At present, COVID-19 is a global pandemic and is seriously harmful to humans. In this retrospective study, the aim was to investigate the interaction between CVD and COVID-19.
MethodsA total of 180 patients diagnosed with COVID-19 in Yichang Central People's Hospital from 29 January to 17 March 2020 were initially included. The medical history, clinical manifestations at the time of admission, laboratory test results, hospitalization time and complications were recorded. According to the medical history, the patients were assigned to the nonsevere group with non-CVD (n=90), the nonsevere group with CVD (n=22), the severe group with non-CVD (n=40) and the severe group with CVD (n=28).
ResultsIn the severe group, compared with non-CVD patients, CVD patients had a significantly higher incidence of fever (P<0.05). However, compared with the nonsevere group, the severe group had significantly higher proportions of patients with hypertension, type 2 diabetes mellitus, CHD and HF (all P<0.05). Among the patients with nonsevere COVID-19, the WBC count and the levels of IL-6, CRP, D-dimer, NT-proBNP, and FBG were significantly higher and the Hb level was significantly lower in the CVD patients than in the non-CVD patients (all P<0.05). However, among the patients with severe COVID-19, only the level of NT-proBNP was significantly higher in CVD patients than in non-CVD patients (P<0.05). In addition, the WBC count and the levels of IL-6, CRP, D-dimer, CKMB, ALT, AST, SCR, NT-proBNP, and FBG were significantly higher and the Hb level was significantly lower in the severe group than in the nonsevere group (all P<0.05). However, among the patients with severe COVID-19, the incidences of acute myocardial injury, acute kidney injury, arrhythmia, and sudden death were significantly higher in the CVD group than in the non-CVD group (all P<0.05). The same results were found in the comparison of the nonsevere group with the severe group. Among the patients with nonsevere COVID-19, those without CVD had a mean hospitalization duration of 25.25 (SD 7.61) days, while those with CVD had a mean hospitalization duration of 28.77 (SD 6.11) days; the difference was significant (P<0.05). The same results were found in the comparison of the severe group.
ConclusionsCVD affects the severity of COVID-19. COVID-19 also increases the risk of severe CVD.
La infección por SARS-CoV-2 está provocando graves consecuencias en la humanidad. El objetivo de este estudio retrospectivo fue investigar el impacto de las enfermedades cardiovasculares (ECV) en la gravedad de dicha infección.
MétodosEntre el 29 de enero y el 17 de marzo de 2020, se diagnosticaron 180 pacientes con neumonía por SARS-CoV-2 en el Hospital Popular Central de Yichang. Se registraron los antecedentes, manifestaciones clínicas, resultados de laboratorio, tiempo de hospitalización y complicaciones. Los pacientes se dividieron en cuatro grupos: 1) infección no grave sin ECV (n=90), 2) infección no grave con ECV (n=22), 3) infección grave sin ECV (n=40) y 4) infección grave con ECV (n=28).
ResultadosLa prevalencia de fiebre en los pacientes con ECV fue significativamente mayor que en aquellos sin ECV (P<0,05). Sin embargo, en comparación con los pacientes no graves, la proporción de pacientes con hipertensión, diabetes mellitus tipo 2, cardiopatía coronaria e insuficiencia cardíaca en los pacientes graves fue significativamente mayor (p<0,05). Los niveles de recuento de leucocitos, IL-6, PCR, dímero D, NT-proBNP y glucemia en ayunas (GA) en pacientes con ECV fueron significativamente mayores que en los de pacientes sin ECV, aunque los niveles de Hb fueron significativamente menores que los de los pacientes sin ECV (p<0,05). Sin embargo, los valores de NT-proBNP en pacientes con ECV fueron significativamente mayores que en los pacientes sin ECV (P<0,05). Además, el recuento de leucocitos y los niveles de IL-6, PCR, dímero D, CK-MB, ALT, AST, creatinina, NT-proBNPy GA en el grupo de pacientes graves fueron significativamente mayores que en el grupo no grave, mientras que los valores de Hb fueron significativamente menores que en el grupo no grave (p<0,05). La prevalencia de lesión miocárdica aguda, lesión renal aguda, arritmia y muerte súbita en el grupo con ECV fue significativamente mayor que en el grupo sin ECV (p<0,05). Los mismos resultados se encontraron al comparar los pacientes no graves con aquellos con infección grave. Entre los pacientes no graves, la duración media de la estancia hospitalaria fue de 25,25 (DE: 7,61) días en los pacientes sin ECV, mientras que la duración media de la estancia hospitalaria fue de 28,77 (DE: 6,11) días en los pacientes con ECV (p<0,05). Los mismos resultados se observaron al comparar los dos grupos con infección grave.
