Edited by: Andre R Brunoni, Marie-Anne Vanderhasselt, Leigh Chavert
More infoOur objective was to review the literature on the parietal cortex and intraparietal sulcus (IPS) in anxiety-related disorders, as well as opportunities for using neuromodulation to target this region and reduce anxiety. We provide an overview of prior research demonstrating: 1) the importance of the IPS in attention, vigilance, and anxious arousal, 2) the potential for neuromodulation of the IPS to reduce unnecessary attention toward threat and anxious arousal as demonstrated in healthy samples; and 3) limited data on the potential for neuromodulation of the IPS to reduce hyper-attention toward threat and anxious arousal among clinical samples with anxiety-related disorders. Future research should evaluate the efficacy of IPS neuromodulation in fully powered clinical trials, as well as the value in augmenting evidence-based treatments for anxiety with IPS neuromodulation.
Anxiety-related disorders are among the leading causes of disability and illness burden globally (Vos et al., 2020). The burden of illness attributable to anxiety-related disorders has remained stable since 1990 despite enormous gains in the evidence-base for effective interventions (Vos et al., 2020). During the global COVID-19 pandemic, prevalence rates of anxiety-related disorders grew to 4802.4 per 100,000 (374 million), up from 3824.9 per 100,000 (298 million people) prior to the pandemic onset (Santomauro et al., 2021). Anxiety disorders also significantly increase risk for comorbid concerns such as mood disorders, substance use disorders, and suicide risk (Brown, Gaudiano & Miller, 2010). There are a few interventions that have a strong evidence base for reducing anxiety disorder severity and risk of relapse, but these treatments are not universally efficacious.
The gold-standard treatment for anxiety-related disorders is cognitive behavioral therapy (CBT; Carpenter et al., 2018). Dozens of meta-analyses have demonstrated that CBT for anxiety has strong evidence for efficacy and effectiveness across the lifespan and that the benefits of CBT are long-lasting (Bandelow et al., 2018; Schwartz, Barican, Yung, Zheng & Waddell, 2019). However, 30 - 50% of patients with anxiety-related disorders do not respond to CBT and 20% drop-out of treatment (Loerinc et al., 2015). Some pharmacotherapy options are also well supported for patients with anxiety, including selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs). Like CBT, up to 45% of patients with anxiety-related disorders do not respond to SSRIs and up to 55% do not achieve remission (Ginsburg et al., 2011).
Neuromodulation is a promising avenue for novel anxiety treatment. There is evidence that prefrontal TMS stimulation may reduce anxiety in MDD patients (Mantovani, Aly, Dagan, Allart & Lisanby, 2013). However, effects are mixed in non-depressed anxiety patients (Lefaucheur et al., 2014; Zwanzger, Fallgatter, Zavorotnyy & Padberg, 2009). One potential explanation is that the mechanisms targeted by prefrontal TMS sessions may not be driving anxiety expression (Baeken et al., 2010). For instance, the subgenual anterior cingulate cortex (sgACC), which is the primary downstream target of left prefrontal TMS treatment protocols, has been implicated in depression. However, anxiety symptoms are more commonly associated with hyperactivity in subcortical regions like the amygdala (Izquierdo, Furini & Myskiw, 2016), bed nucleus of the stria terminalis (Davis, Walker, Miles & Grillon, 2010), and locus coeruleus (Ross & Van Bockstaele, 2021). Recently, encouraging findings have emerged about the role of the intraparietal sulcus (IPS) in attention and anxious arousal, and this region might offer a critical target for neuromodulation.
The parietal cortex is generally involved in tasks related to sensory processes and integration of sensory details. The IPS divides the parietal lobe into the inferior and parietal lobules. Functionally, the IPS plays a critical role in a wide variety of tasks, from salience detection and orienting, to anxious arousal and behavioral intention and motor coordination. The range of function and connectivity to other brain regions has resulted in referring to the IPS as a “connectivity hub” for arousal (Balderston et al., 2017). Specifically, the IPS shows strong functional connectivity with other regions of the frontoparietal attention network, which is known to be critical for cognitive control and attention (M. W. Cole, Repovš & Anticevic, 2014). Similarly, it also has functional connections with subcortical regions like the locus coeruleus, which are known to be critical for anxiety expression (Liebe, Kaufmann, Hämmerer, Betts & Walter, 2022).
