After the COVID-19 pandemic, the Omicron variant of the SARS-CoV-2 virus became the dominant lineage in Spain in 2022. Although it possesses a milder pathogenicity than previous variants, it still poses a high risk of causing severe COVID-19 for immunocompromised populations.
A systematic review was conducted to assess the burden of COVID-19 in Spain among immunocompromised patients during the Omicron predominance (1/04/2022–1/04/2023), using PubMed, Cochrane Library, and EPICOVIDEHA between May and July 2023.
The search retrieved 217 articles, of which a total of 5 were included. Upon analysis, it was observed that immunocompromised patients during the Omicron lineage predominance continue to exhibit higher rates of hospitalizations, ICU admissions, and mortality compared to the general population affected by COVID-19. Although the pandemic has ended, the risk persists for immunocompromised individuals.
Tras la pandemia de la COVID-19, la variante Ómicron del virus SARS-CoV-2 se convirtió en el linaje dominante en España en 2022. Aunque posee una patogenicidad más leve que las anteriores variantes, sigue suponiendo un riesgo elevado de ocasionar una forma de COVID-19 grave para la población inmunodeprimida.
Se realizó una revisión sistemática para evaluar la carga de la COVID-19 en España en pacientes inmunocomprometidos durante la predominancia de Ómicron (1/04/2022- 1/04/2023), en PubMed, Cochrane Library y EPICOVIDEHA entre mayo y julio de 2023.
La búsqueda recuperó 217 artículos, incluyéndose un total de 5. Tras su análisis, se observó que los pacientes inmunocomprometidos durante el predominio del linaje Ómicron siguen presentando una mayor tasa de hospitalizaciones, ingresos en la UCI y mortalidad que la población general afectada por COVID-19. Aunque la pandemia ha terminado, para los inmunodeprimidos continua el riesgo.
The 2019 coronavirus disease pandemic (COVID-19), caused by the severe acute respiratory syndrome type 2 coronavirus (SARS-CoV-2), declared by the World Health Organization (WHO) in 2020, had devastating global health consequences.1 By March 2023, around 700 million cases were reported worldwide and almost seven million deaths.2 In Spain, nearly 14 million people were reported affected and there were more than 120,000 deaths.3
COVID-19 mortality is more prevalent in older people with underlying diseases such as diabetes mellitus (DM), hypertension, chronic kidney disease (CKD) or cardiovascular disease.4 Several studies have identified that patients with haematological malignancies, immunodeficiencies, solid organ transplants or undergoing chemotherapy, i.e. the severely immunocompromised, are at increased risk of low response to vaccination and longer persistence of the virus when infected with SARS-CoV-2. This is of key importance in the emergence of new variants of the virus. Moreover, these patients have more severe forms of COVID-19, longer hospitalisation and higher mortality.5–7 The COVID-19 pandemic has hit immunocompromised patients the hardest because of their impaired immune response to infection and vaccination, and their co-morbidities.8 It is currently difficult to quantify the population of immunocompromised people in Spain. However, according to the National Transplant Organisation, 5,385 solid organ transplants were performed in Spain, mainly kidney transplants9 and 3,630 haematopoietic stem cell transplants.10 Furthermore, the Spanish Network of Cancer Registries (REDECAN) estimates that the most diagnosed cancers in Spain in 2023 will be: colon and rectum (42,721), breast (35,001), lung (31,282), prostate (29,002) and bladder (21,694).11
To date, several variants of SARS-CoV-2 have been identified, including the omicron variant (B.1.1.529) in late 2021.12 Omicron had a high public health impact and was considered by the WHO as a variant of concern (VOC) due to its high transmissibility but lower virulence and its ability to evade all or part of the immune response to previous variants.13 The omicron variant gave rise to five distinct lineages (BA.1, BA.2, BA.3, BA.4 and BA.5) and was predominant from March to June 2022. In October 2022, circulation of the BQ.1 variant and other closely related sublineages was detected, responsible for the umpteenth epidemic wave of COVID-19.14 Unlike previous variant pandemic waves, this latest one was characterised by a high proportion of asymptomatic carriers,15 milder symptoms, shorter incubation and illness periods, less lower respiratory tract involvement and lower likelihood of hospitalisation.16 This reduced severity in the population is explained by prior acquired immunity and attenuation of virulence of current omicron variants and sublineages.17
Immunocompromised patients remain a distinct risk population today, as their vulnerability to clinical response is compounded by the difficulty of generating a protective response through vaccination. Some studies have shown that immunocompromised patients have a higher risk of hospitalisation and death in COVID-19 for the omicron variant and subsequent lineages.18,19 In Spain, the disease burden of these variants in immunocompromised patients has not yet been adequately reviewed and synthesised, and there is no review of the accumulated evidence in this regard. The aim of the present study was to conduct a systematic review of the literature to assess the burden of the Omicron COVID-19 variant in Spain in immunocompromised patients during the seventh epidemic wave, declared as of 28/3/2022, when the new surveillance strategy20 came into force.
