce list. In addition, there was a 100% rate of agreement between the two investigators for the study search and selection.
Voxel-wise meta-analysisWe used the seed-based d Mapping (SDM) software (available at http://www.sdmproject.com) to analyse the differences between patients with schizophrenia and HC. The approach details are described by Müller et al. (2018) and Radua and Mataix (2012). First, we chose the reported peak coordinates of all functional differences that were significant at whole brain level in these studies. We ensured that all included studies used the same statistical threshold throughout the whole brain to avoid possible bias towards regions with liberal thresholds. Thus, we considered the minimum threshold to be defined by a p-value of .001 and Student's t reference value (to minimise the probability of type I error) with the degrees of freedom (df) of each study estimated by the conventional expression (n1 + n2 – 2). Second, we recreated peak coordinates for each study with a standard MNI map of the group differences effect size based on their peak t value by means of a non-normalised Gaussian kernel to the voxels near the peak, which assigns higher values to the voxels closer to peaks. Third, we obtained the mean map by voxel-wise calculation of the study maps random-effects mean, weighted by the sample size. Fourth, to balance sensitivity and specificity correctly, we used a p-value of .05 as the main threshold with an additional peak height of z = 1. We performed jackknife sensitivity analysis to test the replicability of the results. Finally, we considered it appropriate to incorporate papers that have used different estimation techniques. Thus, to the usual estimates based on ALFF, fALFF, or ReHo, we included studies that have proposed some more secondary techniques such as long short functional connectivity (LSFCS), voxel-mirrored homotopic connectivity (VMHC), global function