This functional work is supported by tract tracing studies showing that the parietal cortex and the IPS is highly connected with frontal, temporal, occipital, and subcortical regions (Caspers & Zilles, 2018). For example, the superior longitudinal fascicle connects the IPS to the prefrontal cortex, while the middle longitudinal fascicle connects it with the temporal cortex (Caspers & Zilles, 2018). Together, this situates the IPS at the intersection between cognitive and emotional processing, representing a critical hub for anxiety/cognition interactions. The IPS is one of a few regions implicated in transdiagnostic psychiatric impairments in cognitive processing (McTeague et al., 2017). In this review, we provide an overview of each IPS-associated cognitive task, underscoring its association with fear and anxiety. Next, we describe some key findings from the literature on IPS neuromodulation and discuss their relevance to arousal reduction as a function of anxiety reduction. We conclude with directions for future research to assist with the translation of findings into clinical practice.
Neurocircuitry of anxiety disordersWhile a detailed description of the neurocircuitry of anxiety disorders is beyond the scope of the current work, it is important to provide a brief overview to contextualize the role of the IPS in anxiety expression and regulation. Most models of anxiety disorders are centered on the extended amygdala, which encompasses the amygdala and bed nucleus of the stria terminalis (BNST; Davis et al., 2010; Fanselow & Gale, 2003). The amygdala receives input from all sensory modalities through the lateral nucleus, which then projects to the central, basal, and other nuclei (Amaral, Price, Pitkänen, & Carmichael, 1992, 2003; Pitkänen & Amaral, 1998). The central nucleus, which is the primary output nucleus then project to the BNST, thalamus, hypothalamus, and brainstem (among other regions). Through activation of these downstream regions, the central nucleus of the amygdala, along with the BNST, controls the biological responses (release of stress hormones, activation of the sympathetic nervous system, startle potentiation, etc.) that form the basis of our emotional experience (Amaral, Price, Pitkänen, & Carmichael, 1992, 2003; Pitkänen & Amaral, 1998). Critically, the amygdala shares strong reciprocal connections with the visual cortex at nearly all levels (Balderston, Schultz, Hopkins & Helmstetter, 2015; Pessoa & Adolphs, 2010). Other regions critical to the expression of emotion include the insula, dorsomedial prefrontal cortex, thalamus, hypothalamus, and hippocampus (Fullana et al., 2015). The lateral and ventromedial prefrontal cortical regions also seem to be important for the regulation of anxiety and other emotions (Sylvester et al., 2012; L. K. White, Makhoul, Teferi, Sheline & Balderston, 2023). The IPS, which is interconnected with both lateral prefrontal and higher order visual processing regions (Balderston et al., 2017; Caspers & Zilles, 2018) is perfectly situated to impact amygdala processing at multiple levels.
Roles of parietal cortexAttention. The IPS is critical for attention across a variety of stimulus types and tasks that tax attentional resources. For instance, the IPS is activated during tasks that require selective attention (Lanssens, Pizzamiglio, Mantini & Gillebert, 2020), such as when attentional resources must focus one stimuli over competing, distractor stimuli. Selective attention is critical to patients with anxiety-related disorders, who tend to preferentially attend to threatening stimuli (e.g., fear or angry faces) over non-threatening stimuli (neutral faces; L. K. L. K. White et al., 2017). Patients with posttraumatic stress disorder (PTSD) selectively attend to trauma-related cues to a greater extent than trauma-exposed controls (Naegeli et al., 2018). Moreover, in non-anxious individuals, induction of an attention bias to threat leads to an increase in anxiety-related behaviors (Suway et al., 2013); training anxiety patients to allocate attention away from threat is associated with a decrease in symptoms (L. K. White et al., 2017). There is a strong correlation between anxiety and right IPS activation during the presentation of task irrelevant threat faces (and instead attend to other stimuli; Ewbank et al., 2009). Compared to non-anxious, trauma-exposed controls, patients with PTSD also have elevated activity in the IPS during the presentation of loud sounds (i.e., threatening information), likely reflecting elevated attention or reactivity to such stimuli (Naegeli et al., 2018). These findings coupled with the series of studies showing the role of IPS in allocation of attention across non-emotional contexts suggests the IPS may be an important part of the neural architecture underling the attentional hypervigilance to threat in anxious individuals.
