Myocardial injury has been identified as a common complication in patients with COVID-19. However, recent research has serious limitations, such as non-guideline definition of myocardial injury, heterogenicity of troponin sampling or very short-term follow-up. Using data from a large European cohort, we aimed to overcome these pitfalls and adequately characterize myocardial damage in COVID-19.
MethodsConsecutive patients with confirmed SARS-CoV-2 infection and available high-sensitive troponin I (hs-TnI), from March 1st to April 20th, 2020 who completed at least 1-month follow-up or died, were studied.
ResultsA total of 918 patients (mean age 63.2±15.5 years, 60.1% male) with a median follow-up of 57 (49–63) days were included. Of these, 190 (20.7%) fulfilled strict criteria for myocardial injury (21.1% chronic, 76.8% acute non-ischemic, 2.1% acute ischemic). Time from onset of symptoms to maximum hs-TnI was 11 (7–18) days. Thrombotic and bleeding events, arrhythmias, heart failure, need for mechanical ventilation and death were significantly more prevalent in patients with higher hs-TnI concentrations, even without fulfilling criteria for myocardial injury. hs-TnI was identified as an independent predictor of mortality [HR 2.52 (1.57–4.04) per 5-logarithmic units increment] after adjusting for multiple relevant covariates.
ConclusionElevated hs-TnI is highly prevalent among patients with SARS-CoV-2 infection. Even mild elevations well below the 99th URL were significantly associated with higher rates of cardiac and non-cardiac complications, and higher mortality. Future research should address the role of serial hs-TnI assessment to improve COVID-19 prognostic stratification and clinical outcomes.
El daño miocárdico parece una complicación frecuente en pacientes con COVID-19. Sin embargo, la literatura al respecto presenta serias limitaciones, como definiciones incorrectas, heterogeneidad de las determinaciones de troponina o seguimientos cortos. Utilizando datos de una cohorte amplia, el objetivo del trabajo fue caracterizar adecuadamente el daño miocárdico en pacientes con COVID-19 utilizando una metodología adecuada.
MétodosSe estudió a pacientes consecutivos con infección confirmada y determinaciones disponibles de troponina I de alta sensibilidad (hs-TnI), desde el 1 de marzo hasta el 20 de abril del 2020, que hubieran completado al menos un mes de seguimiento o fallecieran durante el periodo de estudio.
ResultadosSe incluyó a 918 pacientes (edad 63,2±15,5 años, 60,1% varones) con un seguimiento mediano de 57 (49-63) días. De estos, 190 (20,7%) cumplían criterios estrictos de lesión miocárdica (21,1% crónica, 76,8% aguda no isquémica, 2,1% aguda isquémica). El tiempo desde el inicio de los síntomas hasta la hs-TnI máxima fue de 11 (7-18) días. Los eventos trombóticos y hemorrágicos, las arritmias, la insuficiencia cardíaca, la necesidad de ventilación mecánica y la muerte fueron significativamente más frecuentes en pacientes con concentraciones elevadas de hs-TnI, incluso por debajo del nivel de lesión miocárdica. La hs-TnI resultó un predictor independiente de mortalidad (HR 2,52 [(1,57-4,04] por cada 5 unidades logarítmicas).
ConclusiónLa hs-TnI elevada es altamente prevalente entre los pacientes con COVID-19. Elevaciones leves muy por debajo del límite para definir lesión miocárdica se asociaron con más complicaciones y mayor mortalidad. La determinación protocolizada de hs-TnI en estos enfermos podría mejorar su estratificación pronóstica y los resultados clínicos.
Myocardial injury has been identified as a common complication in patients with COVID-19 and has been associated with poorer outcomes. However, recent research focused on troponin in patients with SARS-CoV-2 infection have serious limitations such as non-guideline definition of myocardial injury, heterogenicity of troponin sampling or very short-term follow-up. Hence, improved methodology to make research more interpretable and comparable has been advocated.1
Furthermore, this condition has been associated with a wide spectrum of cardiovascular diseases such as pulmonary embolism, arrhythmias and heart failure.2 Hence, the potential links between these clinical events and myocardial injury is not well defined.
Using data from a large cohort of patients with confirmed SARS-CoV-2 infection, we aimed to overcome these pitfalls and adequately characterize myocardial damage in COVID-19 patients using high sensitivity cardiac troponin with strict definitions and a prolonged period of follow-up. Specifically, we aimed to investigate the association of high sensitivity troponin I (hs-TnI) and all-cause mortality after the diagnosis of SARS-CoV-2 infection.
MethodsStudy design and participantsWe screened all consecutive patients with clinical suspicion of COVID-19 attended at the Emergency Room in a tertiary care center in Madrid from March 1st 2020 to April 20th, 2020. Patients were only considered to be included in the study if they had confirmation of SARS-CoV2 infection by RNA reverse-transcriptase-polymerase-chain-reaction (RT-PCR) assay of nasal or pharyngeal swab specimens and have at least one hs-TnI determination after hospital admission. We aimed to include patients who have completed a follow-up of at least 30 days since their diagnosis. Therefore, patients who were alive and diagnosed less than 30 days before the lock of the database were excluded from the present analysis. The present study was approved by our Institutional Review Board. Individual written Informed consent was waived based on legal standards for national healthcare alarm situations.
Data collectionEpidemiological, demographic, clinical, laboratory, treatment, and outcome data were extracted from electronic medical records from the index and subsequent hospital admissions using a standardized electronic data collection form. In addition, the central healthcare record system, which collects information and medical reports from all public hospitals and primary healthcare centers from the Madrid region was reviewed for additional information and follow-up. All data were thoroughly reviewed by a team of 13 cardiologists. Special care was given to the identification of cardiovascular (CV) baseline characteristics and outcomes. Any disagreements regarding data classification were reviewed by the whole team, and a decision was finally made by consensus.
