Direct oral anticoagulants (DOACs) could effectively prevent the occurrence of cancer-associated venous thromboembolism (CAVTE), which incidence rate was estimated to be 4–20%. But the efficacy and safety remain controversial between DOACs and low molecular weight heparin (LMWH).
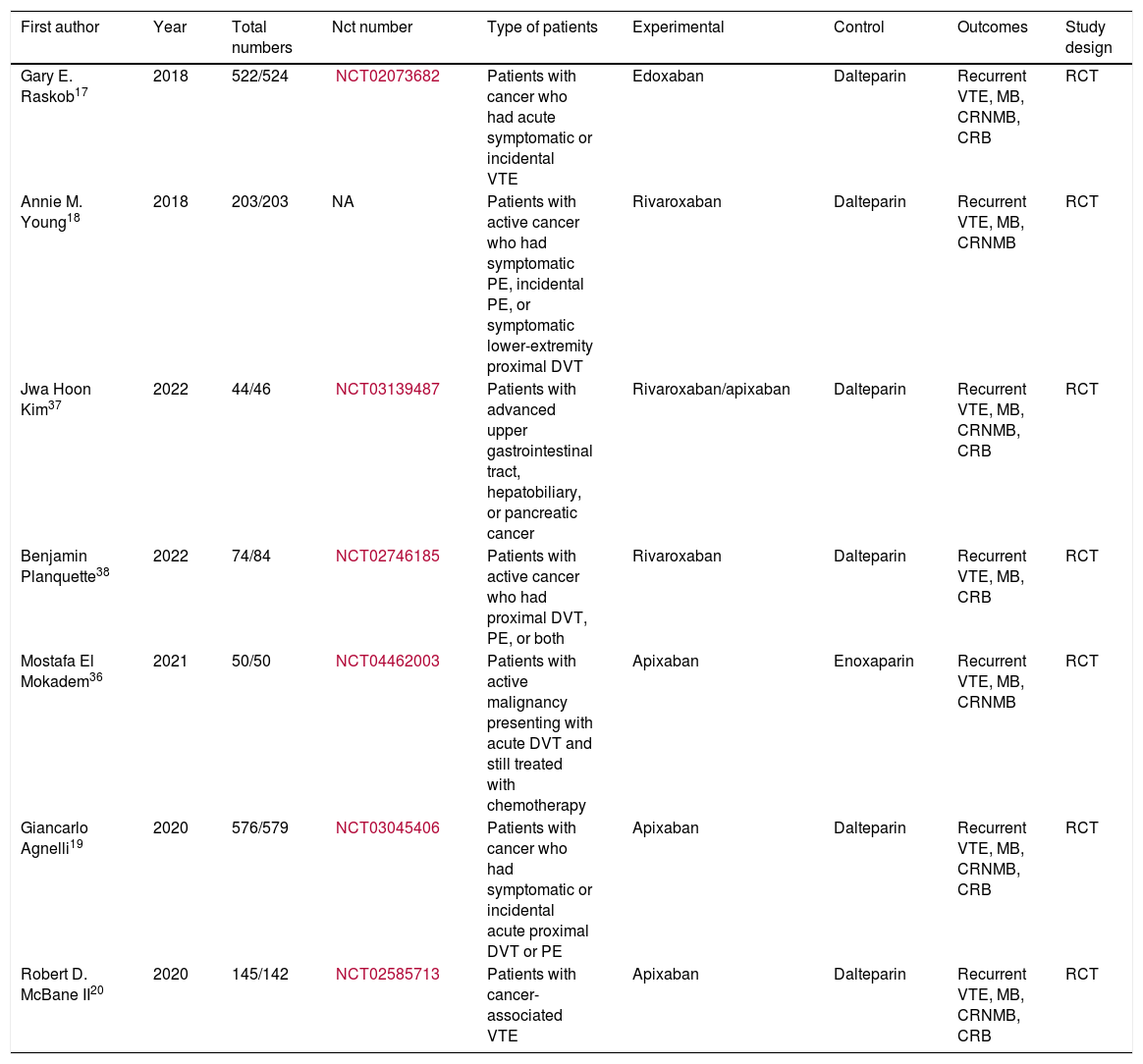
Materials and methodsPubMed, Cochrane Library, Embase, ClinicalTrials.gov databases for randomized controlled trials (RCTs) were systematically searched from inception to March 15, 2022. A random-effects model was used to report the odds ratio (OR) and 95% confidence interval (CI) for both direct and network meta-analyses.