The IPS is involved in the top-down regulation of multiple sensory inputs (S. Huang, Chang, Belliveau, Hämäläinen & Ahveninen, 2014). Like the preference to allocate attentional resources to visual threats, the IPS likely underlies a broader allocation of processing resources (i.e., a hypervigilance) to process all potentially threatening inputs in individuals with high levels of anxiety. For example, in panic disorder, low-grade physical sensations become paired with the perception of danger; these incoming sensory inputs are interpreted as an indication that a panic attack is imminent. Accordingly, patients with panic disorder engage in near constant body scanning to attend to all potentially incoming sensory sources of threat (with a goal of intervening to reduce an impending panic attack). This tendency to scan one's body, requiring selective attention toward bodily sensations, is a core function of the IPS (Rabellino, Frewen, McKinnon & Lanius, 2020).
Attention toward value-driven rewards is also a function of the IPS (Anderson, 2017). Deficits in reward processing and atypical attention toward rewards are reliably observed in depression (Admon & Pizzagalli, 2015). Although there is a paucity of work looking at reward processing in relation to anxiety, growing evidence links anxiety to aberrant reward processing. In youth at risk for anxiety, a bias to attend positive stimuli was associated with decreased anxiety symptoms (L. K. L. K. White et al., 2017). Patients with social anxiety show decreased risk aversion (a construct related to reward processing) and risk-based decisions were related to connectivity between the IPS and the insula and anterior cingulate cortex (Tang, van den Bos, Andrade & McClure, 2012). There is also some evidence that patients with PTSD exhibit reduced reward functioning (both in the anticipation and value placed on rewards) compared to controls; this is especially true for women, although this effect might be attributable to anhedonia (Nawijn et al., 2015).
Vigilance and anxious arousal. Beyond attentional resources, the parietal cortex and the IPS are critical to vigilance and arousal (Lee et al., 2022), particularly in the context of the IPS connectivity to other brain regions (Balderston et al., 2017). For example, IPS and the parietal cortex exhibit vigilance detection decrements under fatigue conditions (Hassanin, Al-Shargie, Tariq & Al-Nashash, 2021). The fronto-parietal network is also implicated in maintaining the benefit of “warning cues,” an indicator of vigilance, in aging adults (Haupt, Ruiz-Rizzo, Sorg & Finke, 2020).
Individuals with higher levels of anxious arousal demonstrate greater parietal activity than individuals with lower anxious arousal (Härpfer, Spychalski, Kathmann & Riesel, 2021). During tasks with uncertain threat (i.e., high arousal), individuals who are higher in trait anxiety demonstrated altered connectivity between the IPS and precuneus (among other regions), and this connectivity was positively correlated with trait anxiety (Geng et al., 2018). Increased IPS activation is also linked to the anticipation of emotional content, even non-social content, in patients with social anxiety (Brühl et al., 2011). Furthermore, global brain connectivity analyses revealed that during such tasks, right and left IPS were significantly more activated during threat versus safety (Balderston et al., 2017). Thus, the extant work highlights that the IPS is a critical region of focus for understanding anxious arousal during potential threat.
Neuromodulation effectsTMS is a noninvasive neuromodulation method that uses strong magnetic pulses to depolarize cortical neurons (Di Lazzaro et al., 2011). Single TMS pulses can induce action potentials in the pyramidal cells directly below the coil and interfere with ongoing neural processing (Di Lazzaro et al., 2011). Repetitive TMS and patterned theta burst stimulation (TBS) can also induce transient changes in cortical excitability and more lasting changes in synaptic plasticity. For instance, rTMS at low frequencies (<5 Hz) can decrease excitability, while rTMS at high frequencies (>5 Hz) can increase excitability (Di Lazzaro et al., 2011). Theoretical models suggest that single TBS pulses produce both excitatory and inhibitory effects but that the net change in synaptic strength is dependent on the amount and pattern of calcium influx into the cell (Dudek & Bear, 1992, 1993; Y. Z. Huang, Rothwell, Chen, Lu & Chuang, 2011; Neves, Cooke & Bliss, 2008). According to these models, intermittent TBS (iTBS), which typically delivers 2-second trains of 5 Hz stimulation every 10 s, leads to increases in cortical excitability and long-term potentiation (LTP)-like synaptic changes. In contrast, continuous TBS (cTBS), which typically consists of a longer (e.g. 40 second) single train of 5 Hz stimulation, leads to decreases in excitability and long term depression (LTD)-like synaptic changes (Y. Z. Huang et al., 2011).