ConclusionesLa infección por SARS-CoV-2 es de evolución más grave en los pacientes con ECV.
Since the first case of unknown pneumonia was identified in early December 2019, coronavirus disease 2019 (COVID-19) has spread worldwide. A novel coronavirus was isolated by the China Center for Disease Control and Prevention on January 7, 2020. COVID-19, which is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was officially named by the World Health Organization.1 Since the start of the outbreak of COVID-19 in 2019, a worldwide pandemic has developed. At present, Europe and the United States are the most strongly affected by the pandemic. Coronary heart disease (CHD), hypertension, heart failure (HF), diabetes and other cardiovascular diseases (CVDs) are common in older adults and may increase the risk of contracting COVID-19 or progressing to severe disease. In addition to typical respiratory symptoms, COVID-19 patients have clinical manifestations of cardiac involvement.2 The data, which have predominantly come from China, show a significant percentage of patients with CVD among those with COVID-19, and CVD substantially accelerates the progression of COVID-19 and complicates treatment.3 At the same time, a large amount of data has shown that COVID-19 patients are prone to myocardial injury and cardiac dysfunction, which substantially increase the risk of death.4 However, the effect of CVD on the progression of COVID-19 is still unclear, and the evidence of myocardial injury caused by COVID-19 is not sufficient. In this study, we analyzed the factors influencing COVID-19 in detail to elucidate the interaction between COVID-19 and CVD, provide guidance regarding effective treatment measures and minimize cardiovascular-related deaths.
Materials and methodsPatient populationThis was a retrospective cohort study including 180 patients with COVID-19 hospitalized in the Central People's Hospital of Yichang, Hubei, from 29 January to 17 March 2020. According to the interim guidance provided by the World Health Organization,5 all included patients had laboratory-confirmed cases of COVID-19 based on a positive result of a real-time reverse-transcriptase polymerase chain reaction (RT-PCR) assay of nasal and pharyngeal swab specimens.6 This study analyzed the relationship between COVID-19 and CVD. The ages of the patients ranged from 18 to 87 years (mean age: 55.38±15.78 years). The study was conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the Central People's Hospital of Yichang. The patients were classified according to disease severity, and the classification criteria were based on the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia released by the National Health Commission of the PRC.7 Patients with mild cases had mild symptoms and no abnormalities in imaging. Those with moderate cases had respiratory infection symptoms, such as a fever and cough, and manifestations indicative of pneumonia in imaging. Patients with severe cases had a respiratory rate ≥30 breaths/min, resting fingertip oxygen saturation level ≤93%, or oxygen partial pressure (PaO2)/fraction of inspired O2 (FiO2)≤300mmHg (1mmHg=0.133kPa). Patients with critical cases had respiratory failure requiring mechanical ventilation, symptoms of shock, or multiple organ dysfunction requiring intensive care. In this study, patients were divided into 4 groups: the nonsevere group (including those with mild and moderate cases) with non-CVD, the nonsevere group with CVD, the severe group (including patients with severe and critical cases) with non-CVD and the severe group with CVD.
Collection of clinical dataThe clinical data for the study patients were extracted from the electronic medical records system and included the following: (I) general information, including sex, age, body mass index (BMI), history of alcohol consumption and smoking history; (II) previous medical history of hypertension, type 2 diabetes mellitus, CHD and HF; (III) the main clinical manifestations at the time of admission, including fever, cough, dyspnea and fatigue; (IV) the results of laboratory tests performed 24h after admission [white blood cell (WBC) count and the levels of hemoglobin (Hb), interleukin (IL)-6, C-reactive protein (CRP), D-dimer, creatine kinase (CK), creatine kinase isoenzyme (CKMB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (SCR), N-terminal prohormone B-type natriuretic peptide (NT-proBNP), fasting blood glucose (FBG) and cardiac troponin I (cTnI)]; (V) hospitalization duration (patients were discharged after their symptoms improved and they had two consecutive negative nucleic acid tests); and (VI) complications, including acute myocardial injury, acute kidney injury, acute liver injury, arrhythmia, and sudden death.
Statistical analysisData analyses were performed using SPSS statistical software (version 22.0). Data for continuous variables are presented as the means±standard deviations (SDs). Independent sample t-tests were used for comparisons between groups. Measurement data with nonnormal distributions are expressed as medians (25th percentile, 75th percentile), and the Mann–Whitney U test was used for comparisons between groups. Discrete variables are expressed as counts and percentages, and differences were analyzed with χ2 tests (or Fisher's exact tests). A two-tailed P<0.05 was considered significant.