connectivity (GFC), and parameter of asymmetry (PAS). The incorporation of different techniques is justified by the following arguments. First, all of them have a common point, which is the use of linear models in their origin, so the parameter estimates, although different, are based on a very similar model. Second, most studies use ALFF, fALFF, or ReHo, so the rest of the techniques are underrepresented and incorporating them would allow for a more realistic average effect size estimate. This underrepresentation prevents their evaluation as categories of a possible moderating variable, because this type of analysis is limited to comparing between ALFF and ReHo, which are the most common. Finally, there are some antecedents that have applied the same criteria as in this meta-analysis but in other populations of interest. For all these reasons, we considered it would be interesting to incorporate the various estimation techniques (Zhang et al., 2020) also in accordance with what was Kühn and Gallinat (2013) proposed with fALFF and ICA.
After calculating Cohen's d and the confidence intervals (CI) for the papers, we conducted a descriptive analysis of each paper to clarify the results. To control false positives, we used the correction described by Albajes-Eizagirre et al. (2021), which is based on the permutation of images to minimise the error.
Quality assessmentWe assessed the quality of the included studies by using a checklist consisting of 11 items that focused on the clinical characteristics of the participants, the neuroimaging and data analysis methodology, the results, and the conclusions of the studies. The Quality Assessment scale is shown in Appendix B. This checklist is based on previous meta-analyses and has been described elsewhere (Chen et al., 2015; Shepherd et al., 2012). One author reviewed the included studies and determined a complete rating, two investigators discussed the resulting scores, and a consensus quality score was obtained.
