Thromboembolic risk is higher in women than men with non-valvular atrial fibrillation (NVAF). Published data indicate variability in antithrombotic use by gender and region. We analyzed gender-specific antithrombotic treatment patterns in Spain and rest of Western Europe (rWE) in patients with NVAF.
MethodsGLORIA-AF (Phase III) is a global, prospective, observational study which enrolled newly diagnosed NVAF patients with CHA2DS2-VAScs≥1 (2014–2016). Analyses were performed comparing antithrombotic treatments by gender in Spain and rWE.
ResultsThis analysis included 1163 and 7972 patients from Spain and rWE, respectively. Stroke risk was higher in women than men in both Spain and rWE. While in rWE, bleeding risk and antithrombotic treatment pattern were similar between genders, in Spain bleeding risk in women was lower and more females compared to men received OACs (95.0% versus 92.4%, d=−0.1078, respectively). Fewer Spanish patients received direct oral anticoagulants (DOACs) (women 32.1%, men 25.3%) than vitamin-K-antagonists (VKAs) (women 63.0%, men 67.1%) vs. rWE patients. In Spain women received more DOACs compared to men (56.0% versus 44.0%).
ConclusionsOAC rates were higher in Spain as compared to rWE. More women received OACs in Spain, while in rWE no difference by gender was observed. DOACs in rWE are the most prescribed OAC while in Spain, due to prescription barriers, its use remains low for both genders and VKAs are preferred. Spanish women received more DOACs compared to men. (NCT01468701).
El riesgo tromboembólico es mayor en mujeres que en varones con fibrilación auricular no valvular (FANV). Existen diferencias en el uso de anticoagulantes (ACO) según sexo y zona geográfica. Se estudiaron los patrones de anticoagulación por sexo en España y el resto de Europa Occidental (rEO) en pacientes con FANV.
MétodosGLORIA-AF es un estudio observacional prospectivo (fase III) que incluyó a pacientes con diagnóstico reciente de FANV y CHA2DS2-VASc>1 (2014-2016). Se analizó la prescripción de anticoagulantes por sexo en España y el rEO.
ResultadosSe incluyó a 1.163 pacientes de España y 7.972 del rEO. El riesgo de ictus fue superior en mujeres tanto en España como en el rEO. El riesgo de hemorragia y el tratamiento antitrombótico fueron similares en ambos sexos en el rEO; en España, el riesgo de hemorragia fue menor en mujeres y estas recibieron más ACO que los varones (95,0% vs. 92,4%, d=–0,1078). En España, menos pacientes recibieron ACO directos (ACOD) (mujeres 32,1%, varones 25,3%) vs. antagonistas de la vitamina K (AVK) (mujeres 63,0%, varones 67,1%), y las mujeres recibieron más ACOD que los varones (56,0% vs. 44,0%).
ConclusionesEn España se emplearon más ACO que en el rEO y más mujeres fueron tratadas con ACO, mientras que en el rEO no hubo diferencias por sexo. En el rEO, los ACOD se emplearon más. En España, los ACOD se emplean menos por restricciones de prescripción y se emplean más los AVK. Las mujeres españolas reciben más ACOD que los varones. (NCT01468701).
Recommendations for long-term oral anticoagulation in patients with atrial fibrillation (AF) are well established in major clinical guidelines and consensus.1–4 Direct oral anticoagulants (DOACs) are preferred over vitamin K antagonists (VKAs) for prevention of stroke due to a better efficacy and safety profile.1,5,6 In 2016, DOACs were used in less than 20% of Spanish patients with non-valvular atrial fibrillation (NVAF).7 In addition, at present there are some local regulatory recommendations in Spain where oral anticoagulants (OACs) are positioned on the basis of a rational use in its National Health System (SNS).8 Thus, DOACs are prescribed in Spain as second line after VKAs while these agents are considered first line therapy in rWE countries.