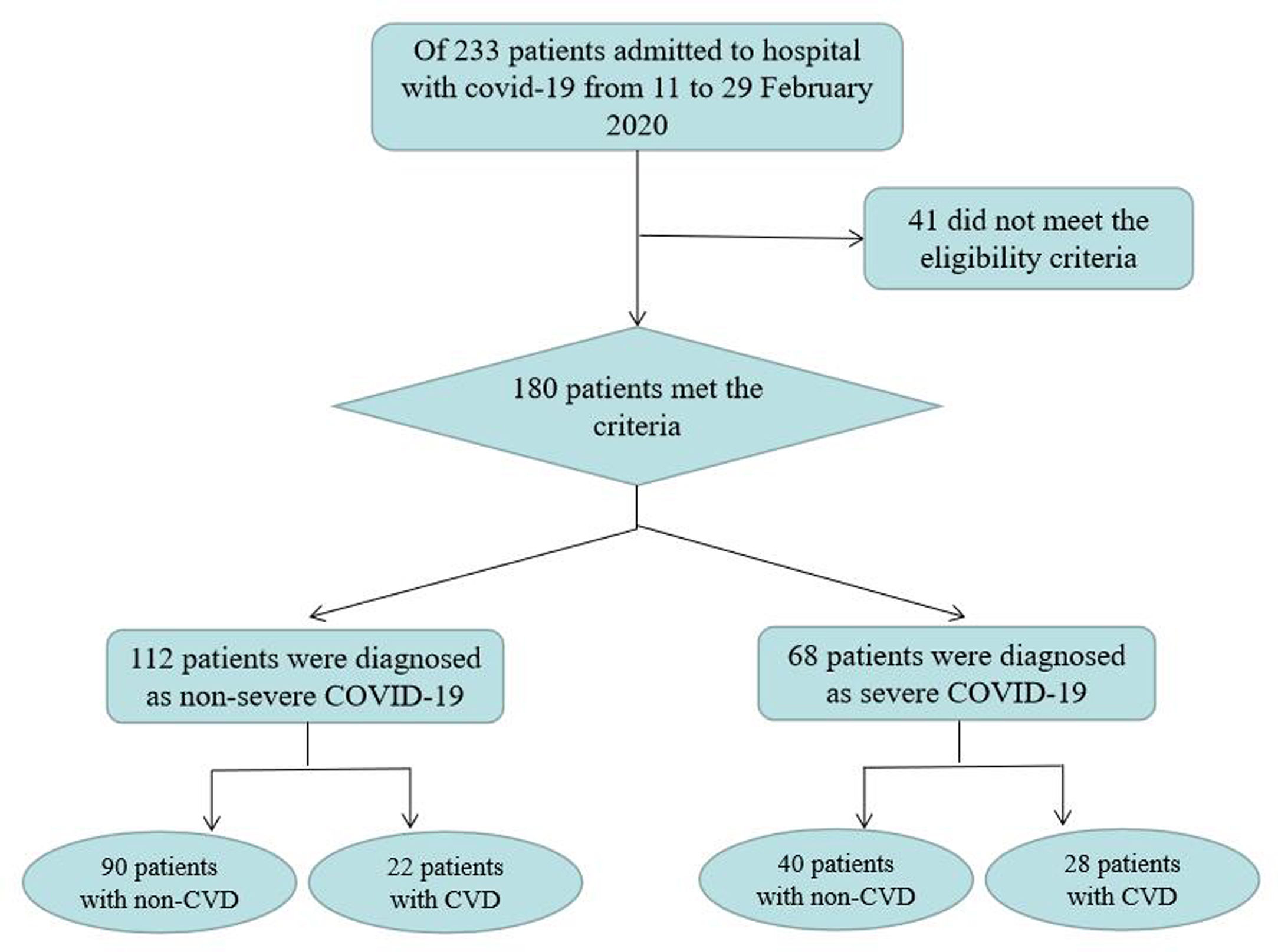
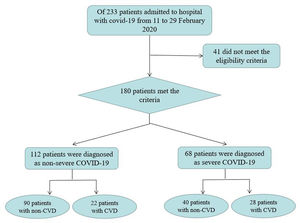
ResultsParticipants’ characteristicsOf the 233 patients admitted to the hospital with COVID-19 from 29 January to 17 March 2020, 41 did not meet the eligibility criteria. The remaining 180 patients were divided into 4 groups: 90 (50%) patients were assigned to the nonsevere group with non-CVD, 22 (12.2%) patients were assigned to the nonsevere group with CVD, 40 (22.2%) patients were assigned to the severe group with non-CVD and 28 (15.6%) patients were assigned to the severe group with CVD (Fig. 1). The clinical characteristics are shown in Table 1. Among the patients with nonsevere COVID-19, there were no significant differences between the groups with and without CVD in age, sex, BMI, alcohol consumption history, smoking history, systolic blood pressure at admission, diastolic blood pressure at admission or clinical manifestations (including fever, cough, dyspnea and fatigue) (all P>0.05). Among the patients with severe COVID-19, there were no significant differences between the groups with and without CVD in age, sex, BMI, alcohol consumption history, smoking history, systolic blood pressure at admission, diastolic blood pressure at admission, cough, dyspnea and fatigue (all P>0.05). However, compared with non-CVD patients, CVD patients had a significantly higher incidence of fever (P=0.003). Moreover, there were no significant differences between the nonsevere group and the severe group in age, sex, BMI, alcohol consumption history, smoking history, diastolic blood pressure at admission, fever, cough, and fatigue (all P>0.05). However, compared with the nonsevere group, the severe group had significantly higher proportions of patients with hypertension, type 2 diabetes mellitus, CHD and HF (all P<0.05). In addition, systolic blood pressure at admission was significantly lower in the severe group than in the nonsevere group (P<0.05), and the incidence of dyspnea was significantly higher in the severe group than in the nonsevere group (P<0.05).