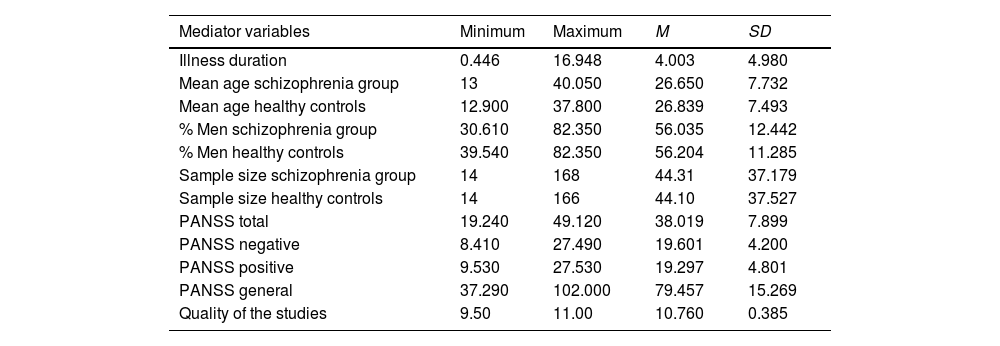
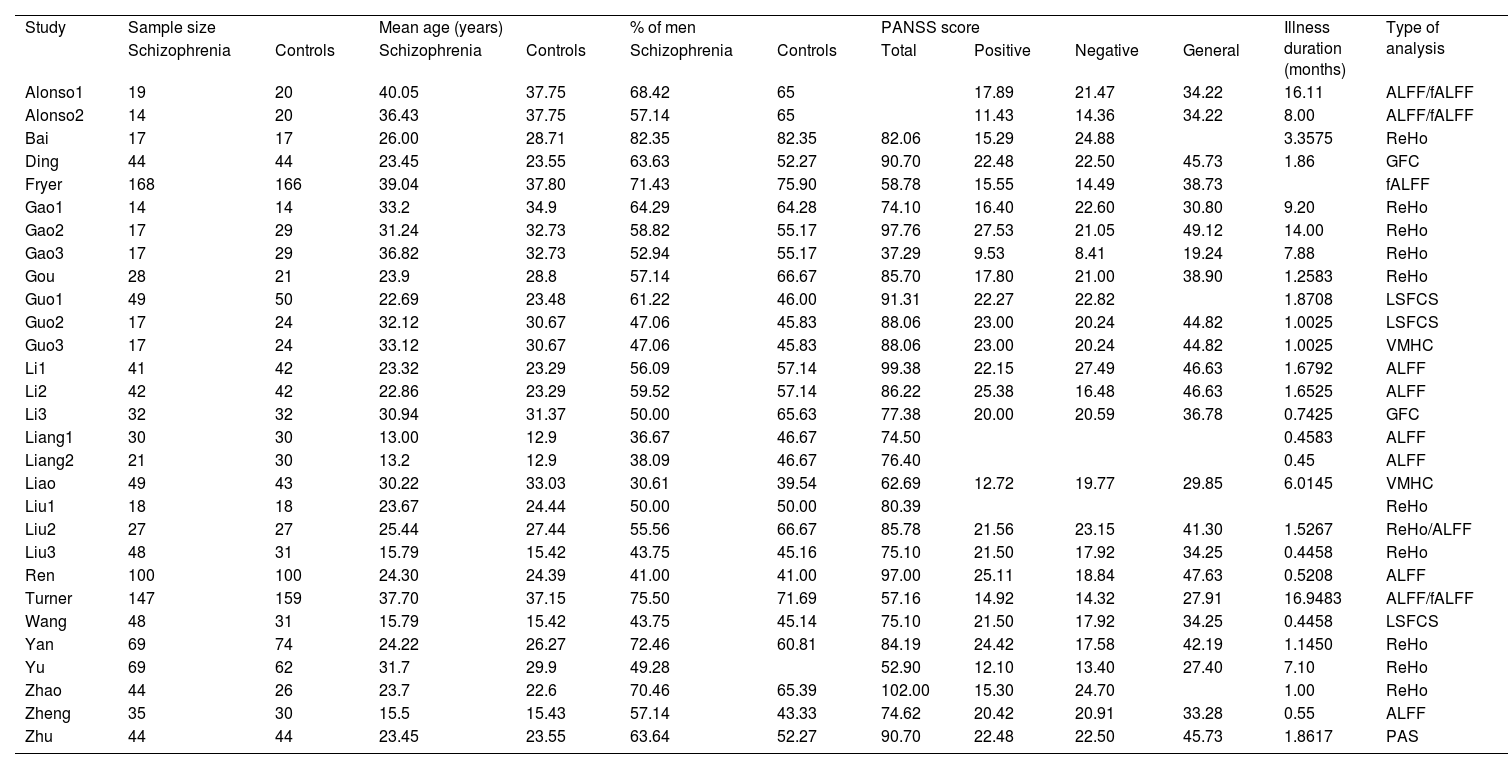
ResultsStudies included in the meta-analysisAppendix C shows the data obtained in each study to describe each mediator variable for each analysed paper. Table 1 shows the basics descriptive statistics of the mediator variables. We want to highlight that the large number of patients with schizophrenia (n = 1285) and HC (n = 1279) in the included studies. Regarding the technique used to estimate connectivity networks, the most common are ALFF (20.68%) and ReHo (34.48%).
Descriptive statistics of the observed distributions of mediator quantitative variables.
Note. PANSS, Positive and Negative Syndrome Scale.
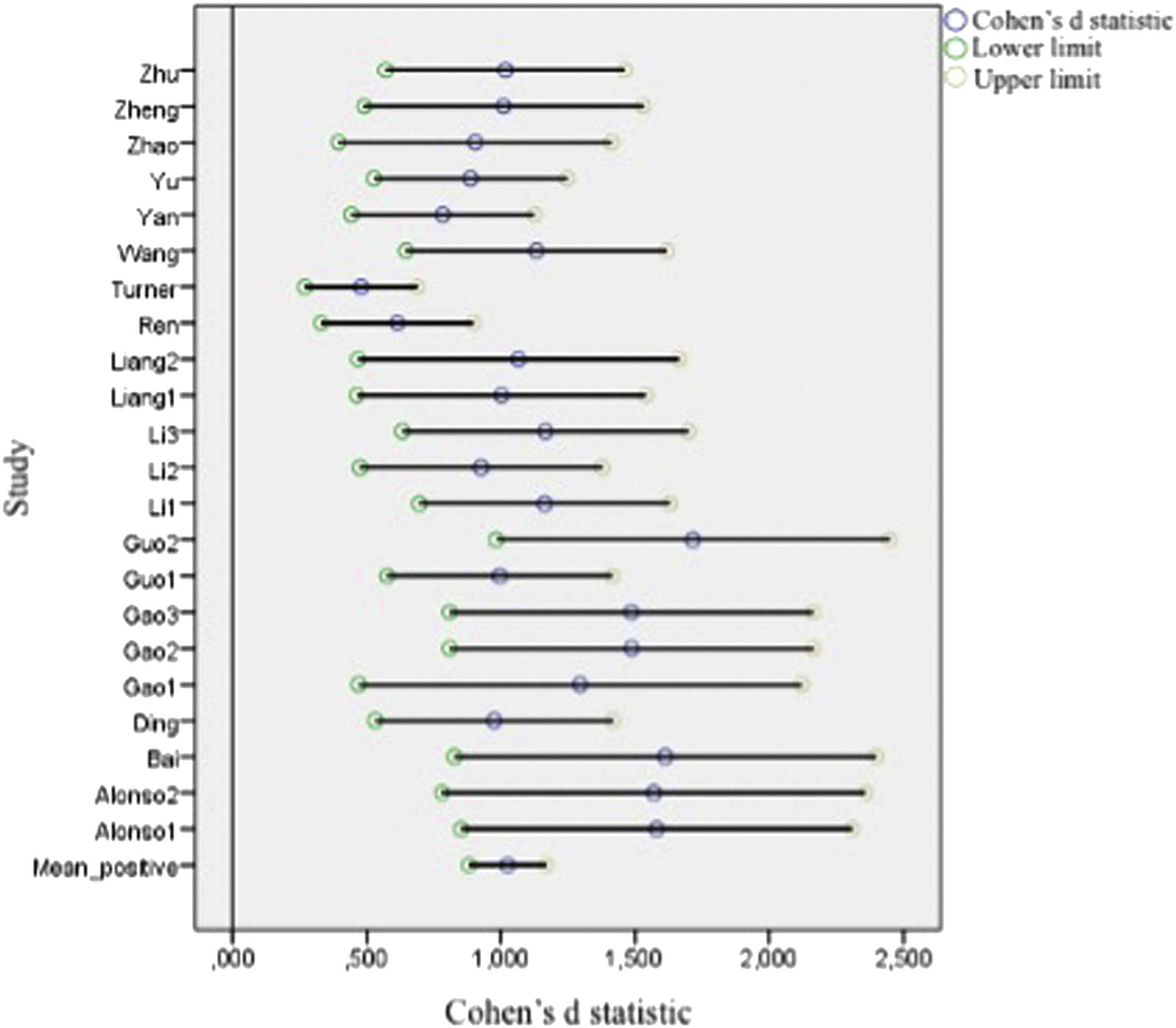
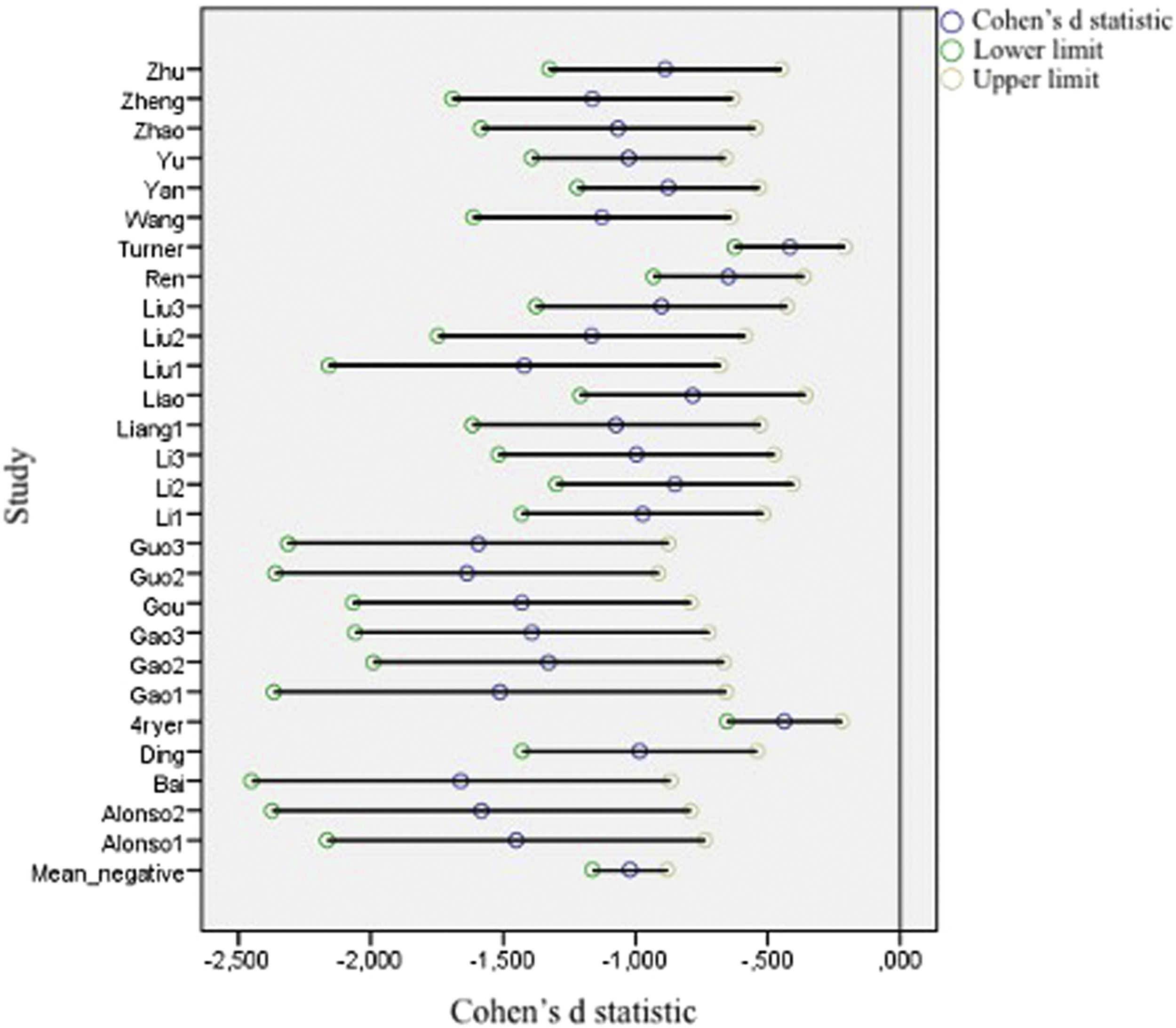
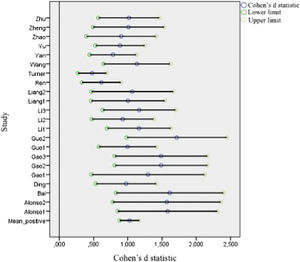
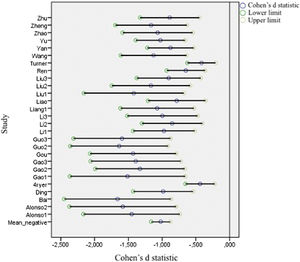
Figs. 2 and 3 present forest plots with the mean effect size and 95% CI for each study. If the effect size is negative, then the patient group shows decreased activation compared with the HC. The most significant positive effect size is 1.715, with an upper limit of 2.448. On the other hand, the highest negative effect size is -1.673, with a lower limit of -2.361. Notably, in the case of negative differences, Fryer et al. (2015) and Turner et al. (2013) show a lower CI width. Turner et al. (2013) shows the most accurate interval in the case of positive differences.
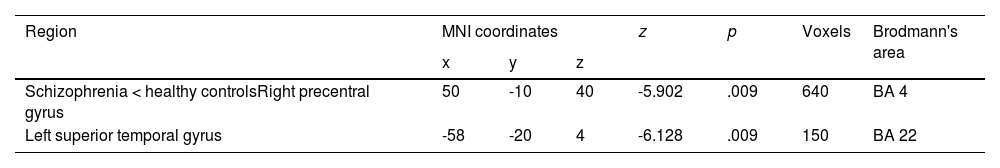
In our meta-analysis, patients with schizophrenia do not show hyperactivation compared with HC. However, they show decreased activation in the right precentral gyrus, specifically in Brodmann area (BA) 4. In addition, they show hypoactivation in the left superior temporal gyrus corresponding to BA 22. We found two clusters: one with 640 voxels and one with 150 voxels. The results are displayed in Table 2.