Neuromodulation work focused on the IPS supports the idea that this region is a hub, potentially providing a critical intersection between the networks mediating cognitive and affective processing. As described above, the IPS is also known to be critical for orienting to salient stimuli in the environment (Du, Chen & Zhou, 2012; Molenberghs, Mesulam, Peeters & Vandenberghe, 2007). Accordingly, disrupting ongoing activity in the IPS can differentially affect task performance depending upon attentional demands (Capotosto, Babiloni, Romani & Corbetta, 2009; Mevorach, Hodsoll, Allen, Shalev & Humphreys, 2010). For instance, disrupting ongoing IPS activity, as indexed by stimulus initiated alpha desynchronization, can interfere with performance on sustained attention tasks (Capotosto et al., 2009). In contrast, performance can be improved by IPS disruption if the task requires filtering out salient distractors (Mevorach et al., 2010). Additionally, down regulating left IPS activity with cTBS can improve attentional neglect symptoms in individuals with right IPS lesions (Koch et al., 2012). Finally, disruption of IPS activity can reduce bottom-up habitual responding (Osada et al., 2019), and reduce risk-taking during an economic decision-making task (Coutlee, Kiyonaga, Korb, Huettel & Egner, 2016).
The role of the IPS in the allocation of attention to emotional, especially threatening, information is supported by neuromodulation studies. Stimulation of IPS facilitates maintenance of socioemotional cues, especially fear cues during WM (Engelen, de Graaf, Sack & de Gelder, 2015). Likewise, excitatory rTMS to the IPS can improve the identification of gender of emotional face during a cued attentional task (Fan, Wan, Zhang, Jin & Li, 2018). In contrast, single pulses to the IPS can also interfere with the online representations of fear cues (Mazzoni, Jacobs, Venuti, Silvanto & Cattaneo, 2017).
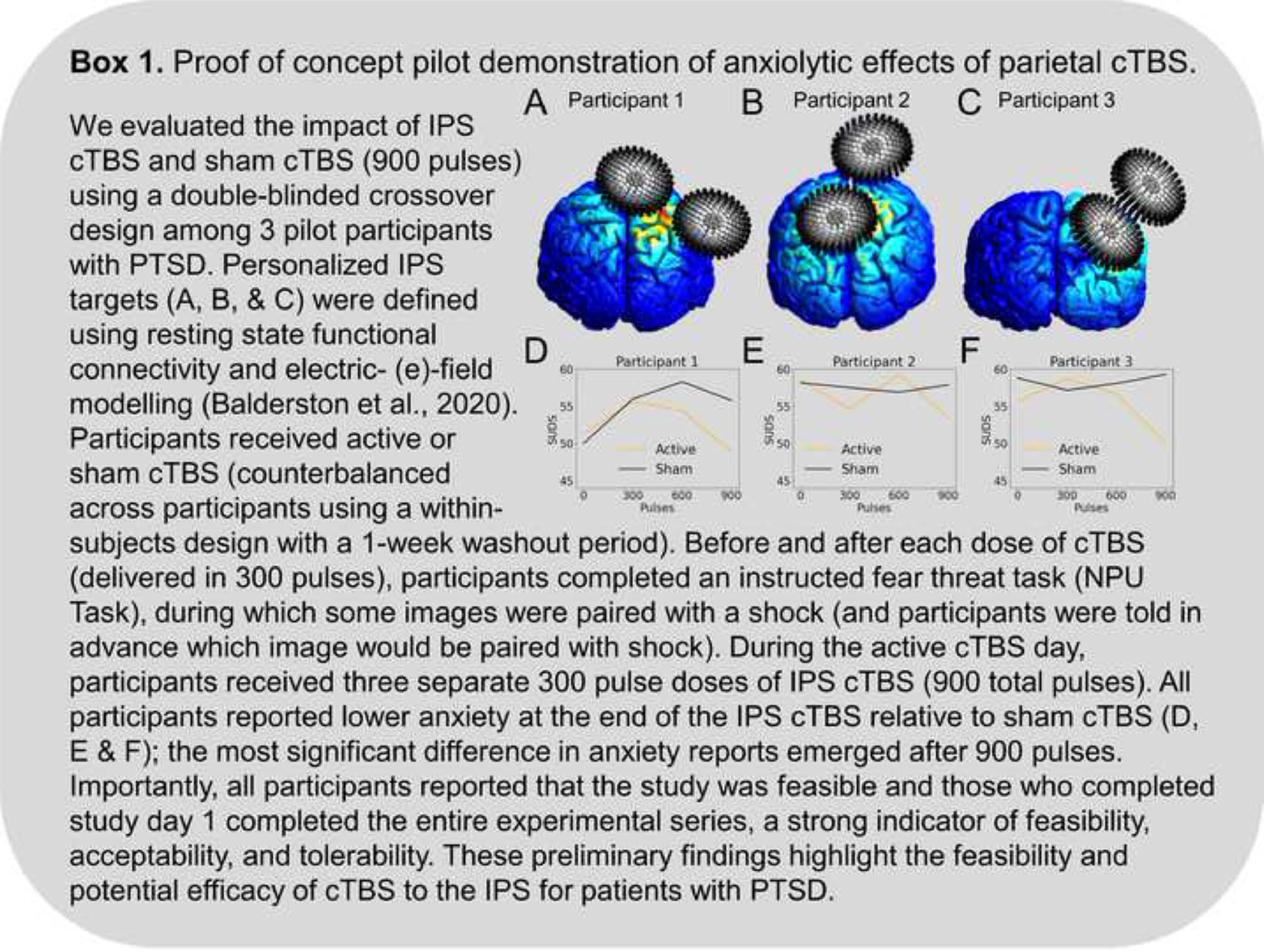