Clinical characteristics of the four groups.
| Characteristics | Non-severe COVID-19 | Severe COVID-19 | P value | ||||
|---|---|---|---|---|---|---|---|
| Non-CVD (n=90) | CVD (n=22) | P value | Non-CVD (n=40) | CVD (n=28) | P value | ||
| Age, mean±SD years | 47.92±14.44 | 61.55±12.38 | 0.236 | 61.90±15.87 | 64.78±10.70 | 0.012 | 0.393 |
| Male, n (%) | 45 (50.0) | 14 (63.6) | 0.341 | 19 (47.4) | 17 (60.7) | 0.330 | 1.000 |
| BMI (kg/m2) | 25.01±3.37 | 24.46±2.88 | 0.465 | 24.62±3.41 | 24.25±2.76 | 0.621 | 0.561 |
| Drinking history, n (%) | 24 (26.7) | 7 (31.8) | 0.606 | 16 (40.0) | 8 (28.6) | 0.441 | 0.318 |
| Smoking history, n (%) | 18 (20.0) | 5 (22.7) | 0.773 | 13 (32.5) | 7 (25.0) | 0.594 | 0.208 |
| Hypertension, n (%) | – | 19 (86.4) | – | – | 23 (82.1) | – | 0.011 |
| Diabetes Mellitus, n (%) | – | 4 (18.2) | – | – | 12 (42.9) | – | 0.002 |
| CAD, n (%) | – | 4 (18.2) | – | – | 9 (32.1) | – | 0.018 |
| HF, n (%) | – | 3 (13.6) | – | – | 7 (25.0) | – | 0.036 |
| Systolic blood pressure at admission (mmHg) | 123.77±10.08 | 128.41±10.91 | 0.311 | 125.35±14.89 | 129.43±12.78 | 0.588 | 0.007 |
| Diastolic blood pressure at admission (mmHg) | 76.33±7.57 | 79.81±9.85 | 0.113 | 76.50±9.79 | 79.29±9.08 | 0.721 | 0.122 |
| Clinical manifestation | |||||||
| Fever, n (%) | 71 (78.9) | 15 (68.2) | 0.397 | 33 (82.5) | 26 (92.9) | 0.003 | 0.122 |
| Cough, n (%) | 56 (62.2) | 14 (63.6) | 0.902 | 26 (65.0) | 19 (67.9) | 0.806 | 0.635 |
| Dyspnea, n (%) | 4 (4.4) | 1 (4.5) | 1.000 | 8 (20.0) | 5 (17.9) | 0.825 | 0.004 |
| Fatigue, n (%) | 18 (20.0) | 7 (31.8) | 0.259 | 14 (35.0) | 10 (35.7) | 0.952 | 0.083 |
Date are presented as mean±SD,M [P25, P75] or number (percentage). CAD: coronary heart disease; HF: heart failure.