Major differences in activation between patients with schizophrenia and healthy controls in the present meta-analysis.
| Region | MNI coordinates | z | p | Voxels | Brodmann's area | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Schizophrenia < healthy controlsRight precentral gyrus | 50 | -10 | 40 | -5.902 | .009 | 640 | BA 4 |
| Left superior temporal gyrus | -58 | -20 | 4 | -6.128 | .009 | 150 | BA 22 |
Note. MNI, Montreal Neurological Institute
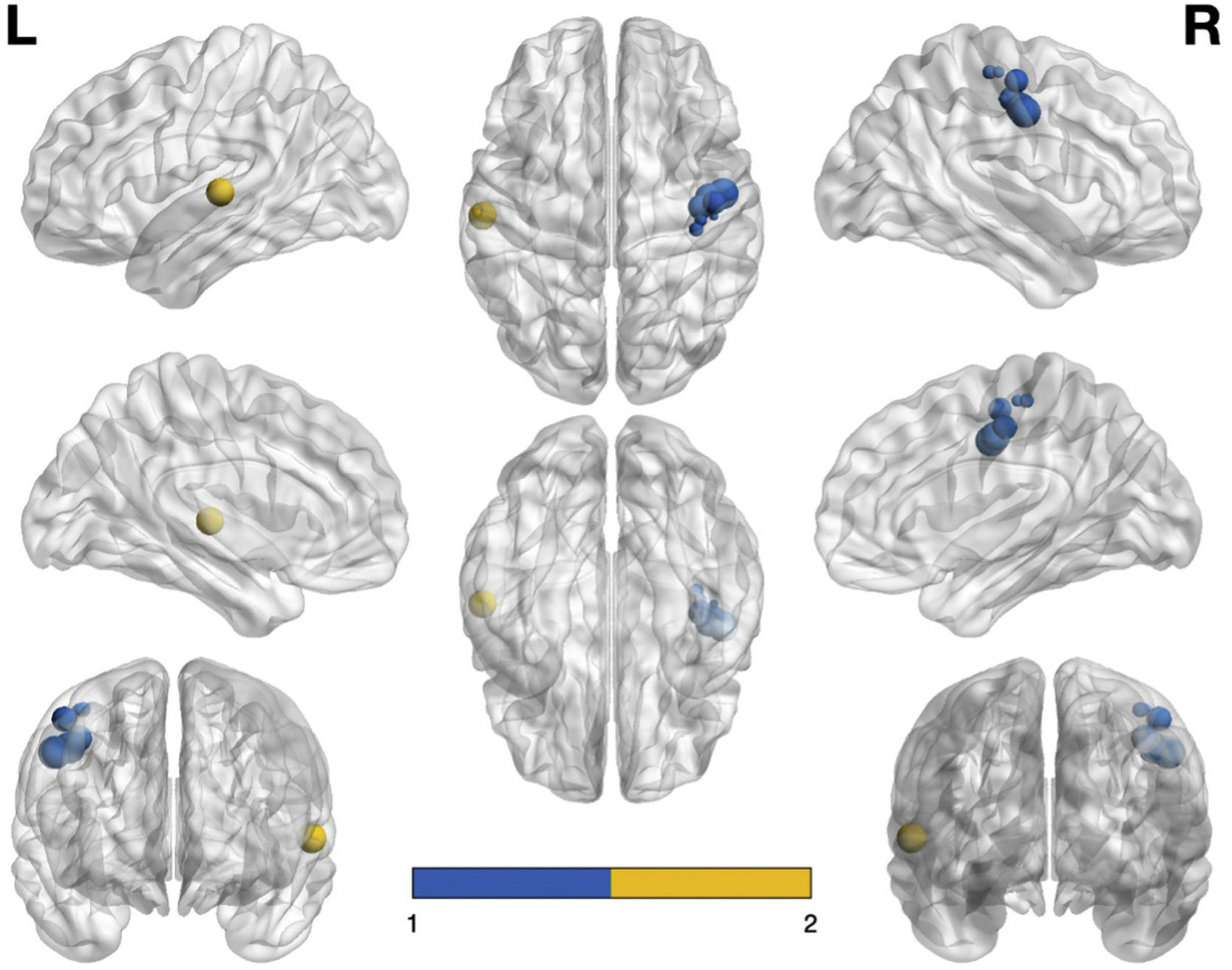
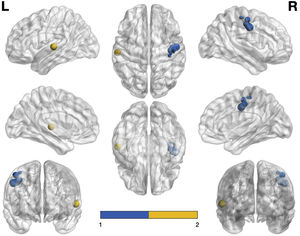
Fig. 4 shows the graphical representation of the areas that are hypoactivated (visualised with the BrainNet Viewer, http://www.nitrc.org/projects/bnv/; Xia et al., 2013). Note that the size of the node is proportional to the voxels it represents – in other words, the larger the node, the more voxels there are in that area and the larger the region it represents. It is also worth noting that the blue nodes correspond to the right precentral gyrus (BA 4) and the yellow nodes refers to the left superior temporal gyrus (BA 22).
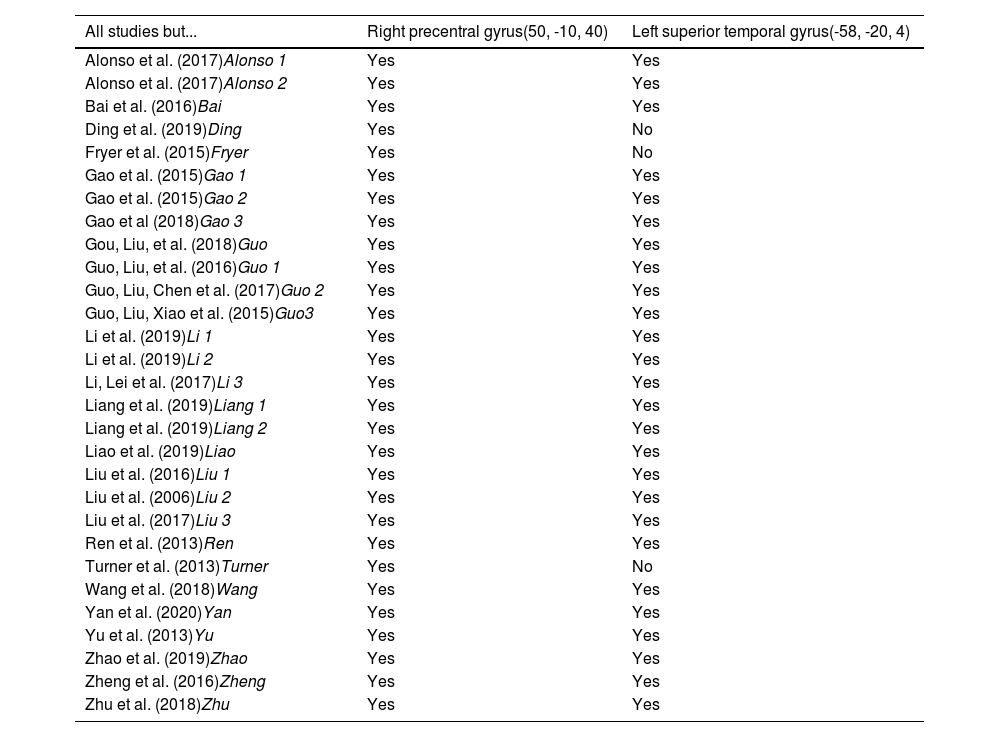
Reliability analysisWe conducted a jackknife sensitivity analysis (Table 3) to check the replicability of the results. This analysis revealed that the right precentral gyrus (with the coordinates 50, -10, 40) is replicable in all 29 datasets (each dataset with one study left out; 100% reliability), and the left superior temporal gyrus (with the coordinates -58, -20, 4) is replicable in 26/29 datasets (89.66% reliability). Therefore, the results have very high reliability.