Troponin assay technical characteristicsTroponin determinations were performed using a dedicated troponin I assay using chemiluminensce (Atellica Solution IM1600, Siemens Healthineers). According to the IFCC (International Federation of Clinical Chemistry) definition, this method is considered as a high sensitivity troponin. The 99th percentile upper reference limit (URL) is 34.1ng/l for males and 53.5 for females, the limit for detection being 2.6ng/l. Analytical imprecision has been identified as 5.5% at 36.4ng/l and 3.8% at 11,711ng/l.
Study definitionsThe primary endpoint of the present study was all-cause mortality. Thromboembolic events during follow-up were defined as the diagnosis of deep vein thrombosis, pulmonary embolism, stroke or acute coronary syndrome based on appropriate imaging criteria. Major bleeding was defined as specified in the Thrombolysis in Myocardial Infarction (TIMI) bleeding classification3 (drop in haemoglobin ≥5g/dl, intracranial or fatal bleeding). Chronic heart failure (CHF) was defined as history of previous congestive decompensation or diagnosis of left ventricular systolic dysfunction (LVEF<40%). Arrhythmias during admission were defined as new onset of atrial (atrial fibrillation/atrial flutter) or ventricular (ventricular tachycardia/ventricular fibrillation) arrhythmias during follow-up. Acute heart failure (AHF) refers to rapid onset or worsening of symptoms and/or signs of HF during the study period. Acknowledging the difficulty to distinguish between respiratory and cardiac causes of dyspnea in COVID-19, acute heart failure events were adjudicated on a case-by-case basis by consensus of all investigators, as previously published.4
Statistical analysisCategorical variables are shown as rates and percentages, and continuous variables as mean (SD) or median (IQR) as appropriate. Means for continuous variables were compared using independent group t tests when data were normally distributed, otherwise, Mann–Whitney test was performed. Normality of distributions was assessed using Shapiro–Wilk test. Proportions for categorical variables were compared using the χ2 test or the Fisher exact test, as appropriate. For the analysis as a continuous variable, the highest measured cardiac troponin I concentrations during the whole study period were log-transformed as a linearizing transformation. Patients were categorized into three groups: hs-TnI below the limit of detection, measurable hs-TnI below the 99th upper reference limit (URL) for each gender and myocardial injury as defined in the fourth universal definition of myocardial infarction.5 Besides, all patients with measurable levels of hs-TnI were split into quartiles for further analysis. Survival during