The laboratory tests at admission are shown in Table 2. Among the patients with nonsevere COVID-19, the WBC count and the levels of IL-6, CRP, D-dimer, NT-proBNP, and FBG were significantly higher and the Hb level was significantly lower in the CVD patients than in the non-CVD patients (all P<0.05). However, there were no significant differences between the non-CVD patients and the CVD patients in terms of the levels of CK, CKMB, ALT, AST, SCR and cTnI (all P>0.05). However, among the patients with severe COVID-19, only the level of NT-proBNP was significantly higher in CVD patients than in non-CVD patients (P<0.05). There were no significant differences between the non-CVD patients and the CVD patients in terms of the WBC count and the levels of Hb, IL-6, CRP, D-dimer, FBG, CK, CKMB, ALT, AST, SCR and cTnI (all P>0.05). In addition, the WBC count and the levels of IL-6, CRP, D-dimer, CKMB, ALT, AST, SCR, NT-proBNP, and FBG were significantly higher and the Hb level was significantly lower in the severe group than in the nonsevere group (all P<0.05). However, there were no significant differences between the nonsevere group and the severe group in terms of the levels of CK and cTnI (all P>0.05).
Laboratory test results in the four groups.
| Laboratory findings | Non-severe COVID-19 | Severe COVID-19 | P value | ||||
|---|---|---|---|---|---|---|---|
| Non-CVD (n=90) | CVD (n=22) | P value | Non-CVD (n=40) | CVD (n=28) | P value | ||
| WBC (×109) | 8.19 (6.02, 9.66) | 9.73 (7.25, 10.75) | 0.010 | 13.75 (9.00, 16.35) | 12.56 (6.20, 16.86) | 0.404 | <0.001 |
| IL-6 (pg/ml) | 5.66 (2.88, 6.38) | 8.11 (5.35, 9.86) | 0.003 | 266.49 (18.87, 74.64) | 79.10 (22.70, 93.99) | 0.307 | <0.001 |
| CRP (mg/L) | 28.50 (6.40, 34.35) | 52.19 (27.95, 81.43) | 0.001 | 86.82 (28.43, 131.23) | 106.10 (46.38, 141.58) | 0.140 | <0.001 |
| Hb (g/L) | 126.61 (115.00, 140.25) | 117.27 (103.75, 132.00) | 0.027 | 13.33 (94.25, 129.25) | 116.29 (108.25, 128.00) | 0.566 | 0.002 |
| D-dimer (μg/ml) | 0.69 (0.30, 0.73) | 1.37 (0.36,1.40) | 0.011 | 1.77 (0.62, 2.00) | 1.36 (0.65, 1.88) | 0.779 | <0.001 |
| CK (IU/L) | 112.51 (47.00,123.25) | 98.41 (39.00, 145.25) | 0.613 | 288.90 (39.00, 217.75) | 291.46 (31.25, 200.50) | 0.474 | 0.287 |
| CKMB (IU/L) | 12.23 (8.00, 14.00) | 9.64 (6.75, 11.75) | 0.091 | 18.80 (9.25, 17.00) | 19.32 (7.50, 22.50) | 0.750 | 0.001 |
| ALT (U/L) | 84.54 (30.75, 107.5) | 72.95 (33.50, 79.50) | 0.575 | 155.75 (45.75, 124.50) | 106.32 (35.25, 134.75) | 0.672 | 0.044 |
| AST (U/L) | 44.54 (25.00, 57.00) | 44.95 (26.50, 46.75) | 0.985 | 170.58 (36.25, 93.75) | 74.64 (35.25, 82.75) | 0.774 | <0.001 |
| SCR (μml/L) | 71.23 (56.13,83.20) | 79.74 (65.80, 81.18) | 0.075 | 92.53 (57.13, 97.88) | 106.71 (69.10, 108.58) | 0.100 | 0.021 |
| NT-proBNP (pg/ml) | 135.88 (62.50, 105.50) | 514.64 (72.75, 493.50) | 0.009 | 1062.30 (118.00, 697.25) | 1323.59 (227.50, 1711.50) | 0.017 | <0.001 |
| FBG (mmol/L) | 6.56 (5.07, 7.31) | 9.03 (5.71, 10.68) | 0.009 | 7.72 (5.87, 9.15) | 7.76 (5.17, 9.43) | 0.529 | 0.034 |
| cTnI (pg/ml) | 0.36 (0.01, 0.05) | 0.20 (0.01, 0.01) | 0.053 | 6.06 (0.01, 0.24) | 8.92 (0.01, 1.00) | 0.843 | 0.072 |
Date are presented as M (P25, P75). WBC: white blood cell; IL-6: interleukin-6; CRP: C-reactive protein; Hb: hemoglobin; CK: creatine kinase; CKMB: creatine kinase isoenzyme; ALT: alanine aminotransferase; AST: aspartate aminotransferase; SCR: serum creatinine; NT-proBNP: N-terminal pro hormone B-type natriuretic peptide; FBG: fasting blood glucose; cTnI: cardiac troponin I.