Jackknife sensitivity analysis.
Note 1. In italics, an abbreviation of each study has been added for identification in the forest plots.
Note 2. Yes, the region is reported if we extract the mentioned study for the analysis; No, the region is not reported if we extract the mentioned study for the analysis
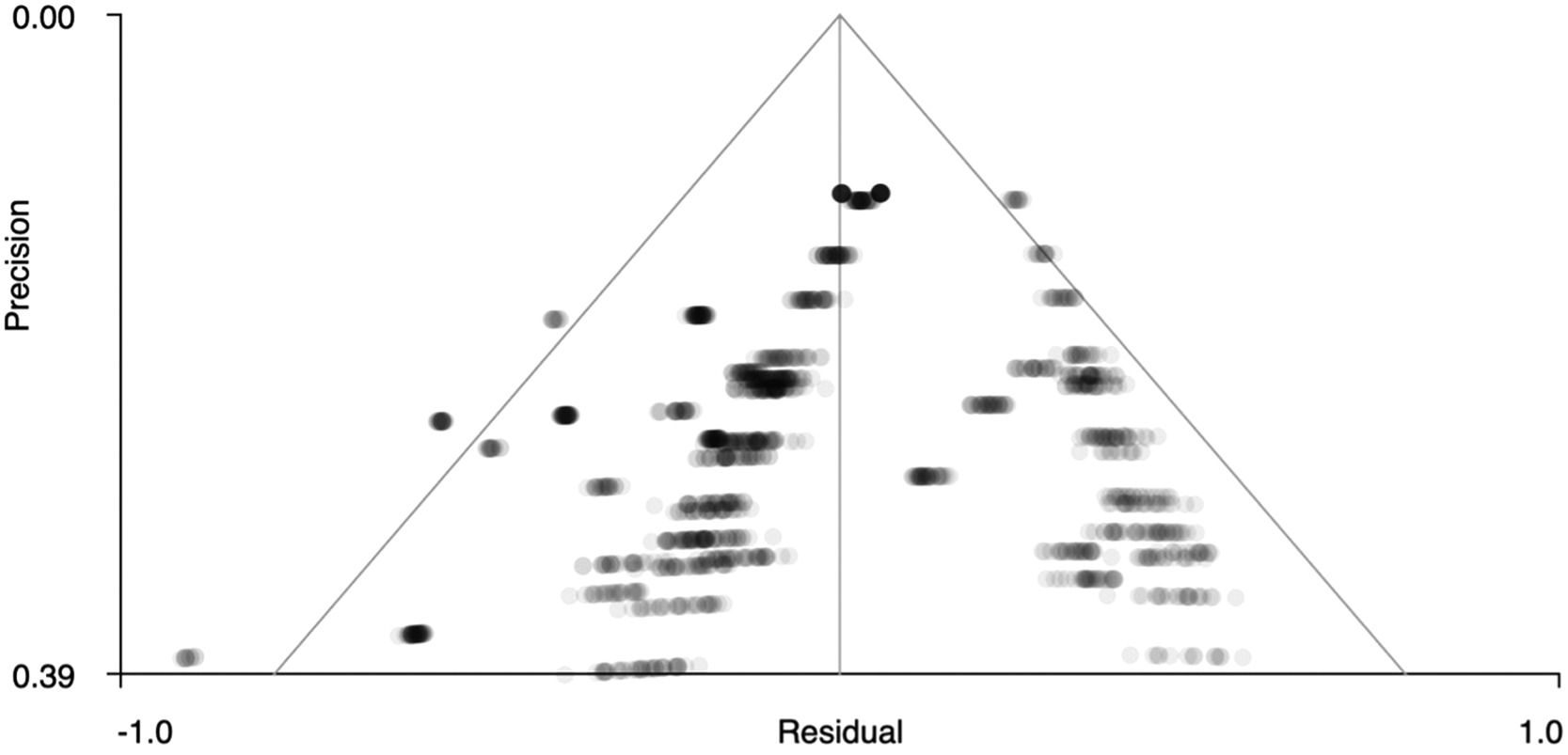
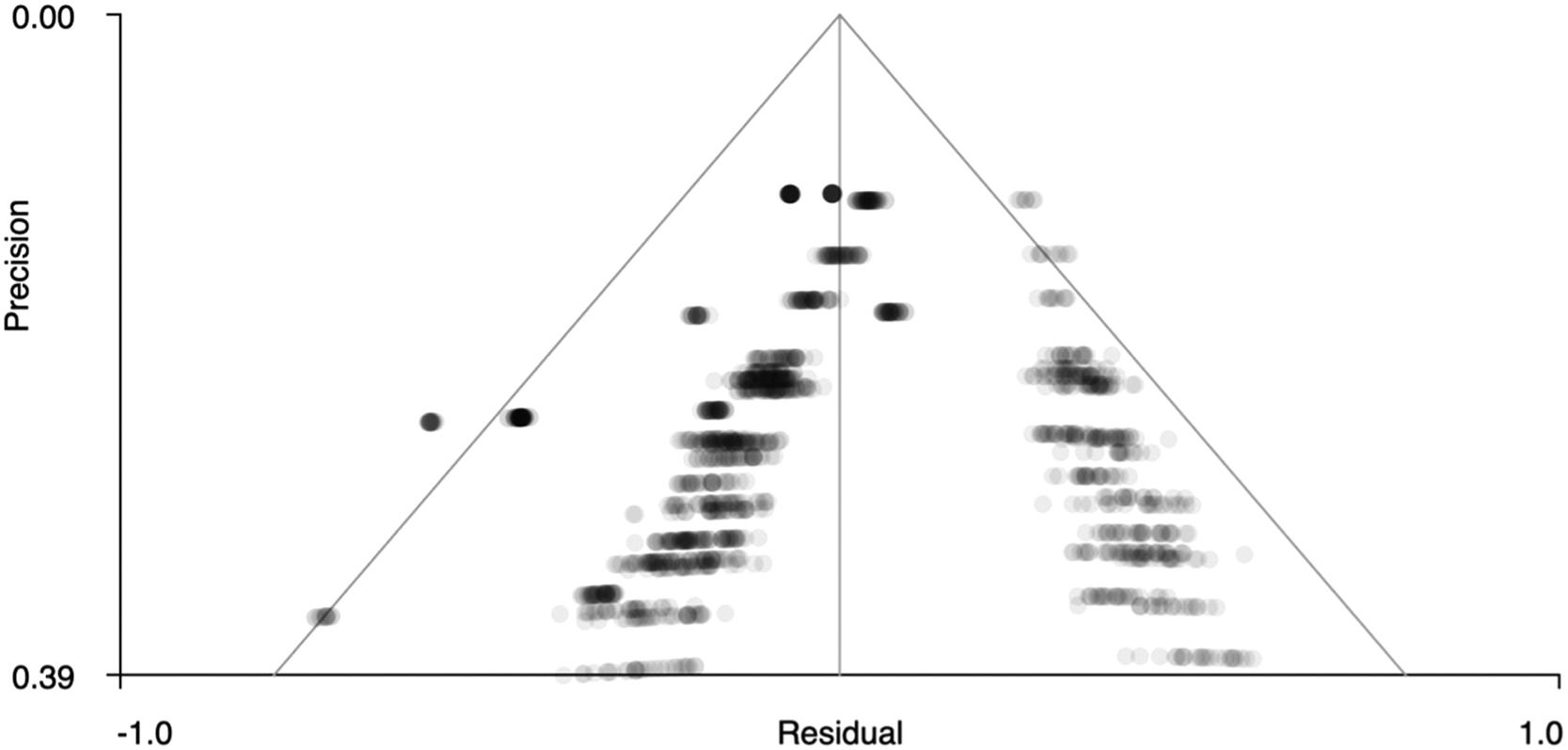
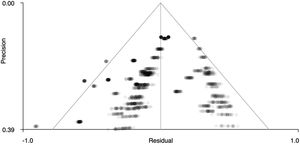
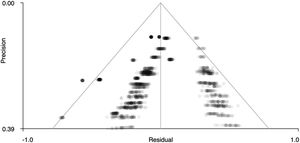
Figs. 5 and 6 show funnel plots that graphically present publication bias. The graphs suggest that there is no publication bias because the points – which correspond to the effect sizes of each study – are distributed uniformly on both sides of ‘0’ on the x-axis (effect size). Therefore, in our case we can studies that have been published with more and less significant effects. In addition, Table 4 shows the z statistics, as well as its significance (p = .504 for the right precentral gyrus and p = .751 for the left superior temporal gyrus), which indicates that there is no bias.
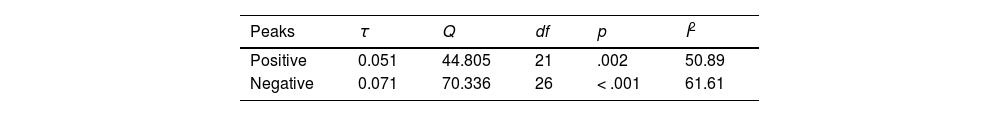
To determine the possible heterogeneity between the studies included in the present meta-analysis, we estimated Q and I2 statistics and well as the values of tau (τ), which represents the variance of the effect size distribution (Table 5). Q statistics from both positive and negative peaks are significant (p < .001). In this sense, there is heterogeneity between the studies, so it is appropriate to explore the mediator variables that could explain this phenomenon. Moreover, the degree of heterogeneity (I2) is 50.89% for positive peaks and 61.61% for negative peaks, indicating a moderate degree of heterogeneity.