MethodsThe systematic review was registered in PROSPERO (CRD42023425793).21
Information sources and search strategyThe published literature was reviewed to identify studies evaluating the burden of COVID-19 in immunocompromised patients during the period of the omicron variants in Spain. The search was conducted between May-July 2023 in electronic databases: PubMed, Cochrane Library and EPICOVIDEHA. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for the study selection process.22 The PRISMA checklist is included as Appendix A of the Supplementary material.
The following related keywords, medical subject headings and text terms were used in the search: (“SARS-CoV-2” OR COVID OR Omicron) in the title AND (Spain OR Spanish OR español OR España) in the full text AND (immunocompromis* OR immunosuppress* OR “hematologic malignan*” OR “hematologic neoplasm*” OR oncohematologic* OR “solid transplant*” OR “onco hematologic*” OR immunodeficient* OR “bone marrow transplant*” OR HSCT OR “CAR T cell therapy” OR Rituximab OR “Anti CD20" OR mAbs OR azathioprine OR Imuran OR mycophenolate OR Belimumab OR Anakinra OR Infliximab OR Abatacept OR Ibrutinib OR Secukinumab OR Tofacitinib OR chemotherap*) in the title, the abstract or the keywords AND (“2022/04/01” [Date of Publication]: “2023/04/01” [Date of Publication]). The decision to select studies with dates of infection or studies conducted between 01/04/2022 and 01/04/2023 was made in order to narrow down the population infected by the omicron variant.
Selection of studiesStudies with original data on the burden of COVID-19 in immunocompromised patients were selected, including randomised clinical trials (CTs) (phase III and IV), cohort/case-control studies, cross-sectional studies, ecological studies, comparative studies, etc. with quantitative or qualitative data with SARS-CoV-2 infection or study date between 01/04/2022 and 01/04/2023, including publications in English or Spanish with available abstracts.
Cochrane protocols and CTs without published results were excluded, as were trials conducted outside Spain and multicentre trials that did not provide data specific to Spain.
Two reviewers independently screened all titles for relevance, removed duplicates and confirmed eligibility criteria on a data collection form. Any disagreement between the two main reviewers was resolved by a third reviewer. The excluded studies and reasons for exclusion are listed in Appendix B.
Measuring resultsA standardised form was used for each manuscript: cumulative incidence of disease one year after vaccination, hospitalisations, intensive care unit (ICU) admissions, oxygen requirement, mortality, symptomatology, pneumonia, risk factors for infection, antibody titre (immunoglobulin G against the receptor binding domain [IgG-RBD] and immunoglobulin A against the receptor binding domain [IgA-RBD]) and other clinical signs and symptoms of interest.