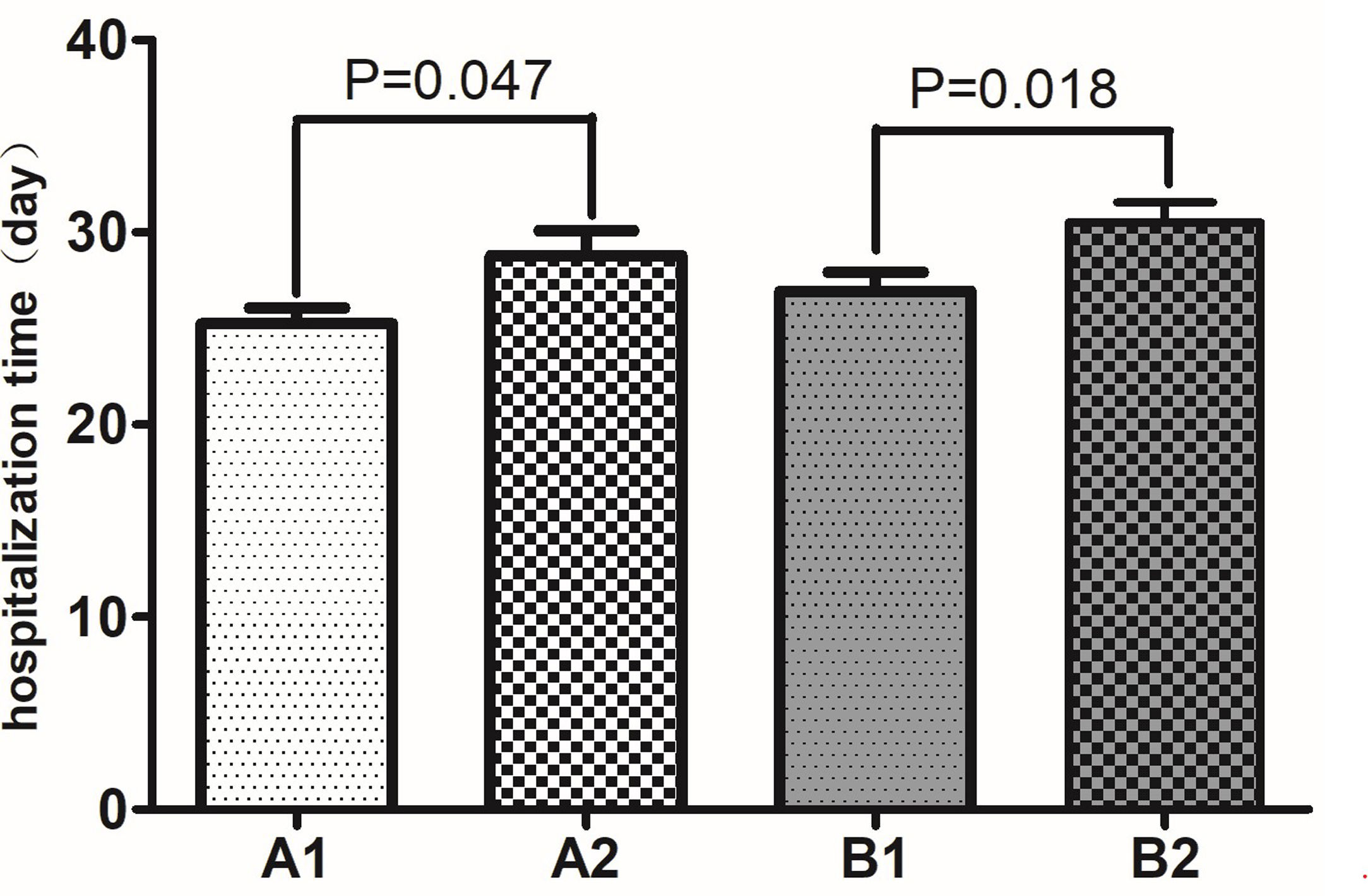
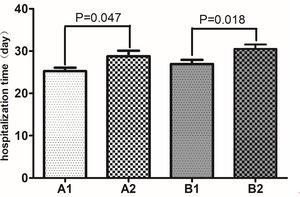
In our cohort, the major complications were acute myocardial injury, acute kidney injury, acute liver injury, arrhythmia, and sudden death. As shown in Table 3, among the patients with nonsevere COVID-19, there was no significant difference in the incidence of complications between the non-CVD patients and the CVD patients (P>0.05). However, among the patients with severe COVID-19, the incidences of acute myocardial injury, acute kidney injury, arrhythmia, and sudden death were significantly higher in the CVD group than in the non-CVD group (all P<0.05), although there was no significant difference in the incidence of acute liver injury (P>0.05). The same results were found in the comparison of the nonsevere group with the severe group. As shown in Figure 2, among the patients with nonsevere COVID-19, those without CVD had a mean hospitalization duration of 25.25 (SD 7.61) days, while those with CVD had a mean hospitalization duration of 28.77 (SD 6.11) days; the difference was significant (P=0.047). Moreover, among the patients with severe COVID-19, those without CVD had a mean hospitalization duration of 26.94 (SD 5.65) days, while those with CVD had a mean hospitalization duration of 30.52 (SD 4.97) days; the difference was significant (P=0.018).