Recent neuromodulation work finds that stimulation of the IPS can directly modulate arousal and anxiety symptoms (Box 1). For instance, online 1 Hz stimulation to the IPS reduces anxiety in the lab, as measured by the potentiated acoustic startle response (Balderston et al., 2020). Critically, potentiated startle is often thought of as a biomarker of anxiety (Davis et al., 2010). In a subsequent study, Wang and colleagues (Wang et al., 2022) found that ten days of 1 Hz stimulation to the IPS was sufficient to improve symptoms relative to sham in a group of individuals with GAD (Wang et al., 2022).
DiscussionHere we have shown that the following: 1) the parietal cortex, specifically the intraparietal sulcus is uniquely situated at the allocation of attention and arousal/hypervigilance, with strong functional connections to networks serving both systems, 2) experimental modulation of IPS activity affects both cognitive and affective processes, 3) IPS neuromodulation may reduce anxiety symptoms. Together, these findings make the IPS a prime target for potential neuromodulatory interventions for anxiety disorders. Given that there is yet to be an FDA-approved neuromodulatory treatment for PTSD or anxiety, and adaptations of depression protocols have had limited efficacy outside of MDD patients (D. White & Tavakoli, 2015), an anxiety-centered treatment option may have untapped potential.
In the sections above, we showed that the IPS is associated with both arousal expression and affect-driven attentional capture, both of which are over-expressed in PTSD and anxiety disorders. While we understand that PTSD is no longer classified as an anxiety disorder (it is classified under “Trauma and other stressor-related disorders” in DSM-5), alterations in arousal are a core symptom cluster of PTSD and are relevant across all anxiety-related disorders. Additionally, we argue that the IPS may be a key node in the network responsible for the expression of these symptoms: the hyperexcitability of the IPS may lead to hyperarousal and hypervigilance to threat leading to increased anxious arousal and anxiety-related behaviors. Based on this mechanism, we argue that decreasing activity in this region may alleviate these symptoms. Indeed, we also showed above that 1 Hz rTMS, thought to be inhibitory reduced arousal and anxiety symptoms (Fig. 1) (Box 1).