follow-up was assessed using Kaplan–Meier analysis and, when appropriate, the log-rank test. The association of hs-TnI with mortality during follow-up was studied using a Cox-proportional hazards model accounting for relevant covariates (age, sex, time from symptoms to diagnosis, CV risk factors, cardiac and non-cardiac comorbidities, vital signs at admission, laboratory results and dedicated COVID-19 treatment). All data were analyzed using the Stata v14.2 statistics package (StataCorp, College Station, TX, USA). Confidence intervals were prioritized in order to interpret statistical significance.
ResultsDuring the study period, 3416 consecutive patients with confirmed SARS-CoV-2 infection attended the Emergency Department of our tertiary care center and were screened for participation in the present study. Of these, 918 [mean age 63.2 (15.5) years, 60.1% male] with available hs-TnI determinations fulfilled all the selection criteria and were ultimately included in the present analysis (supplementary Fig. 1, full study flow chart). Differences between patients with and without hs-TnI determinations are showed in the supplementary Table 1.
The median follow-up was 57 (49–63) days. Time from onset of symptoms and from COVID-19 diagnosis to maximum hs-TnI was 11 (7–18) days and 4 (1–9) days respectively. The majority of the included patients finally required hospital admission (830, 90.4%), the leading reason being respiratory failure associated with pneumonia (776 patients, of whom 607 had bilateral infiltrates). A total of 184 patients (20.0%) died during the study period.
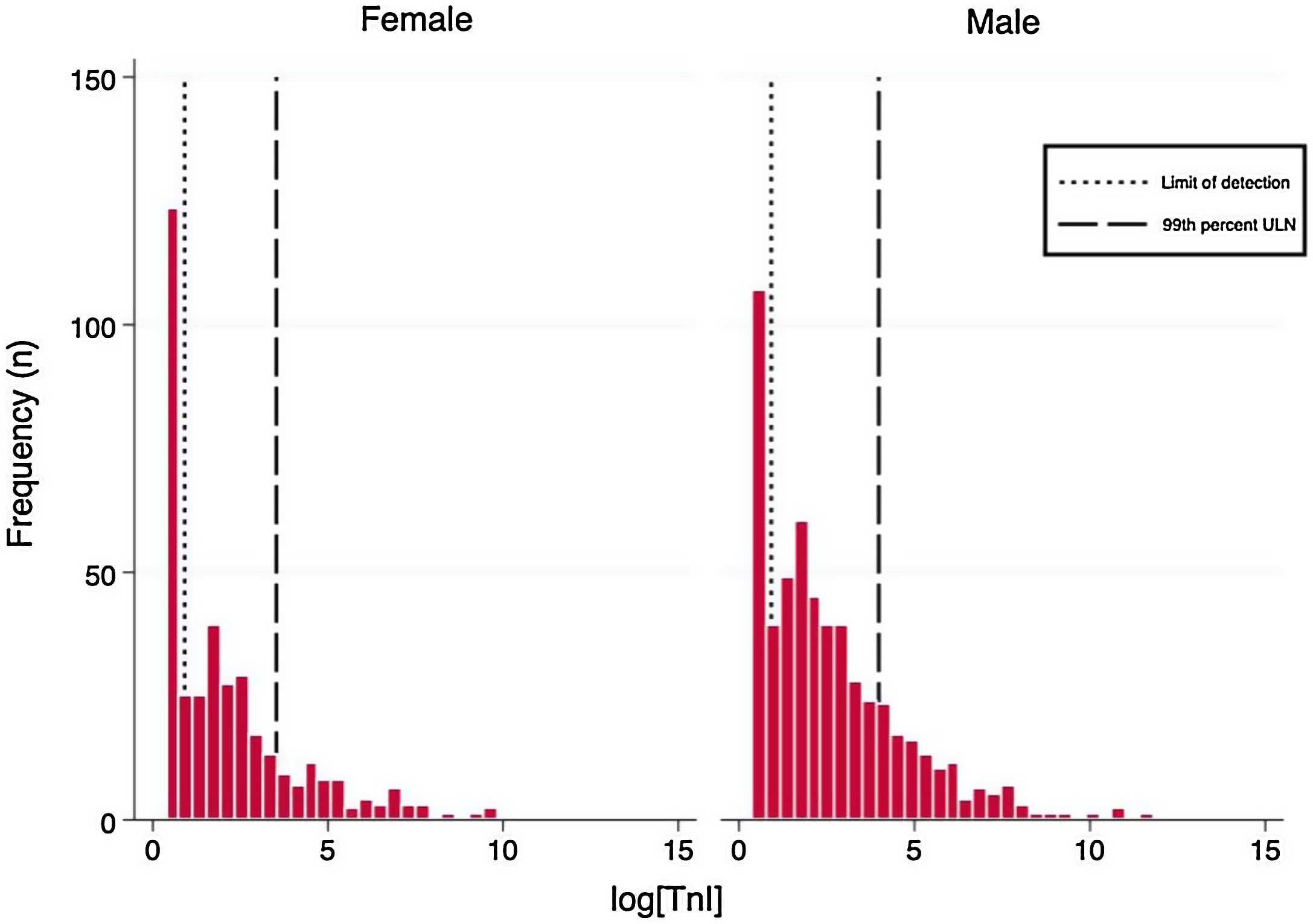
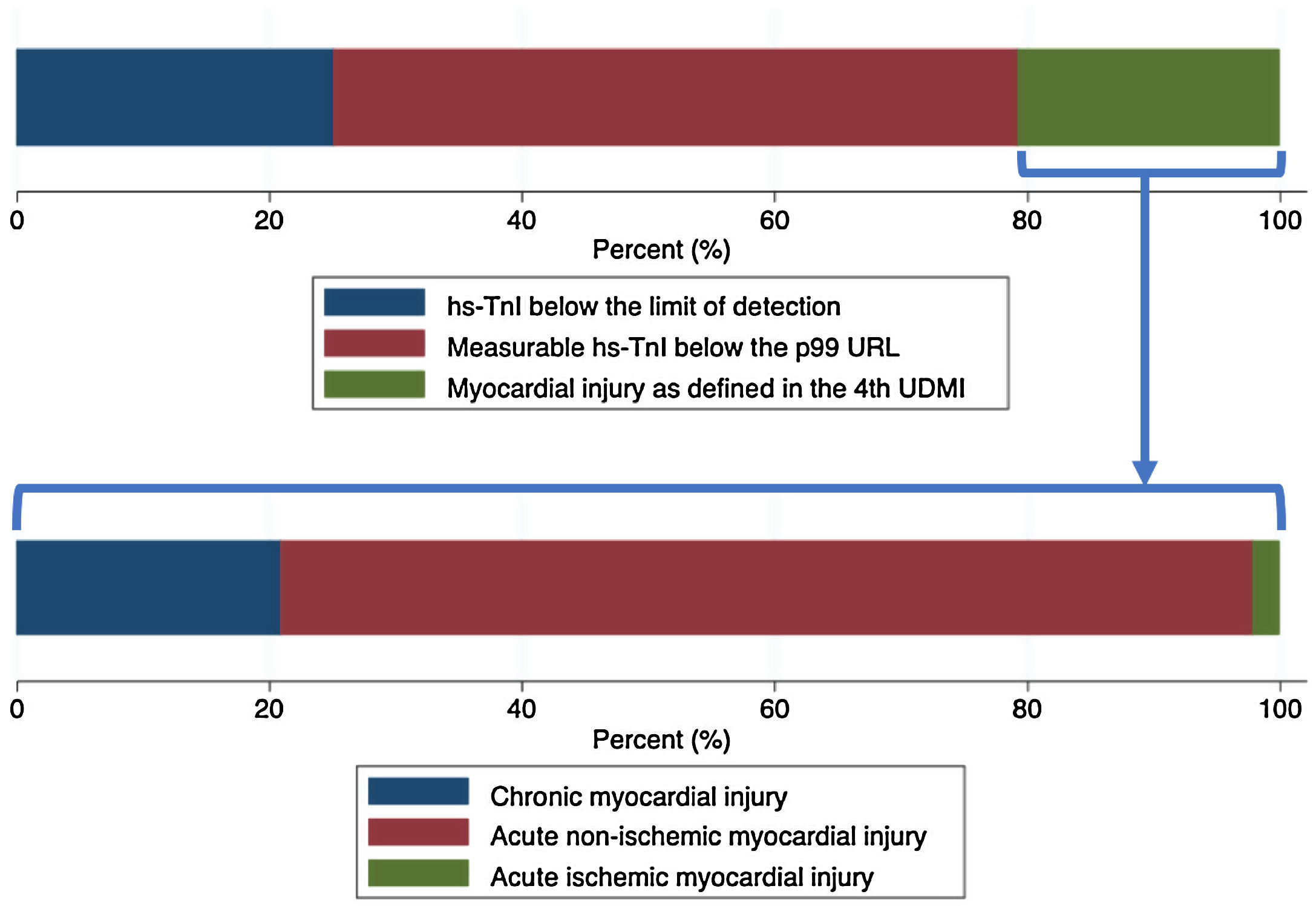
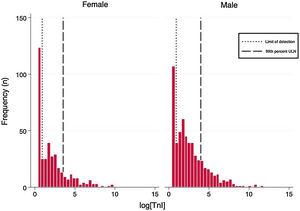
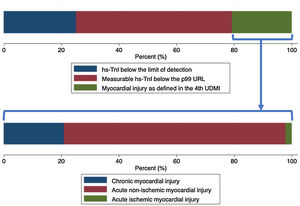
Laboratory resultsThe distributions of the logarithmic hs-TnI concentration among males and females are displayed in Fig. 1. A total of 231 patients (25.2%) presented hs-TnI levels below the limit of detection, 497 (54.1%) had measurable hs-TnI levels below the 99th URL and 190 (20.7%) fulfilled criteria for myocardial injury as defined in the fourth universal definition of myocardial infarction.2 Further categorization of this latter group can be found in Fig. 2. A total of 40 patients (21.1%) fulfilled criteria for chronic myocardial injury (presence of chronic conditions associated with hs-TnI concentrations above the 99th URL, but without serial changes >20%). The remaining 150 patients received a diagnosis of acute myocardial injury: 146 (76.8%) with acute non-ischemic myocardial injury and 4 (2.1%) with acute ischemic myocardial injury (3 patients with non-ST elevation acute coronary syndrome and 1 patient with ST elevation acute coronary syndrome).