Results of related complications in the four groups.
| Complications | Non-severe COVID-19 | Severe COVID-19 | P value | ||||
|---|---|---|---|---|---|---|---|
| Non-CVD (n=90) | CVD (n=22) | P value | Non-CVD (n=40) | CVD (n=28) | P value | ||
| Acute myocardial injury, n (%) | 2 (2.2) | 1 (4.5) | 0.485 | 10 (25.0) | 14 (50.0) | 0.042 | <0.001 |
| Acute kidney injury, n (%) | 6 (6.7) | 3 (13.6) | 0.376 | 8 (20.0) | 13 (46.4) | 0.032 | <0.001 |
| Acute liver injury, n (%) | 52 (57.8) | 13 (59.1) | 1.000 | 34 (85.0) | 20 (71.4) | 0.227 | 0.642 |
| Arrhythmia, n (%) | 8 (8.9) | 4 (18.2) | 0.247 | 11 (27.5) | 15 (53.6) | 0.043 | <0.001 |
| Sudden death, n (%) | 0 (0.0) | 0 (0.0) | – | 1 (2.5) | 5 (17.8) | 0.028 | <0.001 |
Date diven as n (%).
In this study, the clinical characteristics of 180 patients with COVID-19 from the Central People's Hospital of Yichang, China, were described, and the related laboratory tests and complications were investigated. A recent study reported that cardiovascular disease was more prevalent among nonsurviving COVID-19 patients than among those with mild cases of COVID-19.8 Another investigation also revealed that the incidence of CVD was higher in COVID-19 patients with a severe clinical course than in patients with a nonsevere clinical course.9 As in those studies, our data indicated that the proportions of patients who had a history of hypertension, type 2 diabetes mellitus, CHD and HF were significantly higher in the group with severe COVID-19 than in the group with nonsevere COVID-19. However, we found that there were no significant differences in the incidences of these diseases between nonsevere patients and the normal population. Therefore, we can infer that COVID-19 patients with CVD may be more likely to progress to severe COVID-19 and that CVD is a predictor of disease severity in patients with COVID-19.
It is well known that the renin–angiotensin system (RAS) plays an important role in the pathophysiology of CVD.10 There has been widespread interest in angiotensin-converting enzyme 2 (ACE2), which is an important RAS regulator. ACE2 is a functional receptor that binds to the S-protein of SARS CoV-2.11 In addition, ACE2 is highly expressed on the surface of cardiovascular endothelial cells and plays important roles in cardiovascular and immune diseases.12 Based on our clinical data, we found that among patients with severe COVID-19, the incidences of acute myocardial injury, acute kidney injury, arrhythmia, and sudden death were significantly higher in patients with CVD than in non-CVD patients. Furthermore, the incidences of acute myocardial injury, acute kidney injury, arrhythmia, and sudden death were significantly higher in patients with severe COVID-19 than in those with nonsevere COVID-19. The levels of CKMB and NT-proBNP were also significantly elevated in patients with severe COVID-19. According to Oudit's study, SARS-CoV viral RNA was detected in 35% of autopsied human heart samples from SARS-CoV-infected patients during the Toronto SARS outbreak,13 and the myocardium was characterized by myocardial fibrosis, inflammation and decreased ACE2 expression.13,14 Therefore, we can infer that SARS-CoV-2 can induce cardiomyocyte injury through a process mediated by ACE2. At the same time, we also found that inflammatory indicators (including the WBC count and levels of IL-6 and CRP) were significantly elevated in these patients. This indicates that SARS-CoV-2 can also lead to cardiomyocyte injury by mediating the inflammatory response. COVID-19 could cause indirect cardiomyocyte injury through the induction of a severe cytokine storm mediated by an imbalanced response among subtypes of T helper cells and hypoxia-induced excessive intracellular levels of calcium, leading to cardiac myocyte apoptosis.15 Xu et al. found that the overactivation of T cells, manifested by an increase in Th17 cells and the death of CD8 T cells, leads to a severe inflammatory immune response in COVID-19 patients.16 Previous studies on SARS suggested that the activation of proinflammatory cytokines may be related to decreased diastolic function of the left ventricle.17 All these results suggest that SARS-CoV-2 can cause myocardial injury through multiple mechanisms. In addition, compared with patients with nonsevere COVID-19, severe COVID-19 patients exhibited significantly increased levels of D-dimer. These results suggest that SARS-CoV-2 can affect coagulation and fibrinolysis in many ways, resulting in disorders of the coagulation cascade and fibrinolysis.18 The formation of microthrombi can aggravate myocardial damage by creating an imbalance in oxygen supply and demand.19 However, the mechanism by which SARS-CoV-2 affects CVD remains unclear and warrants further investigation.