As mentioned above, the fact that there is heterogeneity between the studies included in the meta-analysis led us to analyse possible mediator variables that could explain the variability between the effect sizes (here considered in absolute value). Because some of the included studies have more than one significant effect, we calculated the mean effect size for each paper. We explored the following variables: the type of data analysis used (ReHo, ALFF, fALFF, etc.); sample size; age; sex; total, general, positive, and negative PANSS scores applied to the patients with schizophrenia; illness duration; and study quality.
Categorical variablesFirst, we used Welch's t-test to analyse the relationship between the effect size and the type of analysis (ReHo or ALFF; the rest of techniques were underrepresented and we eliminated them from this analysis). The results indicate that there is a significant relationship between the effect size and the type of analysis used, t = -2.381, df = 13.927, punivariate = .016, r = .538. In fact, the mean effect size (d) is 1.242 for ReHo studies and 0.964 for ALFF studies, which suggests that studies using ReHo can obtain a greater effect size than those using ALFF, and this effect has high intensity according to Cohen's criteria.
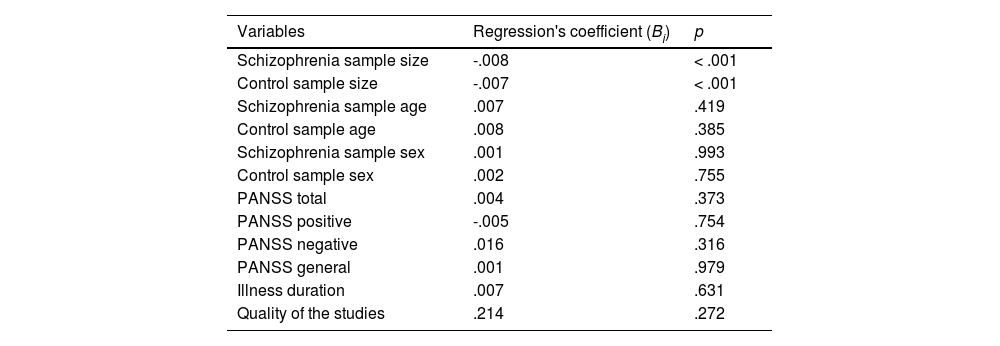
Quantitative variables: meta-regression analysisWe performed meta-regression to analyse whether the quantitative variables described above (Table 1) have a significant impact. Table 6 presents the minimum quadratic estimates of each meta-regression to evaluate the effect of each mediator variable on the effect size. Only sample sizes are significant predictors of the effect size heterogeneity. The negative value of the regression coefficients indicates that studies with larger sample sizes have a lower effect size. In summary, our analysis of mediator variables indicates that, in the analysed studies, the effect size is higher for ReHo estimated with smaller samples.