On the other hand, a subgroup analysis including those patients who had available determinations of hs-TnI and NT-proBNP during the study period, showed a moderate but highly significant positive correlation between both biomarker concentrations (correlation coefficient 0.591, p<0.001, supplementary Fig. 2).
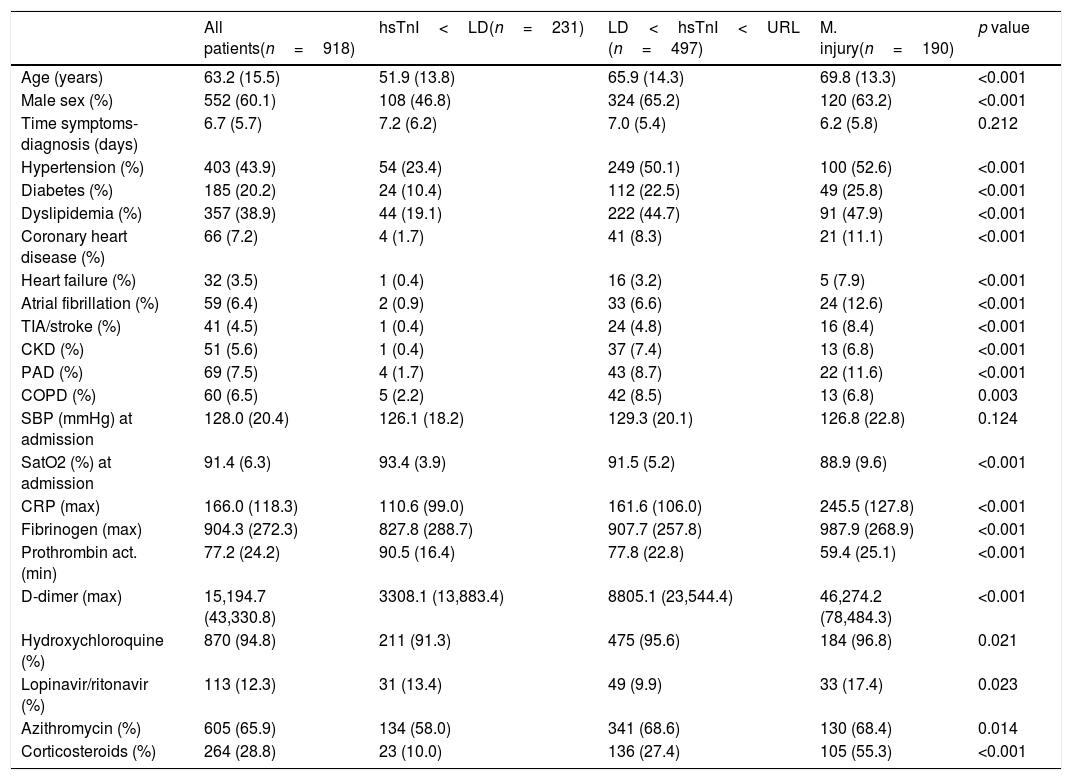
The categorization of the hs-TnI values (Table 1 and Supplementary Table 2) showed statistically significant differences in all baseline characteristics, except in the interval between the onset of symptoms and diagnosis and the systolic pressure on admission. Thus, higher hs-TnI values were associated with older age, more comorbidities, worse respiratory status upon admission, greater alterations in inflammatory and thrombotic markers, and more frequent use of COVID-19 dedicated medications.
Baseline characteristics among different categories of myocardial damage according to the criteria stated in the Fourth Definition of Myocardial Infarction.