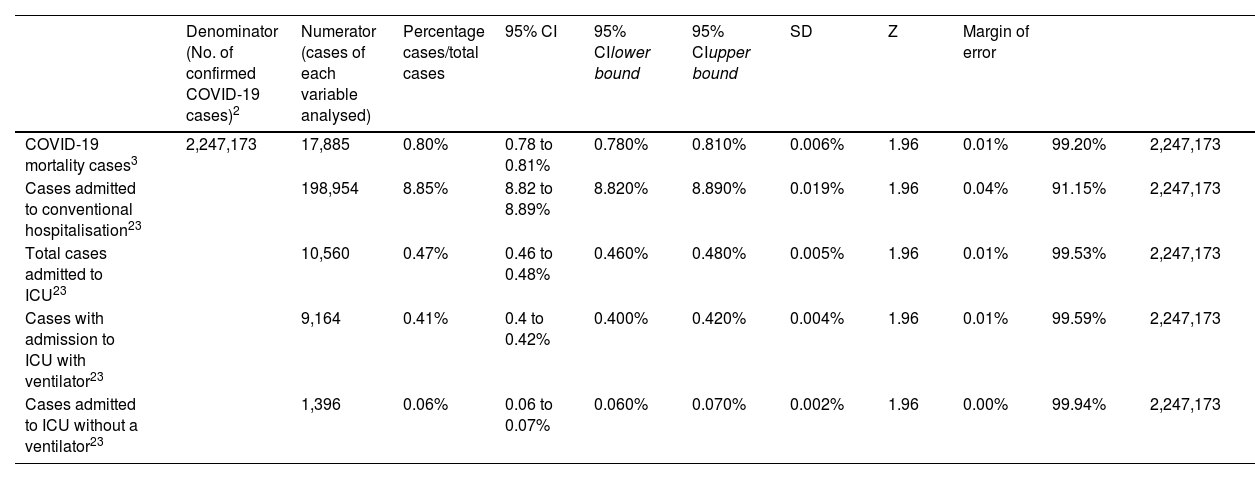
During data extraction, the absence of comparator (control) groups of non-immunocompromised patients was detected. Therefore, data from general population statistics in Spain on mortality, hospitalisations and ICU admissions were reviewed to extract information on the study variables in the general population and for the same study period (Table 1).3,23 To compare data from the immunocompromised population with the general population, 95% confidence intervals were estimated for the reference population and analyses were performed to determine whether or not the results obtained in the trials were within these reference intervals.
Population data in Spain during the study period (01/04/2022 to 31/03/2023).
| Denominator (No. of confirmed COVID-19 cases)2 | Numerator (cases of each variable analysed) | Percentage cases/total cases | 95% CI | 95% CIlower bound | 95% CIupper bound | SD | Z | Margin of error | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 mortality cases3 | 2,247,173 | 17,885 | 0.80% | 0.78 to 0.81% | 0.780% | 0.810% | 0.006% | 1.96 | 0.01% | 99.20% | 2,247,173 |
| Cases admitted to conventional hospitalisation23 | 198,954 | 8.85% | 8.82 to 8.89% | 8.820% | 8.890% | 0.019% | 1.96 | 0.04% | 91.15% | 2,247,173 | |
| Total cases admitted to ICU23 | 10,560 | 0.47% | 0.46 to 0.48% | 0.460% | 0.480% | 0.005% | 1.96 | 0.01% | 99.53% | 2,247,173 | |
| Cases with admission to ICU with ventilator23 | 9,164 | 0.41% | 0.4 to 0.42% | 0.400% | 0.420% | 0.004% | 1.96 | 0.01% | 99.59% | 2,247,173 | |
| Cases admitted to ICU without a ventilator23 | 1,396 | 0.06% | 0.06 to 0.07% | 0.060% | 0.070% | 0.002% | 1.96 | 0.00% | 99.94% | 2,247,173 |
COVID-19: coronavirus disease 2019; SD: standard deviation; CI: confidence interval; n: sample size; Z: Z-score; ICU: Intensive Care Unit; q: quantile value.
Two independent reviewers analysed the quality of the studies with the Mixed Methods Appraisal Tool (MMAT)24 to assess the risk of bias. In case of disagreement, a third reviewer assessed the quality of the discordant study.