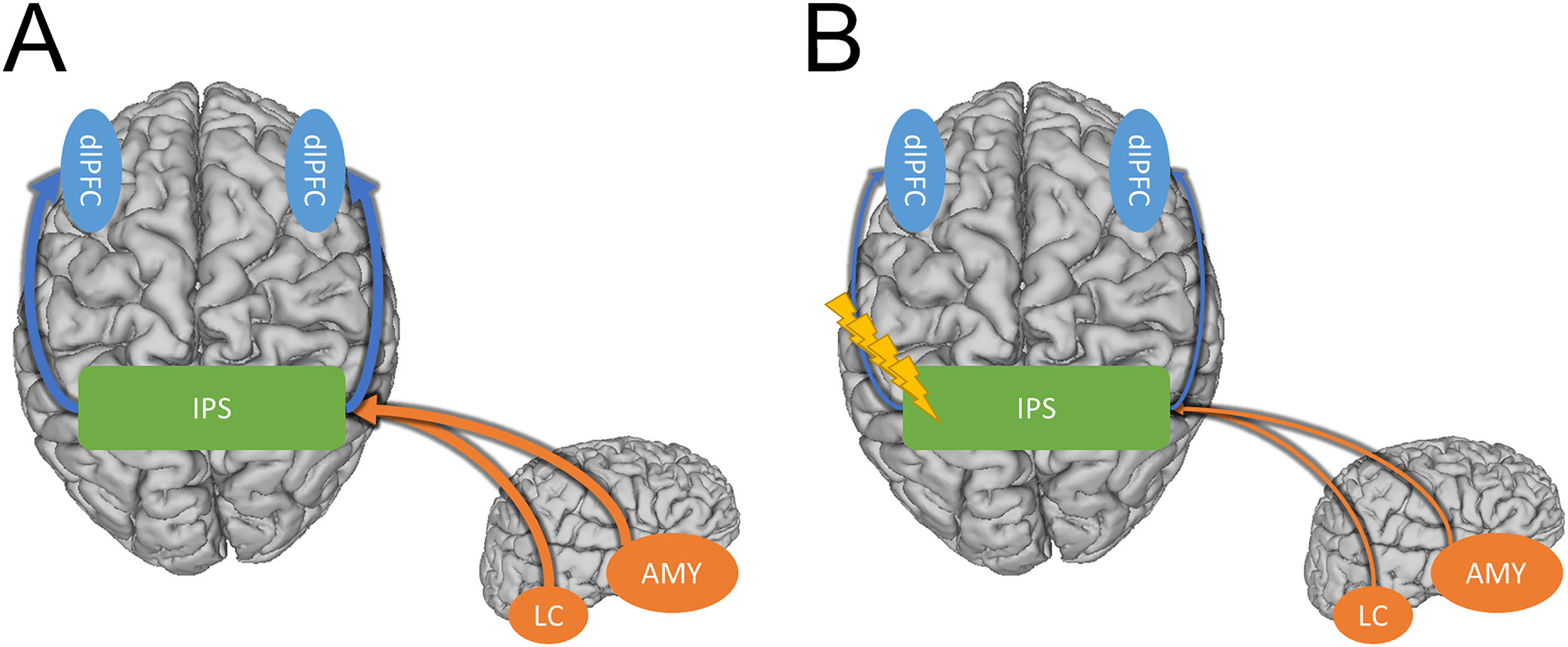
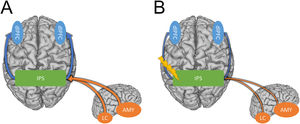
Potential model showing how the IPS may mediate hyperarousal and hypervigilance symptoms in anxiety disorders. A) Strong connectivity between the IPS and the dlPFC facilitates bottom-up processing of threat-related stimuli leading to hypervigilance (Blue arrows). Strong connectivity between subcortical regions like the amygdala and locus coeruleus and the IPS elevate arousal (Orange arrows). B) Inhibitory TMS protocols may alleviate hypervigilance and hyperarousal symptoms in PTSD and anxiety by weakening the connections between the IPS and the cortical/sub-cortical regions involved in these symptoms.
Compared with prefrontal TMS, the standard treatment protocol for depression, inhibitory parietal stimulation likely targets a different mechanism. Although the mechanism of action of left prefrontal TMS is not fully known, one candidate mechanism is the strengthening of anti-correlations between the prefrontal cortex and the subgenual anterior cingulate cortex (sgACC), a hub region of the default mode network (DMN; Mayberg et al., 2005). In contrast, our previous work suggests that inhibitory parietal stimulation may reduce hyperexcitability leading to a decrease in anxious arousal (Balderston et al., 2017, 2020). It could be helpful to consider these two forms of stimulation as targeting top-down vs. bottom-up systems. For prefrontal TMS, it could be argued that the mechanism of action is the strengthening of the top-down connections between the executive control network and the DMN, lessening the verbal, self-referential symptoms of depression (Chen et al., 2013). In contrast, for the inhibitory parietal stimulation, it could be argued that the mechanism is a direct reduction in the bottom-up expression of anxiosomatic anxiety symptoms, thus resulting in a decrease in the allocation of attention to threat cues (i.e., hypervigilance; Cisler et al., 2010). Although this top-down vs. bottom-distinction is preliminary, the framework is helpful in that it leads to testable hypotheses. Specifically, we predict that inhibitory parietal stimulation will primarily improve anxiosomatic symptoms, while left prefrontal stimulation will primarily improve symptoms like worry. Additionally, we predict that left prefrontal stimulation will be most beneficial in those with comorbid depression, while inhibitory parietal stimulation will be most beneficial in those with a primary diagnosis of anxiety or PTSD.
