The criteria for the use of proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) more restrictive than those approved were established in Catalonia by the Health System (CatSalut) to improve their efficiency, with different LDL-C values from which to start treatment according to risk factors. The aim of the study is to analyse adherence to these criteria and results.
MethodsA retrospective study of patients treated with PCSK9i at Vall d’Hebron University Hospital between 2016 and 2021 was performed using data from the Registry of Patients and Treatments and medical records. The degree of agreement with the CatSalut criteria, LDL-C-responders (decrease ≥30%), cardiovascular events and discontinuations were analysed.
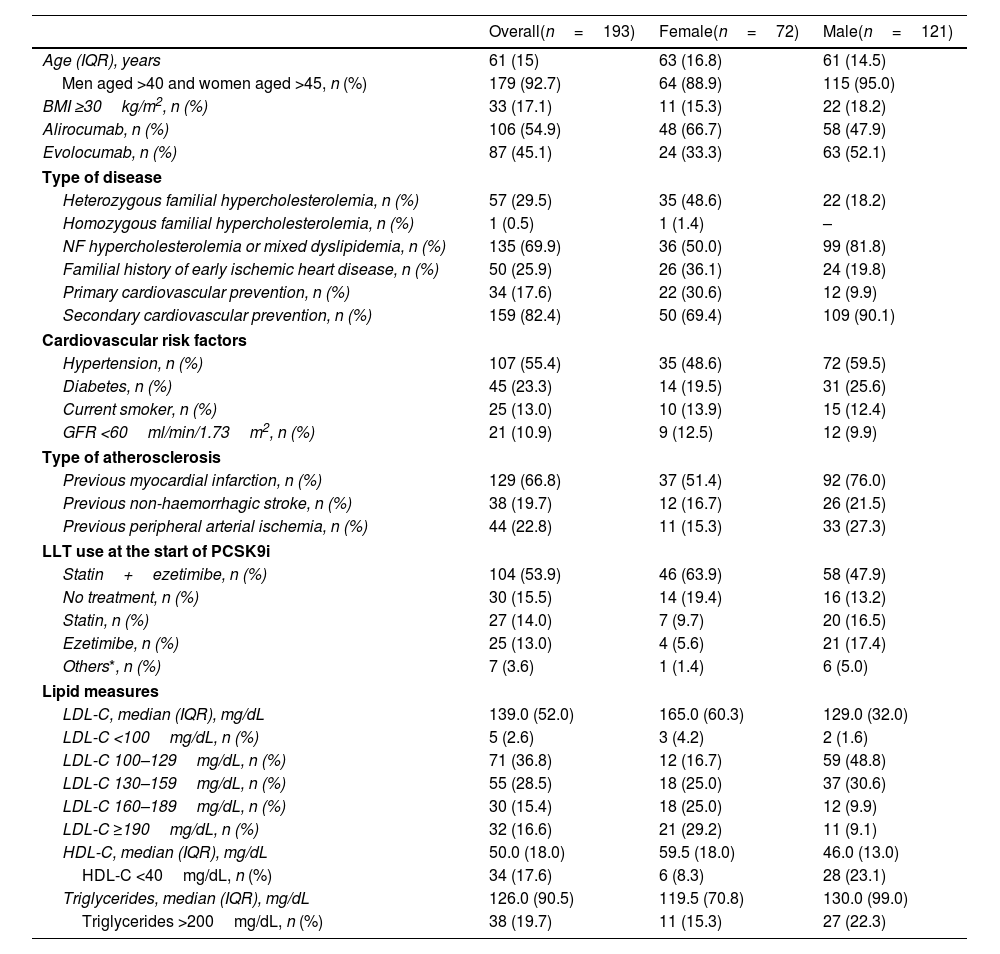
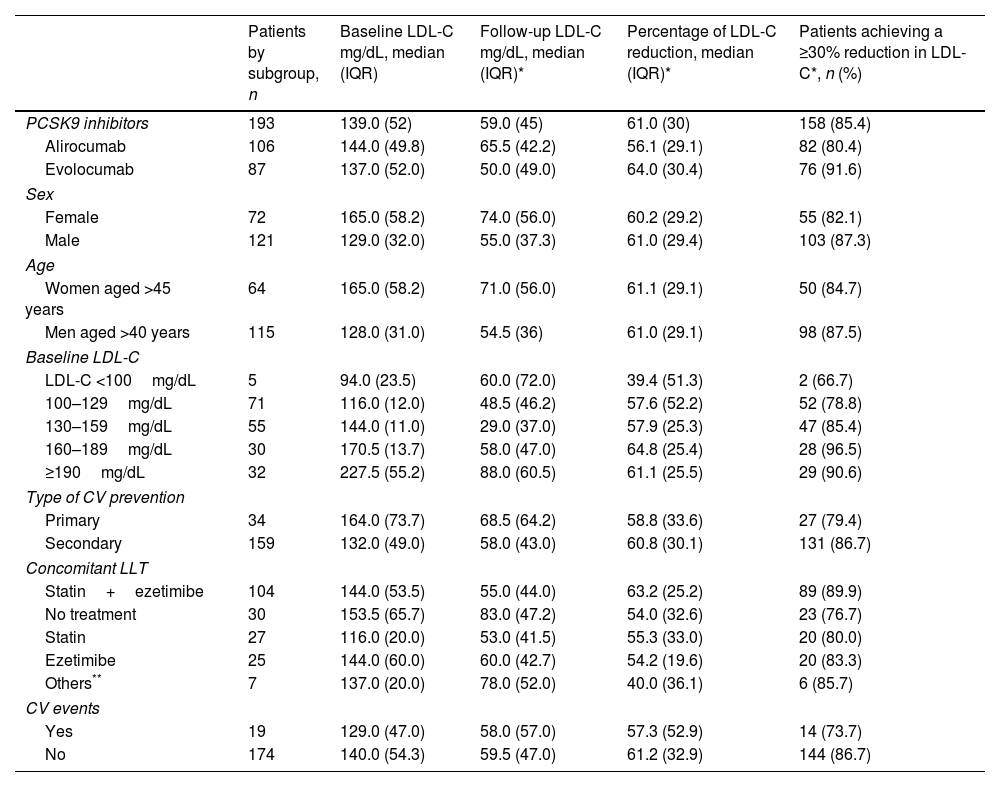
ResultsA total of 193 patients treated with PCSK9i were followed for a median of 27 months (IQR 23). The median age was 61 (IQR 15); 62.7% were men. Seventy percent of the patients had non-familial hypercholesterolemia. Treatment was for secondary prevention of cardiovascular disease in 82.4% of cases. The median LDL-C decreased from 139 (IQR 52) to 59 (IQR 45) mg/dL. The percentage of LDL-C reduction was 61.0% (IQR 30). In 72.5% of patients, all CatSalut criteria for starting treatment were met. The rate of responders was 85.4%. During follow-up, 19 patients (9.8%) had a cardiovascular event, and 15 (7.7%) discontinued treatment, in two cases due to toxicity.
ConclusionPCSK9i were used according to CatSalut criteria in three out of four cases. In this high-risk population, incidence of cardiovascular events was similar to that in clinical trials.