Meta-regression of the possible mediator variables and effect sizes.
Note. PANSS, Positive and Negative Syndrome Scale
Our meta-analysis on rsFC using whole-brain studies showed hypoactivation of the left superior temporal gyrus and the right precentral gyrus in patients with schizophrenia compared with HC. In this sense, our results are partially consistent with previously published meta-analyses that employed different data analysis techniques. Li et al. (2019) analysed studies using ICA and found hypoactivation in the same brain areas in patients with schizophrenia. Xiao et al. (2017) evaluated studies using ReHo and found decreased ReHo in the left superior temporal gyrus and the right precentral gyrus in patients with schizophrenia compared with HC. Interestingly, they also suggested that unaffected relatives of patients with schizophrenia show decreased ReHo in the right superior temporal gyrus. Dong et al. (2018) included only connectivity studies using ALFF and reported reduced connectivity in the left superior temporal gyrus in patients with schizophrenia. In addition, our results are consistent with Mwansisya et al. (2017). In their systematic review, these authors concluded that patients who experience their first episode of schizophrenia present consistent alterations in the left superior temporal gyrus in both task-related activities and rest-connectivity conditions. However, it should be noted that these authors reported connectivity and task-related abnormalities in both directions (hyper- and hypoactivation) in this brain area.
The left superior temporal gyrus is part of the sensorimotor network (Li et al., 2019) and pertains to the language network (Friederici & Gerhan, 2013). This brain area has shown a progressive decrease in grey matter in longitudinal MRI studies of patients with schizophrenia (Kasai et al., 2003; Takahashi et al., 2009). Its malfunction in schizophrenia has been related to verbal auditory hallucinations (Wolf et al., 2012) and formal thought disturbances, two symptoms often experienced by patients with schizophrenia (Owen et al., 2016). Auditory hallucinations are experienced as a perception of speech without any stimulus, and brain imaging studies revealed that the left superior temporal gyrus is activated during the hallucination experienced by patients with schizophrenia (Plaze et al., 2006). Other studies suggest that in patients with chronic hallucinations, the left superior temporal gyrus shows hypoconnectivity with the left operculum, the left parietal opercular area, and the left hippocampus (Sommer et al., 2012). In their review, Alderson-Day et al. (2016) concluded that auditory hallucinations in schizophrenia are associated with alterations in the brain connectivity of the left superior temporal gyrus with several other brain structures. However, they also concluded that this symptom in schizophrenia and other disorders may be related to complex interactions between the brain areas of the sensorimotor network, including the left superior temporal gyrus, the DMN and the CCN (Alderson-Day et al., 2016). Structural abnormalities in the arcuate fasciculus have also been consistently reported in patients with schizophrenia experiencing auditory hallucinations (Geoffroy et al., 2014). This fasciculus communicates the temporal areas of the language network, including the left superior temporal gyrus, with the frontal areas of the same network (Friederici & Gerhan, 2013). Formal thought disturbances in schizophrenia are problems in the coherence and organisation of language. Various studies suggest that the presence of thought disorders and their severity in patients with schizophrenia is related to abnormal activations in the left superior temporal gyrus during language comprehension tasks (Weinstein et al., 2006) and language production tasks (Kircher et al., 2001, 2002). More recent work shows that thought disorders in schizophrenia are associated with structural abnormalities in the left superior temporal gyrus and other areas of the language network and with hyper- and hypoactivation in this same brain area during language processing and language producing tasks, such are irony comprehension, passive language perception tasks, semantic processing tasks, and free language production tasks (for a review, see Cavelti et al., 2018). Likewise, the left superior temporal gyrus consistently shows connectivity alterations in the resting state in patients with schizophrenia who experience formal thought disturbances. However, in their revision, more studies have reported resting state connectivity increases than hypoconnectivity in these patients (Cavelti et al., 2018).
We found that both the left superior frontal gyrus and the right precentral gyrus show hypoactivation in patients with schizophrenia. These brain areas are part of the praxis or gesture network mentioned in the Introduction (Andric & Small, 2012; Walther, Mittal, et al., 2020; Yang et al., 2015). In their review, Andric & Small (2012) found that the left superior temporal gyrus is an essential structure for understanding the movements of the arms and hands that accompany speech, known as co-speech gestures. Yang et al. (2015) suggest that both the left superior temporal gyrus and the right precentral gyrus are important structures to understand ‘co-speech gestures’ and gestures with meaning independent of language. Patients with schizophrenia often have problems understanding and executing gestures (Walther et al., 2015; Walther, Mittal, et al., 2020; Wüthrich et al., 2020). They also have difficulty detecting discrepancies between gestures and speech (Nagels et al., 2019). These problems tend to worsen with the course of the disease and do not improve even if patients experience clinical improvements and a reduction in the general symptoms of schizophrenia with their treatment (Wüthrich et al., 2020). The significant hypoactivation of the left superior temporal gyrus and the right precentral gyrus that we found could explain, at least in part, why patients with schizophrenia often present this type of deficit. Some neuroimaging work supports this statement, at least partially. Viher et al. (2018) showed the existence of associations between the presence of deficits in a gesture recognition task in patients with schizophrenia and a reduction in cortical thickness in structures of the praxis network of the left hemisphere, such as the inferior parietal lobe, the superior frontal gyrus, the inferior frontal gyrus, and the superior temporal gyrus.
Patients with schizophrenia also present problems in gesture production (Walther, Mittal et al., 2020). Several studies suggest that patients with schizophrenia present different types of errors in producing gestures much more frequently than HC. However, most of them do not have sufficient alterations to fulfil a diagnosis of apraxia (Walther, Mittal et al., 2020). However, another study found that around 27% of a sample of patients with schizophrenia submitted to the Test of Upper Limb Apraxia (TULIA) qualified for a diagnosis of apraxia (Walther, Alexaki, et al., 2020). Wutrich et al. (2018) showed that patients with schizophrenia who score worse than healthy participants in the TULIA present alterations in resting brain connectivity in the praxis network, including hyperconnectivity between the right precentral gyrus and the parietal lobe and hypoconnectivity between the left and right superior temporal gyrus. Likewise, Viher et al. (2018) showed that patients with schizophrenia and deficits in gestural production have a reduction in cortical thickness in structures of the praxis network, very similar to what is presented by patients with problems understanding gestures, including a reduction in the cortical thickness of the left superior temporal gyrus.