Furthermore, although studies suggest that women with NVAF are at increased risk of stroke than men,9–11 published data indicate variability in anticoagulation by gender.12–17 The Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA-AF), is a large, three-phased, global prospective registry on patients with newly diagnosed NVAF. This sub-analysis within Phase III of GLORIA-AF explored whether there were any gender differences in the prescription of antithrombotic therapies and to explore the overall differences in antithrombotic treatment patterns among NVAF patients from Spain and the rest of Western Europe (rWE).
The objectives of the present analysis include: (i) To explore the use of different antithrombotic strategies across gender in Spanish versus rWE, (ii) to determine the possible factors related to the use of OACs versus non-use of OACs in the Spanish population, and (iii) to analyze gender differences in the use of DOACs in Spain vs the rWE.
MethodsStudy designThe design of the GLORIA-AF registry program has been published.18 Briefly GLORIA-AF is a prospective, three-phase, global registry program, which assessed the patient characteristics influencing the choice of antithrombotic agent and its outcomes in NVAF patients with ≥1 stroke risk factors.18
Adult patients with newly diagnosed NVAF (<3 months from arrhythmia onset) and a CHA2DS2-VASc score19 of >1 were consecutively enrolled. Globally, subjects were recruited at 935 clinical sites in 38 countries reflecting a wide variety of health care settings (general practices, specialist offices, community hospitals, university hospitals, outpatient care centers, anticoagulation and clinics).
Demographic and baseline characteristics, including stroke and bleeding risk scores (CHA2DS2-VASc and HAS-BLED20) and concomitant medications were collected for all eligible patients by prescribed antithrombotic treatment at the baseline visit.
Patients from Spain consecutively enrolled at 29 clinical sites were compared with patients from rWE enrolled at 305 clinical sites from the following 13 countries: Austria, Belgium, Denmark, France, Germany, Greece, Ireland, Italy, The Netherlands, Norway, Portugal, Switzerland and the United Kingdom.
Data collection and timelinesThe enrolment of Phase III GLORIA-AF was conducted from January 2014 to December 2016. All clinical data were collected and processed using a web-based Electronic Data Capture System (Florida, USA) over a secure network and a complete electronic audit trail. Quality of data entered into the electronic database was monitored and audited during the course of the program.
Statistical analysisBaseline data were summarized descriptively. Continuous variables were reported as mean and standard deviation (SD). Categorical variables were reported as absolute frequencies and percentages. To compare baseline characteristics, antithrombotic therapy and OACs use by gender within Spain and rWE, standardized differences (d) were used. The standardized difference with absolute value <0.1 was considered as balance between groups.
ResultsBaseline characteristicsThe analysis included a total of 9135 eligible patients, 1163 and 7972 from Spain and rWE, respectively (Table 1). In Spain, 583 (50.1%) of patients were female vs 3503 (43.9%) in rWE. Women were older in both Spain and rWE compared to men. The mean (±SD) age was 75.3±9.1 (Spanish females) vs. 71.7±10.5 (Spanish males) (d=−0.3585) and 74.0±9.7 (rWE females) vs. 70.9±10.0 (rWE males) (d=−0.3114).
Baseline characteristics and comorbidities for all eligible patients in Spain and rest of Western Europe (rWE).