En Cataluña, CatSalut estableció criterios de uso de inhibidores de la proproteína convertasa subtilisina/kexina tipo 9 (iPCSK9) más restringidos que los aprobados, para mejorar su eficiencia, con diferentes valores de cLDL a partir de los cuales iniciar el tratamiento según factores de riesgo. El objetivo del estudio es analizar la adherencia a estos criterios y los resultados.
MétodosEstudio retrospectivo de los pacientes tratados con iPCSK9 en el Hospital Universitario Vall d’Hebron entre 2016 y 2021 a partir de los datos del Registro de Pacientes y Tratamientos y de la historia clínica. Se analizó el grado de concordancia con los criterios de CatSalut, cLDL-respondedores (disminución ≥30%), acontecimientos cardiovasculares y abandonos.
ResultadosUn total de 193 pacientes tratados con iPCSK9 fueron seguidos durante una mediana de 27 meses (IQR 23). La mediana de edad era de 61 años (IQR 15); un 62,7% eran hombres. Un 70% tenía hipercolesterolemia no familiar. En un 82,4% el tratamiento fue en prevención secundaria cardiovascular. La mediana de cLDL disminuyó de 139 (IQR 52) a 59 (IQR 45) mg/dL. El porcentaje de reducción fue del 61,0% (IQR 30). En el 72,5% de los pacientes se cumplieron todos los criterios de CatSalut para iniciar el tratamiento. La tasa de respondedores fue del 85,4%. Durante el seguimiento, 19 pacientes (9,8%) sufrieron un acontecimiento cardiovascular y 15 (7,7%) interrumpieron el tratamiento, en 2 casos por toxicidad.
ConclusiónLos iPCSK9 se utilizaron según criterios de CatSalut en 3 de cada 4 casos. En esta población de alto riesgo, la incidencia de acontecimientos cardiovasculares fue similar a la de los ensayos clínicos.