Another important aspect of our results that deserves to be highlighted is the poor significance found in brain areas other than those already mentioned. After applying the type I error rate correction, the other significant results in our whole-brain meta-analysis disappeared. Hence, our whole brain analysis does not provide sufficiently strong evidence to show other brain areas that present aberrant connectivity in patients with schizophrenia based on the previously published meta-analyses (Dong et al., 2018; Gong et al., 2020; Li et al., 2019; Xiao et al., 2017).
Regarding the effect of several variables as potential mediators of the obtained effect sizes, the sample size shows, as in previous studies, a significant effect in the sense that the effect size decreases as the sample size of patients with schizophrenia (βi = -.008, p < .001) or HC (βi = -.007, p < .001) increases. This effect is not new: it has been found in other meta-analyses that evaluated connectivity in rs-fMRI in other populations (Cañete et al., 2021). In general, it is assumed that the increase in the sample size increases a regression to the mean effect due to the reiteration of the use of the linear model in the estimation of the representative scores of each brain area considered in each registered brain volume. Concerning the effect associated with the estimation technique that indicates that ReHo presents larger effect sizes than ALFF, this effect is because ReHo is based on an estimation centered on the centrality of each ROI. In contrast, ALFF relies on studying low-frequency fractional amplitude. ALFF, therefore, is a much more restrictive technique than ReHo, so it is more conservative in terms of significance (Yue et al., 2020).
None of the other variables we considered as potential mediators have a significant effect. Of note, we found no effect of the illness duration on the rsFC of our patients. In contrast, Dong et al. (2018) found several significant relationships with disease duration in their effect estimations. The connectivity within the DMN of their patients with schizophrenia gradually decreases with longer disease duration, and the negative connectivity between the DMN and VAN increases across illness duration. We also did not find an effect of the PANSS scores as a moderator variable. Gong et al. (2018) found that a higher PANSS total score correlates with increases in ALFF in the right putamen and the superior occipital gyrus and smaller decreases in the inferior temporal gyrus and anterior cingulate cortex in patients experiencing their first schizophrenic episode. These authors also found that patients with chronic schizophrenia and a higher PANSS total score exhibit smaller decreases in ALFF in the right inferior frontal gyrus, the anterior cingulate cortex, and the left inferior temporal gyrus.
There are some limitations to this meta-analysis. We did not include studies that did not report the three-dimensional activation coordinates. We also excluded studies that did not report the corresponding statistical contrasts associated with the three-dimensional activation coordinates. In addition, we did not consider either the different subtypes of schizophrenia or the disease duration. Some previously published meta-analyses showed different connectivity effects related to this last variable when assessed qualitatively (Gong et al., 2020) or quantitatively (Dong et al., 2018). In addition, some analysis techniques used by the studies we included were underrepresented and could not be considered when evaluating whether they could predict the effect size. Finally, several original papers in our meta-analysis did not report the PANSS scores, which impeded proper analysis of these scores as potential moderator variables of the obtained effect sizes.
Our work also has some strengths. As far as we know, this is the first meta-analysis of brain rsFC in patients with schizophrenia using a whole-brain analysis approach. We must note that two independent researchers carried out a thorough search of the literature by following the PRISMA guidelines. In addition, we did not find any publication bias. We also analysed the quality of the studies: all of the included studies in our meta-analysis are high quality. In addition, the jackknife sensitivity analysis showed that our results are replicable in almost all included studies in our meta-analysis, demonstrating that our results are highly reliable.
In conclusion, our results showed that patients with schizophrenia show hypoactivation of left superior temporal gyrus and the right precentral gyrus. In other studies, these changes been associated with some clinical symptoms usually present in patients with schizophrenia, including the presence of auditory verbal hallucinations, formal thought disorder, and the comprehension and production of gestures.
This study was funded by the Ministry of Science, Innovation and State Research Agency (Project code: PGC2018-095829-B-I00).
Keyword Combinations Used in the Search
SCOPUS
Title: schizophreni*
AND
Title/Abstract/Keywords: fMRI OR ‘functional magnetic resonance imag*’ OR ‘functional neuroimag*’ OR ‘functional MRI’ OR ‘functional connectivity’
AND
Title/Abstract/Keywords: ‘resting state’ OR rest
Web Of Science
Title: schizophreni*
AND
Topic: fMRI OR ‘functional magnetic resonance imag*’ OR ‘functional neuroimag*’ OR ‘functional MRI’ OR ‘functional connectivity’
AND
Topic: ‘resting state’ OR rest
PubMed (new version)
Title: schizophreni*
AND
Title/Abstract: fMRI OR ‘functional magnetic resonance imag*’ OR ‘functional neuroimag*’ OR ‘functional MRI’ OR ‘functional connectivity’
AND
Title/Abstract: ‘resting state’ OR rest
The 11-point checklist concentrates on both the clinical and neuroimaging methodology of each study and mainly assesses the quality from three aspects: subjects, methods for image acquisition and analysis, and results and conclusions. Each item is matched with the study and then assigned a score of 1 (fully satisfied), 0.5 (partially satisfied), or 0 (otherwise).
Category 1: Subjects
- 1
Patients were evaluated prospectively, specific diagnostic criteria were applied, and demographic data were reported.
- 2
Healthy comparison subjects were evaluated prospectively; psychiatric and medical illnesses were excluded.
- 3
Important variables (e.g., age, sex, illness duration, onset time, medication status, comorbidity, severity of illness) were checked, either by stratification or statistically.
- 4
Sample size per group > 10.
Category 2: Methods for image acquisition and analysis
- 1
Magnet strength ≥ 1.5 Tesla.
- 2
For resting-state functional magnetic resonance imaging studies, whole brain analysis was automated with no a priori regional selection.
- 3
Coordinates reported in a standard space.
- 4
Processing of the imaging technique was described clearly enough to be reproduced.
- 5
Measurements were described clearly enough to be reproduced.
Category 3: Results and conclusions
- 1
Statistical parameters were provided for significant and important non-significant differences.
- 2
Conclusions were consistent with the results obtained, and the limitations were discussed.
Demographic and clinical characteristics of the included studies
Note. ALFF, amplitude of low-frequency fluctuation; fALFF, fractional amplitude of low-frequency fluctuation; GFC, global function connectivity; PAS, parameter of asymmetry; PANSS, Positive and Negative Syndrome Scale; LSFCS, long short functional connectivity; ReHo, regional homogeneity; VMHC, voxel-mirrored homotopic connectivity.