| All patients(n=918) | hsTnI<LD(n=231) | LD<hsTnI<URL (n=497) | M. injury(n=190) | p value | |
|---|---|---|---|---|---|
| Age (years) | 63.2 (15.5) | 51.9 (13.8) | 65.9 (14.3) | 69.8 (13.3) | <0.001 |
| Male sex (%) | 552 (60.1) | 108 (46.8) | 324 (65.2) | 120 (63.2) | <0.001 |
| Time symptoms-diagnosis (days) | 6.7 (5.7) | 7.2 (6.2) | 7.0 (5.4) | 6.2 (5.8) | 0.212 |
| Hypertension (%) | 403 (43.9) | 54 (23.4) | 249 (50.1) | 100 (52.6) | <0.001 |
| Diabetes (%) | 185 (20.2) | 24 (10.4) | 112 (22.5) | 49 (25.8) | <0.001 |
| Dyslipidemia (%) | 357 (38.9) | 44 (19.1) | 222 (44.7) | 91 (47.9) | <0.001 |
| Coronary heart disease (%) | 66 (7.2) | 4 (1.7) | 41 (8.3) | 21 (11.1) | <0.001 |
| Heart failure (%) | 32 (3.5) | 1 (0.4) | 16 (3.2) | 5 (7.9) | <0.001 |
| Atrial fibrillation (%) | 59 (6.4) | 2 (0.9) | 33 (6.6) | 24 (12.6) | <0.001 |
| TIA/stroke (%) | 41 (4.5) | 1 (0.4) | 24 (4.8) | 16 (8.4) | <0.001 |
| CKD (%) | 51 (5.6) | 1 (0.4) | 37 (7.4) | 13 (6.8) | <0.001 |
| PAD (%) | 69 (7.5) | 4 (1.7) | 43 (8.7) | 22 (11.6) | <0.001 |
| COPD (%) | 60 (6.5) | 5 (2.2) | 42 (8.5) | 13 (6.8) | 0.003 |
| SBP (mmHg) at admission | 128.0 (20.4) | 126.1 (18.2) | 129.3 (20.1) | 126.8 (22.8) | 0.124 |
| SatO2 (%) at admission | 91.4 (6.3) | 93.4 (3.9) | 91.5 (5.2) | 88.9 (9.6) | <0.001 |
| CRP (max) | 166.0 (118.3) | 110.6 (99.0) | 161.6 (106.0) | 245.5 (127.8) | <0.001 |
| Fibrinogen (max) | 904.3 (272.3) | 827.8 (288.7) | 907.7 (257.8) | 987.9 (268.9) | <0.001 |
| Prothrombin act. (min) | 77.2 (24.2) | 90.5 (16.4) | 77.8 (22.8) | 59.4 (25.1) | <0.001 |
| D-dimer (max) | 15,194.7 (43,330.8) | 3308.1 (13,883.4) | 8805.1 (23,544.4) | 46,274.2 (78,484.3) | <0.001 |
| Hydroxychloroquine (%) | 870 (94.8) | 211 (91.3) | 475 (95.6) | 184 (96.8) | 0.021 |
| Lopinavir/ritonavir (%) | 113 (12.3) | 31 (13.4) | 49 (9.9) | 33 (17.4) | 0.023 |
| Azithromycin (%) | 605 (65.9) | 134 (58.0) | 341 (68.6) | 130 (68.4) | 0.014 |
| Corticosteroids (%) | 264 (28.8) | 23 (10.0) | 136 (27.4) | 105 (55.3) | <0.001 |
Note: Quantitative date are expressed in mean (SD). Abbreviatures: TIA: transient ischemic attack; CKD: chronic kidney disease; PAD: peripheral artery disease; COPD: chronic pulmonary obstructive disease; SBP: systolic blood pressure; CRP: C-reactive protein.
All patients with a diagnosis of acute coronary syndrome received dual antiplatelet therapy according to the European Society of Cardiology Clinical Practice Guidelines.6,7 Two patients (including the one with ST-elevation ACS) underwent coronary angiography and stent implantation during hospital admission, while the other two were managed conservatively due to severe COVID-19 disease.
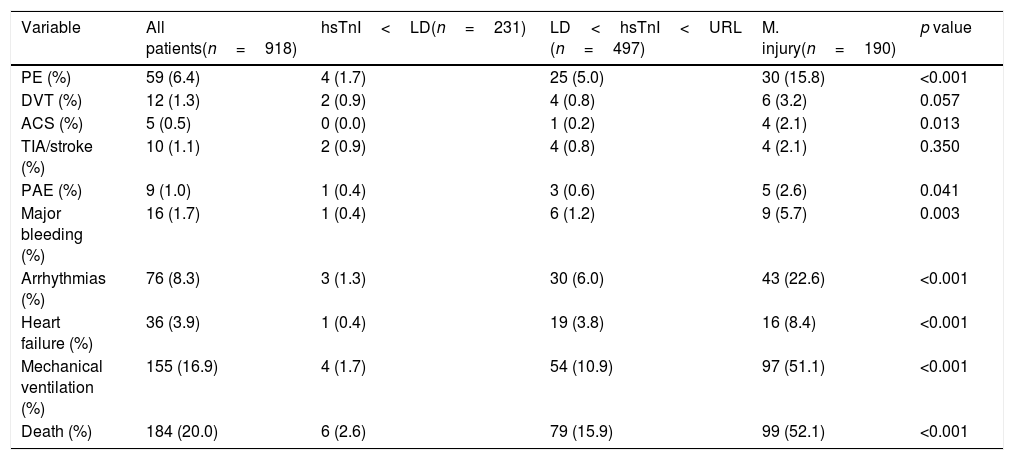
All relevant clinical events (thrombotic and bleeding events, arrhythmias, heart failure decompensations, need for mechanical ventilation and death) were significantly more prevalent in patients with higher hs-TnI concentrations (Table 2 and Supplementary Table 3), exception made of deep vein thrombosis and cerebrovascular events.
Clinical outcomes among different categories of myocardial damage according to the criteria stated in the Fourth Definition of Myocardial Infarction.
| Variable | All patients(n=918) | hsTnI<LD(n=231) | LD<hsTnI<URL (n=497) | M. injury(n=190) | p value |
|---|---|---|---|---|---|
| PE (%) | 59 (6.4) | 4 (1.7) | 25 (5.0) | 30 (15.8) | <0.001 |
| DVT (%) | 12 (1.3) | 2 (0.9) | 4 (0.8) | 6 (3.2) | 0.057 |
| ACS (%) | 5 (0.5) | 0 (0.0) | 1 (0.2) | 4 (2.1) | 0.013 |
| TIA/stroke (%) | 10 (1.1) | 2 (0.9) | 4 (0.8) | 4 (2.1) | 0.350 |
| PAE (%) | 9 (1.0) | 1 (0.4) | 3 (0.6) | 5 (2.6) | 0.041 |
| Major bleeding (%) | 16 (1.7) | 1 (0.4) | 6 (1.2) | 9 (5.7) | 0.003 |
| Arrhythmias (%) | 76 (8.3) | 3 (1.3) | 30 (6.0) | 43 (22.6) | <0.001 |
| Heart failure (%) | 36 (3.9) | 1 (0.4) | 19 (3.8) | 16 (8.4) | <0.001 |
| Mechanical ventilation (%) | 155 (16.9) | 4 (1.7) | 54 (10.9) | 97 (51.1) | <0.001 |
| Death (%) | 184 (20.0) | 6 (2.6) | 79 (15.9) | 99 (52.1) | <0.001 |
Abbreviatures: PE: pulmonary embolism; DVT: deep vein thrombosis; ACS: acute coronary syndrome; TIA: transient ischemic attack; PAD: peripheral arterial events.