| Baseline characteristics | Spain | Rest of Western Europe | ||||
|---|---|---|---|---|---|---|
| Female (n=583) | Male (n=580) | d | Female (n=3503) | Male (n=4469) | d | |
| Age, mean±SD, years | 75.3±9.1 | 71.7±10.5 | −0.3585 | 74.0±9.7 | 70.9±10.0 | −0.3114 |
| CHA2DS2-VASc, mean±SD | 4.0±1.3 | 3.0±1.5 | −0.7379 | 3.8±1.5 | 2.8±1.4 | −0.6974 |
| CHA2DS2-VASc score=1, n (%) | 10 (1.7) | 101 (17.4) | 0.5539 | 135 (3.9) | 862 (19.3) | 0.4972 |
| HAS-BLED score, mean±SDa | 1.2±0.7 | 1.3±0.9 | 0.1400 | 1.3±0.8 | 1.4±0.9 | 0.0806 |
| CrCl, mean±SD, mL/minb | 71.4±31.1 | 83.0±35.8 | 0.3465 | 71.6±30.9 | 85.5±39.7 | 0.3915 |
| Symptomatic AF, n (%) | 205 (35.2) | 151 (26.0) | −0.1991 | 1230 (35.1) | 1223 (27.4) | −0.1677 |
| Paroxysmal AF, n (%) | 251 (43.1) | 206 (35.5) | −0.1548 | 1897 (54.2) | 2067 (46.3) | −0.1585 |
| Persistent AF, n (%) | 217 (37.2) | 251 (43.3) | 0.1237 | 1240 (35.4) | 1913 (42.8) | 0.1522 |
| Previous stroke, n (%) | 35 (6.0) | 57 (9.8) | 0.1420 | 470 (13.4) | 598 (13.4) | −0.0011 |
| Myocardial infarction, n (%) | 29 (5.0) | 81 (14.0) | 0.3108 | 221 (6.3) | 632 (14.1) | 0.2607 |
| Coronary artery disease, n (%) | 40 (6.9) | 99 (17.1) | 0.3185 | 340 (9.7) | 945 (21.1) | 0.3208 |
| Congestive heart failure, n (%) | 134 (23.0) | 170 (29.3) | 0.1443 | 483 (13.8) | 919 (20.6) | 0.1804 |
| Hypertension history, n (%) | 461 (79.1) | 417 (71.9) | −0.1674 | 2518 (71.9) | 3211 (71.9) | −0.0007 |
| Diabetes mellitus, n (%) | 140 (24.0) | 166 (28.6) | 0.1048 | 618 (17.6) | 961 (21.5) | 0.0974 |
| Antiplatelet use, n (%) | 67 (11.5) | 102 (17.6) | 0.1735 | 505 (14.4) | 887 (19.8) | 0.1445 |
AF, atrial fibrillation; CrCl, creatinine clearance; d, standardized difference (male patients minus female patients); SD, standard deviation.
Based on the CHA2DS2-VASc score, stroke risk was higher in Spanish females vs males (4.0±1.3 and 3.0±1.5; d=−0.7379) and also in rWE females vs males (3.8±1.5 and 2.8±1.4; d=−0.6974). Bleeding risk was lower in Spanish females vs males (1.2±0.7 and 1.3±0.9 respectively, d=0.1400); a similar trend was observed among rWE patients (1.3±0.8 and 1.4±0.9 respectively), as assessed by the HAS-BLED score. Bleeding risk was unknown for 5.0% of Spanish women and for 8.1% for men, and for 11.1% of rWE women and for 13.4% for men. In Spain, mean creatinine clearance was lower in women than in men (71.4+31.1mL/min vs 83.0+35.8mL/min; p=0.3465) and the same occurred in the rWE (71.6+30.9mL/min vs 85.5+39.7mL/min; p=0.3915). In the rWE group, prevalence of previous stroke, hypertension history and diabetes, were balanced between genders. A higher proportion of men, more than double, had coronary artery disease and prior myocardial infarction compared to women in both Spanish and rWE populations. Among the Spanish population females had a higher proportion of hypertension, however males had a higher proportion of previous stroke, congestive heart failure and diabetes mellitus. Among rWE patients, males had a higher proportion for congestive heart failure, myocardial infarction, and coronary artery disease as compared to females.