As a stand-alone treatment, one might consider enrolling high-anxious patients in a trial where 1 Hz rTMS or cTBS is administered to the IPS. There are a number of potential protocols that could be adapted for this purpose, such as standard clinical TMS protocols where patients are given 5 TMS session per week for 4–6 weeks (O'Reardon et al., 2007). This course of treatment has been shown to be safe and effective when applied to the dlPFC for depression, albeit with excitatory protocols (i.e., 10 Hz and iTBS; O'Reardon et al., 2007). Alternatively, newer accelerated approaches have begun to show promising results for depression as well, suggesting that similar efficacies may be achieved in as little as 1 week by administering multiple sessions per day (E. J. Cole et al., 2022). Like the other FDA-approved treatments for depression, this approach targets the left dlPFC with excitatory stimulation (iTBS; E. J. Cole et al., 2022). Although there have been studies examining the efficacy of a similar accelerated approach with inhibitory stimulation, there are substantially fewer clinical trials using inhibitory stimulation, especially outside of the prefrontal cortex (Miron et al., 2021).
Pairing inhibitory IPS stimulation with another form of therapy, like prolonged exposure (PE), an extinction-based therapy for PTSD, may have complementary effects on symptom reduction. Patients with PTSD demonstrate reliable deficits in extinction learning, which is the ability to learn that a previously threatening stimulus is no longer a threat (Lissek et al., 2005). Hyperarousal during extinction may interfere with extinction learning in PTSD patients, as studies have shown that arousal should be in a moderate range to maximize therapeutic outcomes with PE (McCormack, Mennies, Silk & Stone, 2020). Therefore, reducing hyperarousal from inhibitory parietal stimulation may increase the efficacy of PE in PTSD patients.
LimitationsAlthough we have provided a framework to guide potential anxiety interventions aimed at the IPS, very few of these studies have been conducted. As such, many of the arguments made in this paper are speculative or based on small pilot studies. We hope that this work will inspire future clinical trials for PTSD and anxiety. Such trials should take care to monitor cognitive processes closely as online parietal stimulation can reduce working memory performance (Harris & Miniussi, 2003; Mackey & Curtis, 2017; Sasanguie, Göbel & Reynvoet, 2013). If offline stimulation has a similar effect, such an effect should be considered when making treatment decisions.
ConclusionsThe IPS is a connectivity hub for anxiety-related arousal due to its role in orienting, attention, salience-detection, reward processing, vigilance. This region has promise for reducing anxious arousal and hyper-attention toward threat among patients with anxiety-related disorders. More research is needed to evaluate the effect of neuromodulation, specifically cTBS, to this region in larger, well-controlled clinical trials. Finally, more research is needed to evaluate whether neuromodulation to the IPS can augment response rates to existing evidence-based treatments for anxiety-related disorders, such as PE.
Author contributionsCRediT author statement according to: https://www.elsevier.com/authors/policies-and-guidelines/credit-author-statement. Conceptualization: LB, LKW, NLB; Writing - Original Draft: LB, NLB; Writing - Review & Editing: LB, LKW, MT, WM, YS, NLB; Visualization: LB, MT, NLB; Supervision: LB, YS, NLB; Funding acquisition: LB, NLB.
Financial supportThis project was supported in part by a NARSAD Young Investigator Grants from the Brain & Behavior Research Foundation (NLB: 2021), a career development grant from the National Institutes of Health: K01MH121777 (NLB), and a research grant from the National Institutes of Health: (U01MH130447).
We would like to thank the following individuals who contributed to Dr. Balderston's K01 project: Dr. Kerry Ressler, Dr. Michael Thase, and Dr. Kristin Linn. The authors would like to thank Maria Prociuk for her expertise and assistance in submitting the paper.