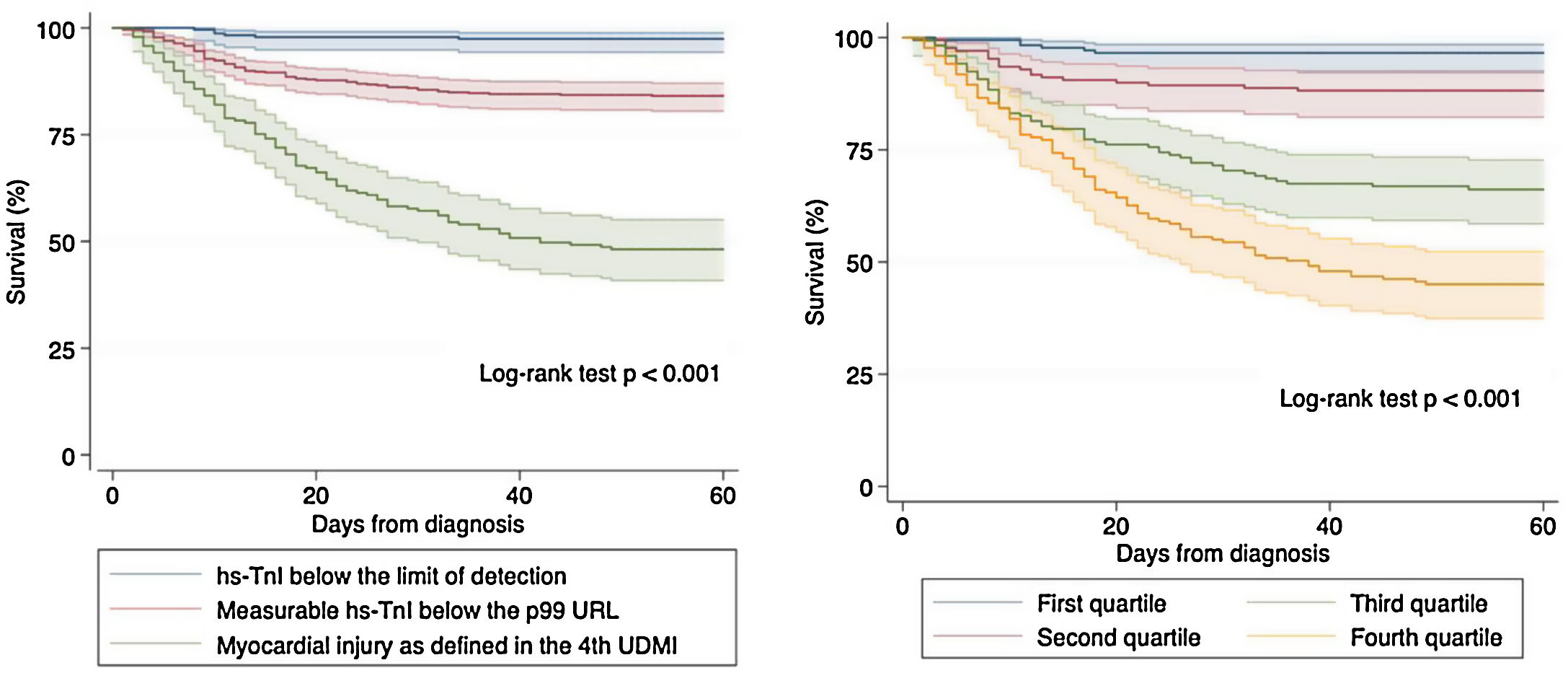
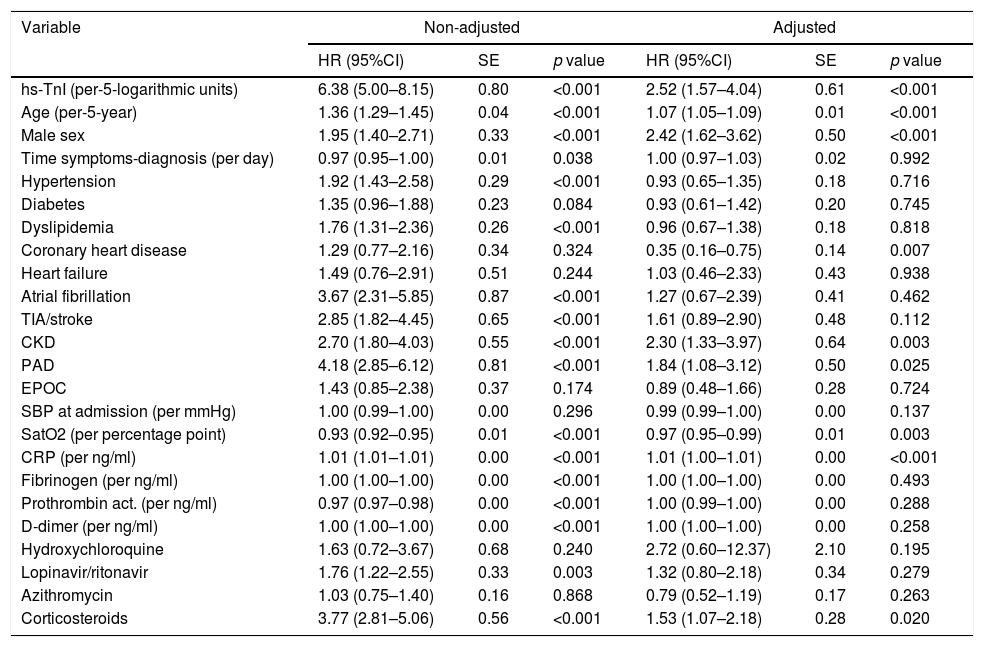
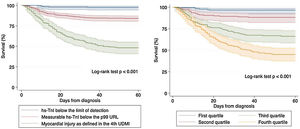
Survival analysis using Kaplan–Meier techniques (Fig. 3) showed a clearly significant relationship between both different categorizations of hs-TnI and higher mortality during follow-up (p<0.001 for the log-rank test regarding both comparisons). In order to adequately characterize the prognostic value of hs-TnI, we performed a multivariable analysis using a Cox proportional hazards model (Table 3) including as dependent covariates the logarithmic transformation of hs-TnI and all potentially relevant confounders (age, sex, time from symptoms to diagnosis, CV risk factors, cardiac and non-cardiac comorbidities, vital signs at admission, laboratory results and dedicated COVID-19 treatment). Thus, hs-TnI was confirmed to be an independent predictor of mortality [HR 2.52 (1.57–4.04) per 5-logarithmic units increment].