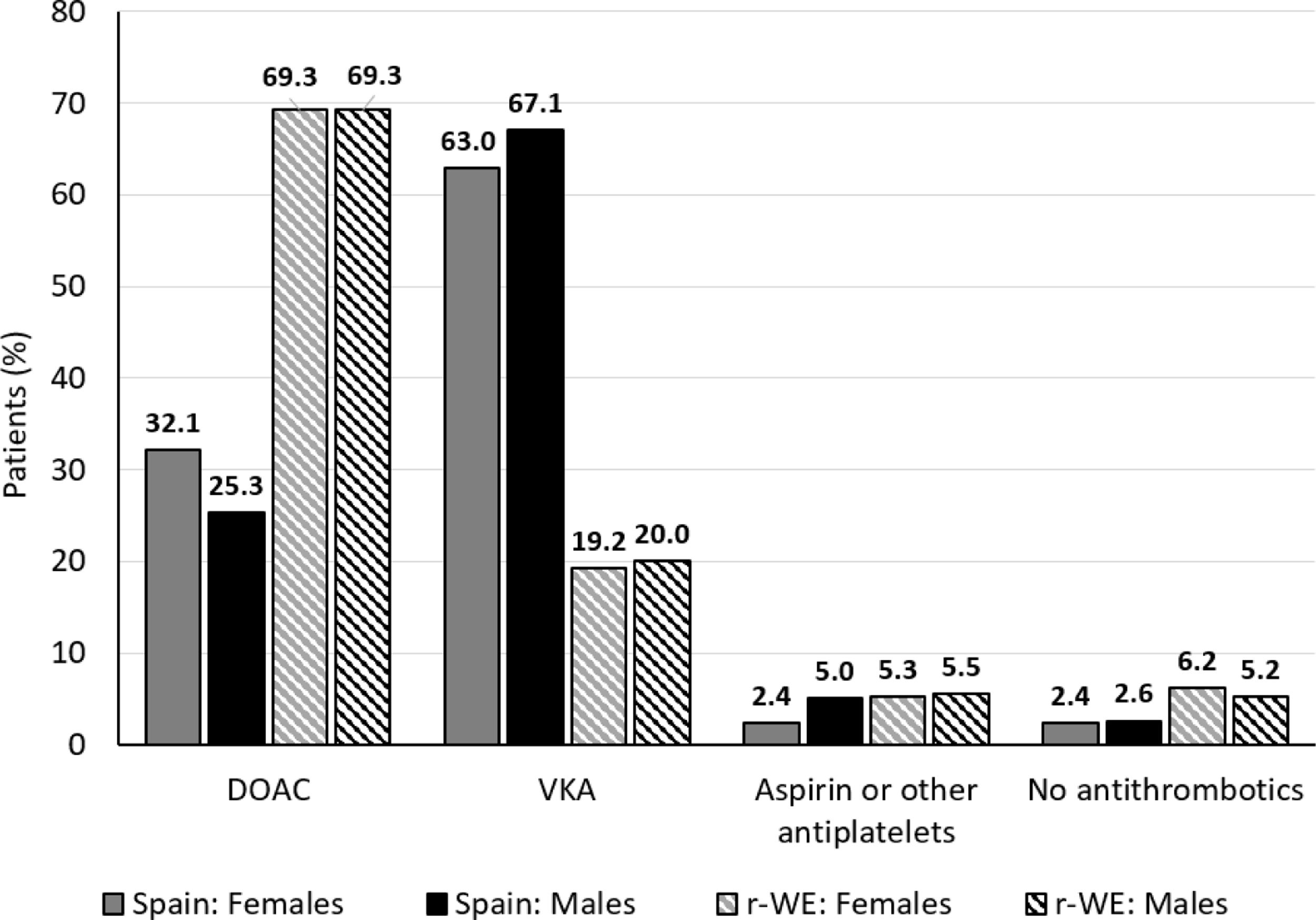
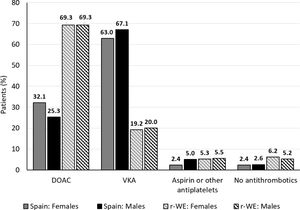
Use of different antithrombotic strategiesAntithrombotic treatment patterns by gender are presented in Fig. 1. Overall, the prevalence of OACs (DOACs and VKAs) in Spain and rWE was high, since 93.7% and 89.0% of the patients, respectively, were prescribed OACs.
When analyzing gender differences in OAC use, Spanish women showed slightly higher figures than men (females 95.0%, males 92.4%; d=−0.1078). On the contrary, OACs use in rWE was balanced between genders (88.6% vs 89.3%, respectively: d=0.0247). However, 7.6% of males in Spain did not receive OACs (no treatment or antiplatelets alone) while it was only 4.8% in females. This gender difference was not observed in rWE since 11.4% females did not receive OACs vs 10.7% in males.