In addition, in our study, we found that COVID-19 patients have different degrees of liver and kidney dysfunction. The levels of SCR, AST and AST were higher in patients with severe COVID-19 than in those with nonsevere COVID-19, but there were no significant differences between CVD patients and non-CVD patients. This may be due to the use of antiviral drugs to treat COVID-19, as these antiviral drugs have different levels of hepatotoxicity and nephrotoxicity,2 especially in some severely ill patients. Therefore, during treatment for COVID-19, especially when antivirals are administered, the liver and kidney indexes must be closely monitored. We also found that compared with patients with nonsevere COVID-19, patients with severe COVID-19 had obvious glucose metabolism disorders. Patients with CVD often have glucose metabolism disorders. Wu et al. found that 68% had abnormalities in lipid metabolism, 40% had cardiovascular system abnormalities and 60% had altered glucose metabolism.20 However, the mechanisms by which infection with SARS-CoV-2 leads to metabolic disturbances are still unclear and need further investigation.
Finally, based on our analysis, the hospitalization duration was significantly higher in patients with CVD than in those without CVD, regardless of whether they had severe COVID-19. The incidences of related complications in these patients were also high. This suggests that patients with CVD are more likely to experience complications of COVID-19, leading to a sudden deterioration in their condition. Some patients with COVID-19 in Wuhan had a history of CVD, and that history was associated with severe disease and a longer hospitalization duration.21 In patients with CVD, SARS-CoV-2 infection might precipitate the deterioration of their condition, leading to a prolonged hospitalization duration. Patients with CVD may have a relatively higher risk of SARS CoV-2 infection, and CVD could also affect the progression and prognosis of COVID-19.
In conclusion, CVD plays a vital role in the severity of COVID-19. Conversely, COVID-19 also increases the risk of severe CVD. SARS-CoV-2 is thought to infect host cells through ACE2 and to cause damage to the myocardium.15 Therefore, in the clinical setting, particular attention should be given to protecting cardiovascular function during treatment of COVID-19.
Study limitationsThis study has some limitations. First, our patients were admitted to the hospital that was designated for the treatment of COVID-19 in Yichang, Hubei Province, rather than in Wuhan, the center of the outbreak. Therefore, the sample size was very small, which may have led to bias in the statistical analysis. Second, potentially important clinical data, such as echocardiography results, 24-h dynamic electrocardiography results, treatment protocols, and long-term follow-up, were not collected. Third, large longitudinal studies that involve the comprehensive analysis of all cardiovascular risk factors and possible confounding factors, as well as the progression and prognosis of COVID-19, are warranted.
Authors’ contributionsZai-qiang Zhang, and Jia-wang Ding conceived and designed the article. Jian-qiao Wan, Sheng-kui Zhu and Man Wang were mainly responsible for collecting the data and follow-up. Zai-qiang Zhang and Xin-an Wang were mainly responsible for analyzing the data. Jian-qiao Wan, Sheng-kui Zhu and Xiao-hong Tong collected related articles. Zai-qiang Zhang wrote the manuscript and drew the figures. Jia-wang Ding, Xin-an Wang and Xiao-hong Tong revised the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participateThe study was approved by the Ethics Committee of the Central People's Hospital of Yichang.
Availability of data and materialsThe data sets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
FundingThis work was supported by the National Natural Science Foundation of China (Grant No. 81770456).
Conflict of interestAll the authors have no financial conflicts of interest.