Cox-proportional hazards model assessing the relationship between hs-TnI and mortality during follow-up adjusted for multiple relevant covariates.
| Variable | Non-adjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| HR (95%CI) | SE | p value | HR (95%CI) | SE | p value | |
| hs-TnI (per-5-logarithmic units) | 6.38 (5.00–8.15) | 0.80 | <0.001 | 2.52 (1.57–4.04) | 0.61 | <0.001 |
| Age (per-5-year) | 1.36 (1.29–1.45) | 0.04 | <0.001 | 1.07 (1.05–1.09) | 0.01 | <0.001 |
| Male sex | 1.95 (1.40–2.71) | 0.33 | <0.001 | 2.42 (1.62–3.62) | 0.50 | <0.001 |
| Time symptoms-diagnosis (per day) | 0.97 (0.95–1.00) | 0.01 | 0.038 | 1.00 (0.97–1.03) | 0.02 | 0.992 |
| Hypertension | 1.92 (1.43–2.58) | 0.29 | <0.001 | 0.93 (0.65–1.35) | 0.18 | 0.716 |
| Diabetes | 1.35 (0.96–1.88) | 0.23 | 0.084 | 0.93 (0.61–1.42) | 0.20 | 0.745 |
| Dyslipidemia | 1.76 (1.31–2.36) | 0.26 | <0.001 | 0.96 (0.67–1.38) | 0.18 | 0.818 |
| Coronary heart disease | 1.29 (0.77–2.16) | 0.34 | 0.324 | 0.35 (0.16–0.75) | 0.14 | 0.007 |
| Heart failure | 1.49 (0.76–2.91) | 0.51 | 0.244 | 1.03 (0.46–2.33) | 0.43 | 0.938 |
| Atrial fibrillation | 3.67 (2.31–5.85) | 0.87 | <0.001 | 1.27 (0.67–2.39) | 0.41 | 0.462 |
| TIA/stroke | 2.85 (1.82–4.45) | 0.65 | <0.001 | 1.61 (0.89–2.90) | 0.48 | 0.112 |
| CKD | 2.70 (1.80–4.03) | 0.55 | <0.001 | 2.30 (1.33–3.97) | 0.64 | 0.003 |
| PAD | 4.18 (2.85–6.12) | 0.81 | <0.001 | 1.84 (1.08–3.12) | 0.50 | 0.025 |
| EPOC | 1.43 (0.85–2.38) | 0.37 | 0.174 | 0.89 (0.48–1.66) | 0.28 | 0.724 |
| SBP at admission (per mmHg) | 1.00 (0.99–1.00) | 0.00 | 0.296 | 0.99 (0.99–1.00) | 0.00 | 0.137 |
| SatO2 (per percentage point) | 0.93 (0.92–0.95) | 0.01 | <0.001 | 0.97 (0.95–0.99) | 0.01 | 0.003 |
| CRP (per ng/ml) | 1.01 (1.01–1.01) | 0.00 | <0.001 | 1.01 (1.00–1.01) | 0.00 | <0.001 |
| Fibrinogen (per ng/ml) | 1.00 (1.00–1.00) | 0.00 | <0.001 | 1.00 (1.00–1.00) | 0.00 | 0.493 |
| Prothrombin act. (per ng/ml) | 0.97 (0.97–0.98) | 0.00 | <0.001 | 1.00 (0.99–1.00) | 0.00 | 0.288 |
| D-dimer (per ng/ml) | 1.00 (1.00–1.00) | 0.00 | <0.001 | 1.00 (1.00–1.00) | 0.00 | 0.258 |
| Hydroxychloroquine | 1.63 (0.72–3.67) | 0.68 | 0.240 | 2.72 (0.60–12.37) | 2.10 | 0.195 |
| Lopinavir/ritonavir | 1.76 (1.22–2.55) | 0.33 | 0.003 | 1.32 (0.80–2.18) | 0.34 | 0.279 |
| Azithromycin | 1.03 (0.75–1.40) | 0.16 | 0.868 | 0.79 (0.52–1.19) | 0.17 | 0.263 |
| Corticosteroids | 3.77 (2.81–5.06) | 0.56 | <0.001 | 1.53 (1.07–2.18) | 0.28 | 0.020 |
Abbreviatures: TIA: transient ischemic attack; CKD: chronic kidney disease; PAD: peripheral artery disease; COPD: chronic pulmonary obstructive disease; SBP: systolic blood pressure; CRP: C-reactive protein.