Spanish patients were prescribed fewer DOACs (women 32.1%, men 25.3%) than VKAs (women 63.0%, men 67.1%) vs. rWE patients. Subjects in rWE received more DOACs (69.3%, both genders) than VKAs (women 19.2%, men 20.0%).
Gender and use of OACsAccording to our study, a total of 8184 patients received OACs in WE (Spain and rWE combined). Of these, 5859 (71.6%) received DOACs and 2325 (28.4%) received VKAs. When analyzing gender differences by geographical region it was observed that 334 patients in Spain received DOACs. Of these, 56.0% were female and 44.0% were male. Of the 5525 patients receiving DOACs in rWE, 44.0% were female and 56.0% were male.
As for patients receiving VKAs, 756 did so in Spain and 1569 in rWE. The percentage of Spanish patients by gender is similar (48.5% women and 51.5% men). In contrast, fewer women (42.9%) received VKAs than men (57.1%) at rWE.
DiscussionGeneral introductionOur study shows high use of OAC in Spain as compared with the rWE, in particular women treated with OACs and DOAC in a higher proportion than men in Spain. However, DOACs are used only in a fraction of patients as compared with the rWE countries.
Use of different antithrombotic strategiesObservational and large registries, as the GLORIA-AF,21 are essential to characterize treatment patterns and responses in clinical practice. These data provide long-term safety and effectiveness information in heterogeneous populations and raise the level of evidence upon which to base treatment recommendations.
The global results of the total population included in the GLORIA registry concluded that the prevalence of anticoagulant use is similar in both genders.22 However, the conclusions of our work differ from this one. In Spain, we have a different scenario since DOACs are prescribed less than VKAs, unlike in the rWE. While the first DOAC in this country was marketed in 2008, it was shown in 2015 a percentage of use of DOACs of 15.8% compared to 63.6% of VKAs.23 Prescription for DOACs in Spain were significantly lower as compared to DOAC prescription in neighboring countries such as France, Italy, Portugal, or Germany (38.7%, 35.1%, 50% and 53.0% respectively). Other authors, early 2016, published that DOACs were used in fewer than 20% of Spanish patients with NVAF due to their impact on the health budget.7
Factors related to the use of OACs in SpainAt present, the prescription of DOACs reimbursed by the Spanish Health system requires an inspection visa to ensure its rational use according to the clinical conditions defined in the current version of the DOACs’ Therapeutic Positioning Report (IPT), published by the Spanish Medicines Agency late in 2016.24 These recommendations differ from those of the ESC (2016), limiting the use of DOACs to second-line treatment in most cases. Hence, VKA remains the recommended treatment option for the majority of patients. DOACs can only be considered as a therapeutic option in the NVAF population if they satisfy following criteria: (1) known hypersensitivity/contraindication to acenocoumarol or warfarin, (2) history of intracranial hemorrhage (ICH), previous ischemic stroke who have clinical and neuroimaging criteria of high ICH risk and (3) patients with VKA who suffer severe arterial thromboembolic events despite good international normalized ratio (INR) control.8 All of this implies that new patients with NVAF requiring anticoagulation and those on VKAs with good INR control are not DOAC candidates.
Gender and use of OACsWomen with NVAF are at increased risk of stroke than men,9–11 and neglecting female sex as a risk modifier may underestimate stroke risks in NVAF patients age >65 or with ≥1 additional stroke risk factors.25 As expected, both in Spain and in rWE, a difference in the CHA2DS2-VASc score between men and women of 1 point was observed, considering female sex as a risk factor for stroke in patients with AF within the same scale. Current European guidelines state that women with CHA2DS2-VASc=1 (1 point for female gender only) are at “truly low-risk” for stroke and should not be anticoagulated because this brings no benefit but may cause harm.26–28 By contrast, anticoagulation should be considered in patients with 1 non-gender-related risk factors for stroke, that is, CHA2DS2-VASc=1 for men and CHA2DS2-VASc=2 for women.29
We observed in the current analysis that women with NVAF have a higher risk of stroke than men and this is reflected in the OAC prescription pattern, i.e., a higher proportion of female patients received OACs as compared to their male counterparts in Spain.