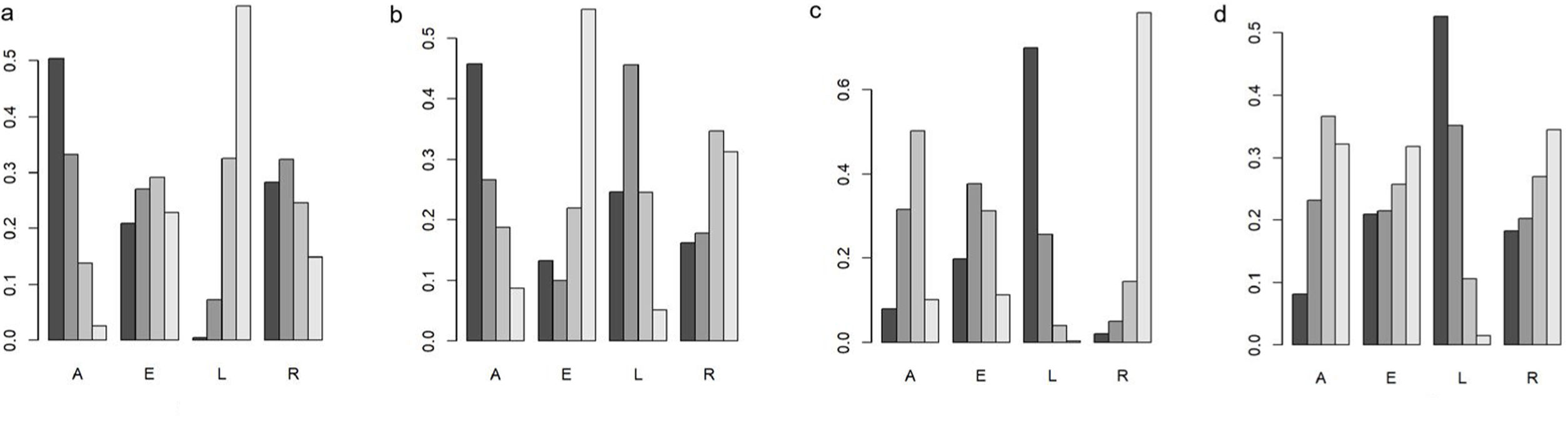
ResultsSeven studies were included totaling 3242 patients. A lower rate of recurrence VTE was noted in the DOACs compared with LMWH (OR 0.62, 95% CI 0.47–0.82, I2=0.0%). The aspect of major bleeding (MB) was similar (OR 1.30, 95% CI 0.77–2.18, I2=34.9%). When assessing clinically relevant nonmajor bleeding (CRNMB) (OR 1.61, 95% CI 1.17–2.22, I2=20.7%) and clinically relevant bleeding (CRB) (OR 1.39, 95% CI 1.11–1.74, I2=0.0%), a higher risk of events was observed in DOACs. In subgroup analyses, the MB of gastrointestinal and genitourinary malignancies had a higher rate in the DOACs. For ranking, apixaban ranked the first in prevention of VTE and reducing MB events. Edoxaban had the highest risk drug in MB. In terms of CRNMB and CRB, LMWH showed the lowest risk.
ConclusionsCompared with LMWH, DOACs seemed to have a decreased risk of recurrence VTE while increasing CRNMB and CRB. DOACs and LMWH were equivalent to the aspect of MB, but DOACs had a higher MB risk in patients with gastrointestinal and genitourinary malignancies. Apixaban may be the lowest risk compared to the other DOACs in precaution of VTE and reducing bleeding events.
Los anticoagulantes orales directos (ACOD) son eficaces en la prevención de la tromboembolia venosa (TEV) relacionada con el cáncer, cuya tasa de incidencia se estima en 4-20%. Sin embargo, la eficacia y seguridad de ACOD y heparina de bajo peso molecular (HBPM) siguen siendo controvertidas.
Materiales y métodosDesde el inicio hasta el 15 de marzo de 2022 se realizaron búsquedas sistemáticas en las bases de datos de ensayos controlados aleatorios (ECA) en PubMed, The Cochrane Library, Embase, ClinicalTrials.gov. Se utilizó el modelo de efectos aleatorios para informar la razón de probabilidades (RP) y los intervalos de confianza (IC) de 95% para los metaanálisis directos y de red.
ResultadosSe incluyeron siete estudios con un total de 3.242 pacientes. En comparación con HBPM, los ACOD tienen una tasa más baja de recurrencia de TEV (OR 0,62, IC 95%: 0,47 a 0,82, I2 = 0,0%). La frecuencia de hemorragias mayores fue similar (OR 1,30, IC 95% 0,77 a 2,18, I2 = 34,9%). Se observó un mayor riesgo de eventos en los ACOD. Cuando se evaluaron las hemorragias no mayores clínicamente relevantes (CRNMB) (OR 1,61, IC 95%: 1,17 a 2,22, I2 = 20,7%) y las hemorragias clínicamente relevantes (OR 1,39, IC 95%: 1,11 a 1,74, I2 = 0,0%), En los análisis de subgrupos, las hemorragias mayores en las neoplasias malignas gastrointestinales y genitourinarias fueron más frecuentes con los ACOD. Apixabán ocupó el primer lugar en la prevención de TEV y la reducción de eventos hemorrágicos mayores. Edoxabán tuvo el mayor riesgo de hemorragias mayores. Las HBPM demostraron tener menor riesgo de hemorragias mayores clínicamente relevantes y hemorragias clínicamente relevantes.
ConclusionesEn comparación con la HBPM, el tratamiento con ACOD se asociaba a un menor riesgo de recurrencia de TEV, mientras que aumentaba el riesgo de sufrir hemorragias mayores clínicamente relevantes y hemorragias clínicamente relevantes. Los ACOD y LMWH comportaban un riego equivalente de sufrir hemorragias mayores. Sin embargo, los ACOD tenían un mayor riesgo de hemorragias mayores en pacientes con neoplasias malignas gastrointestinales y genitourinarias. En comparación con otros ACOD, apixabán tuvo un riesgo más bajo de eventos hemorrágicos cuando se usaba en prevención de la TEV.