Since the early days of the pandemic, cardiac damage has been identified as a relatively common finding and a prognostic marker of poor outcomes in COVID-19 patients. Thus, Shi et al. described a cohort of 416 patients in Wuhan, in whom 19.7% had hs-TnI concentrations above the 99th URL, a condition that was associated with higher risk of death.8 Guo et al. showed similar results in 187 patients using TnT.9 Subsequent research from China has been assessed in a recent meta-analysis including 22 studies and 3684 patients.10 However, the definition employed for myocardial damage in this latter work not only included troponin, but also elevated levels of lactate dehydrogenase and creatine kinase MB. Indeed, the issue of myocardial damage definition has been a common problem in all COVID-19 literature, with some studies using non-guideline myocardial damage definition or even criteria based on electrocardiography.11 In fact, technical details of the specific analytical assays used to quantify troponin concentrations have been almost universally omitted even in research papers specifically focused on cardiac damage. On the other side, timing of hs-TnI sampling has been extraordinary heterogeneous between studies. Most studies report only single point early determinations, like a recent large cohort study from the United States, that included only hs-TnI measurements during the first 24h from hospital admission.12 This may result in reduced sensitivity for the detection of elevated troponin and underdiagnosis of cardiac damage, specifically in patients with progressive clinical deterioration during admission.
We report here data from a large cohort of consecutive patients with confirmed SARS-CoV-2 infection and prolonged follow-up, using a rigorous definition of myocardial damage as stablished after the Fourth Universal Definition of Myocardial Infarction.5 In our series, the majority of patients with available troponin determinations had detectable levels of hs-TnI. Almost a third of these patients fulfilled criteria for myocardial injury, the majority of them exceeding the 20% delta that stablishes the diagnosis of acute cardiac injury.5 However, only 4 patients (2.1% of the total population) during the study period suffered a classical acute coronary syndrome. In our study, in which the decision to request a troponin determination was left to the discretion of the treating physician, the moment at which the maximum value of hs-TnI was identified was well beyond the first 24h of admission. This underscores the fact that cardiac involvement in COVID-19 may present late during the course of the disease, and the development of cardiac signs or symptoms should prompt further investigations.
Many different mechanisms leading to cardiac damage in COVID-19 patients have been described, including acute coronary syndromes,13,14 Tako-tsubo,15 myocarditis16 and even pulmonary embolism.17 However, the actual incidence of these specific cardiac complications does not seem to be so high. Several authors have tried to characterize cardiac damage in patients recovered from the disease using cardiac magnetic resonance, showing that cardiac abnormalities such as elevated T1 and T2, late gadolinium enhancement, myocardial inflammation and edema are common.18,19 Interestingly, a small pathological study including 22 autopsies of patients with SARS-CoV-2 infection, found a pattern of scattered cell apoptosis/necrosis not associated with any lymphocytic infiltrate.20
In our series, patients with higher TnI showed increasingly higher levels of C-reactive protein, fibrinogen, D-dimer and decreased prothrombin activity. This is in accordance with the findings from a prospective cohort study showing that COVID-19-related acute respiratory distress syndrome was associated with a significant increase in procoagulant mediators which closely correlated with the elevation of acute phase reactants.21 These authors also suggested a potential role of pulmonary endothelial cell dysfunction in the local regulation of coagulation/fibrinolysis balance and in situ pulmonary thrombosis. Indeed, histological studies have confirmed the presence of virus material within endothelial cells associated with severe endothelial injury and widespread microthrombi,22,23 that may explain the systemic repercussions in different organs, including the heart. Patients with prior cardiovascular risk factors or stablished cardiovascular disease may be more vulnerable to this endothelial damage and that condition would partially explain their poor outcomes after SARS-CoV-2 infection. Moreover, infection of endothelial cells and host immune and inflammatory response may also play a role in the development of heart failure after COVID-19.4,24,25
Even though hs-TnI above the 99th URL have been identified as an independent predictor of mortality,26 our results highlight that milder elevations are also associated with poorer outcomes and suggest that serial TnI measurement in COVID-19 may improve prognostic stratification. However, elevated hs-TnI concentrations without a proper cardiac diagnosis may also trigger unnecessary tests and procedures that may also lead to unnecessary complications. Therefore, whether a proactive strategy of cardiac damage detection may result in better outcomes remains unknown and should be the focus of future research.
Some limitations should be considered. This was an observational study and therefore may be affected by the inherent bias of this type of design (i.e. the impossibility to establish definitive causal associations). On the other hand, hs-TnI was not routinely assessed in every patient after the diagnosis of SARS-CoV-2 infection and that fact may have introduced a certain selection bias: patients included in the present analysis were older, had more comorbidities and ultimately underwent much more frequent hospitalization than those who did not underwent natriuretic peptides assessment. Thus, our sample should not be viewed as representative of the whole COVID-19 population. Several complications, such as pulmonary embolism, may have been underdiagnosed due to prioritization of hospital resources and isolation protocols established to avoid the spread of the disease among healthcare providers and leading to restricted non-invasive studies.27
To conclude, elevated hs-TnI is highly prevalent among patients with SARS-CoV-2 infection. Around a fifth of the study population fulfilled strict criteria for the diagnosis of myocardial damage. Even mild elevations well below the 99th URL were significantly associated with higher rates of cardiac and non-cardiac complications, and higher mortality. Future research should address the role of serial hs-TnI assessment to improve COVID-19 prognostic stratification and clinical outcomes.
Ethics approvalThe present study was approved by our Institutional Review Board and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Individual written Informed consent was waived based on legal standards for national healthcare alarm situations.
FundingThis work has not received funding.
Conflict of interestJ.L.M. reports grants and personal fees from Bayer, grants and personal fees from Correvio, grants from Daiichi-Sankyo, personal fees from Sanofi, outside the submitted work; E.L.S reports grants from Zoll Medical Corporation, Boehringer Ingelheim and Servier; personal fees from Daiichi Sankyo, Rovi, Servier, BARD and Astra Zeneca, all outside the submitted work.
We thank Sylvia Merino, M.D, for reviewing the manuscript and Inmaculada Haro and Milagros Lopez Nieto, from the IT department, for their support extracting electronic medical records.