Although the percentage of DOACs use of 28.7% in Spain shown by the current analysis is slightly higher than other previously published data, it continues to be inadequate. These figures are lower than the ones observed in neighboring countries and also lower than expected, given current guidelines and given the number of patients uncontrolled while taking VKAs. Therefore, all those barriers that make access to DOACs difficult should be addressed to improve the outcomes in patients requiring anticoagulation. Greater attention to gender-specific risks and treatment patterns will improve the effectiveness of stroke prevention in women and ultimately reduce stroke-related severe outcomes.
LimitationsGLORIA-AF included patients only from participating sites and most patients came from Cardiology practices. There are limitations in generalizing the finding of this data and the results may not be representative of the overall AF population; however, as a representative of other registries which included newly diagnosed AF population. Also, male patients with a low risk of stroke (CHA2DS2-VASc score=0) were not recruited (per protocol) so no data on this subpopulation is available. The current analysis is only based on the prescription pattern at time of AF diagnosis (baseline); therefore, no conclusions can be drawn on the quality of anticoagulation or potential changes of OAC use over time. Only new-onset AF patients were enrolled preventing extrapolating findings to patients with >3 months from arrhythmia onset. Despite the patients recruited in the registry are high, only 1163 Spanish patients (583 women) were analyzed so numbers can be somewhat limited to extrapolate the information to the entire population. Subjects and investigators knew they joined a registry program so this might have led to higher overall compliance/anticoagulation rates compared with the general population. It needs to be considered that WE countries may have some degree of varying healthcare systems, reimbursement policies and enrolling sites. In this sense, a bias arising from direct comparison of antithrombotic treatment pattern between rWE and Spain cannot be ruled out. It also should be noted that the current DOAC prescription rates, both in Spain and in the rWE, are higher than the ones reported in the GLORIA registry 5 years ago.
ConclusionsIn rWE DOACs have been established as the preferred OAC treatment, while in Spain due to prescription barriers, DOAC use remains low for both genders. In Spain for both genders, fewer patients were prescribed DOACs than VKAs. While in the rWE population there appears no difference in OAC use by gender. In Spain, a higher percentage of women appears to be treated with OAC particularly taking into consideration the higher stroke risk in women.
Ethical considerationsThe procedures used in the patients have been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki), with the authorization of the Clinical Research Ethics Committees of the institutions of origin of the patients.
FundingThe GLORIA-AF Registry Program was funded by Boehringer Ingelheim. ClinicalTrials.gov Identifier: NCT01428765.
Conflict of interestJose L. Lopez Sendon report research grants from Bayer, Merck, Pfizer-BMS, Sanofi, Boheringer-Ingleheim and personal fees from Menarini and Daiichi Sankyo.
David Alonso has been a speaker for Bayer, BMS/Pfizer, Boehringer Ingelheim, and Daiichi-Sankyo.
Gonzalo Barón-Esquivias. Francisco Marín report consultant fees from Boehringer Ingelheim and speaker fees from Bayer, BMS/Pfizer, Boehringer Ingelheim, Astra-Zeneca and Daiichi-Sankyo.
Juan Cosin-Sales has been a consultant and speaker for Bayer, BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo.
Natalia Jiménez and Sabrina Marler are employees of Boehringer Ingelheim.
Professor Huisman reports grants from ZonMW Dutch Healthcare Fund, grants, and personal fees from Boehringer-Ingelheim, Pfizer-BMS, Bayer Health Care, Aspen, Daiichi-Sankyo, outside the submitted work.
Professor Lip has been a consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi-Sankyo. He has been a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees directly received personally.
The authors would like to acknowledge María Romero who provided medical writing support on behalf of Springer Healthcare.