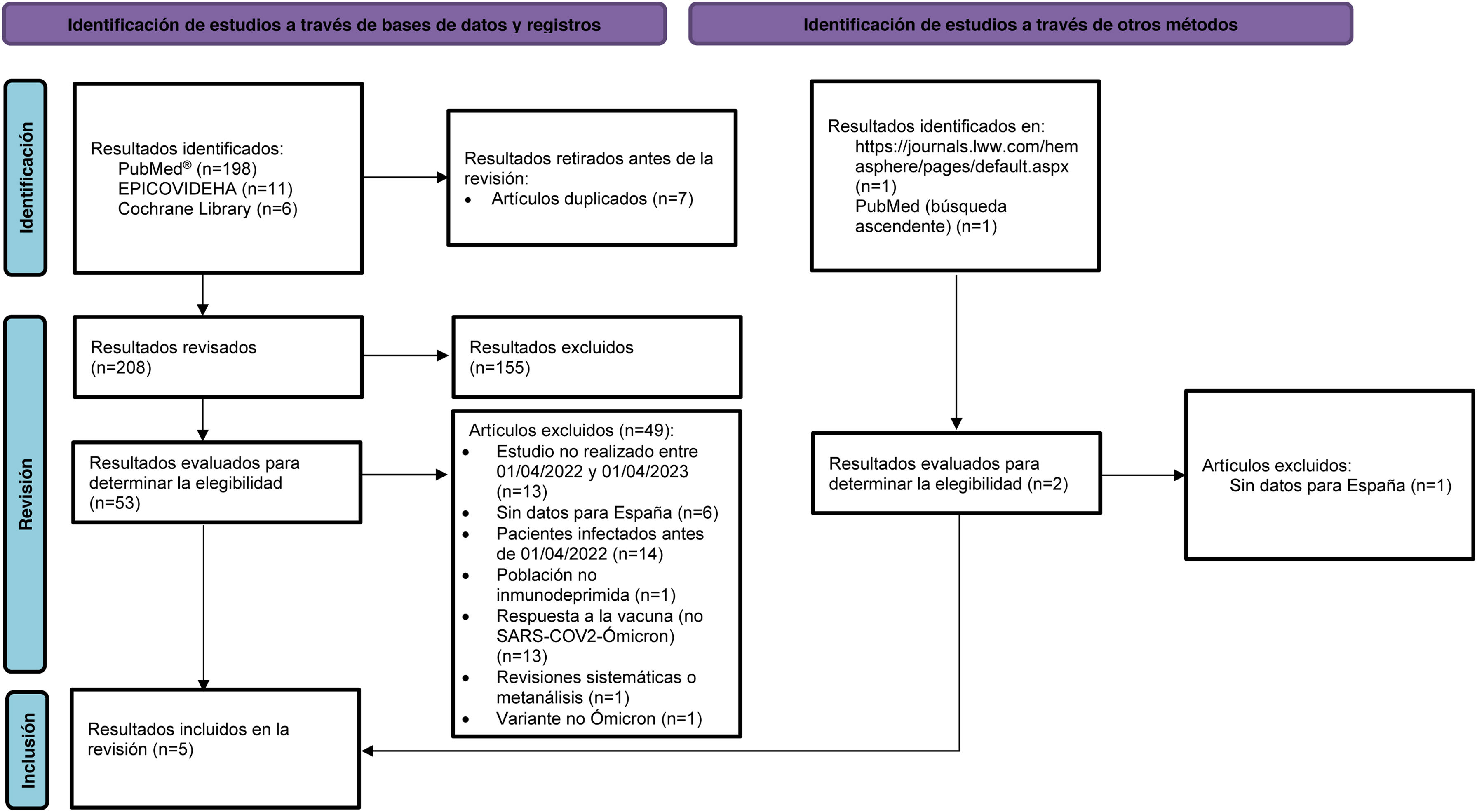
ResultsDescription and characteristics of the studiesWe identified 198 results in PubMed, 11 in the EPICOVIDEHA database and six in the Cochrane Library. Of these 215, seven duplicates were removed. After an initial review of the title and abstract, 155 articles were excluded for not meeting the selection criteria (Appendix B Supplementary material). Concurrently, two studies were identified through other methods (Fig. 1). After checking the pre-selected articles against the eligibility criteria, five articles were selected for the systematic review.25–29Fig. 1 shows the flow of article selection according to PRISMA and the reasons for exclusion.22
PRISMA flowchart of publication selection. Adapted from Page et al.22
n: number; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SARS-CoV-2: severe acute respiratory syndrome 2 coronavirus type 2.
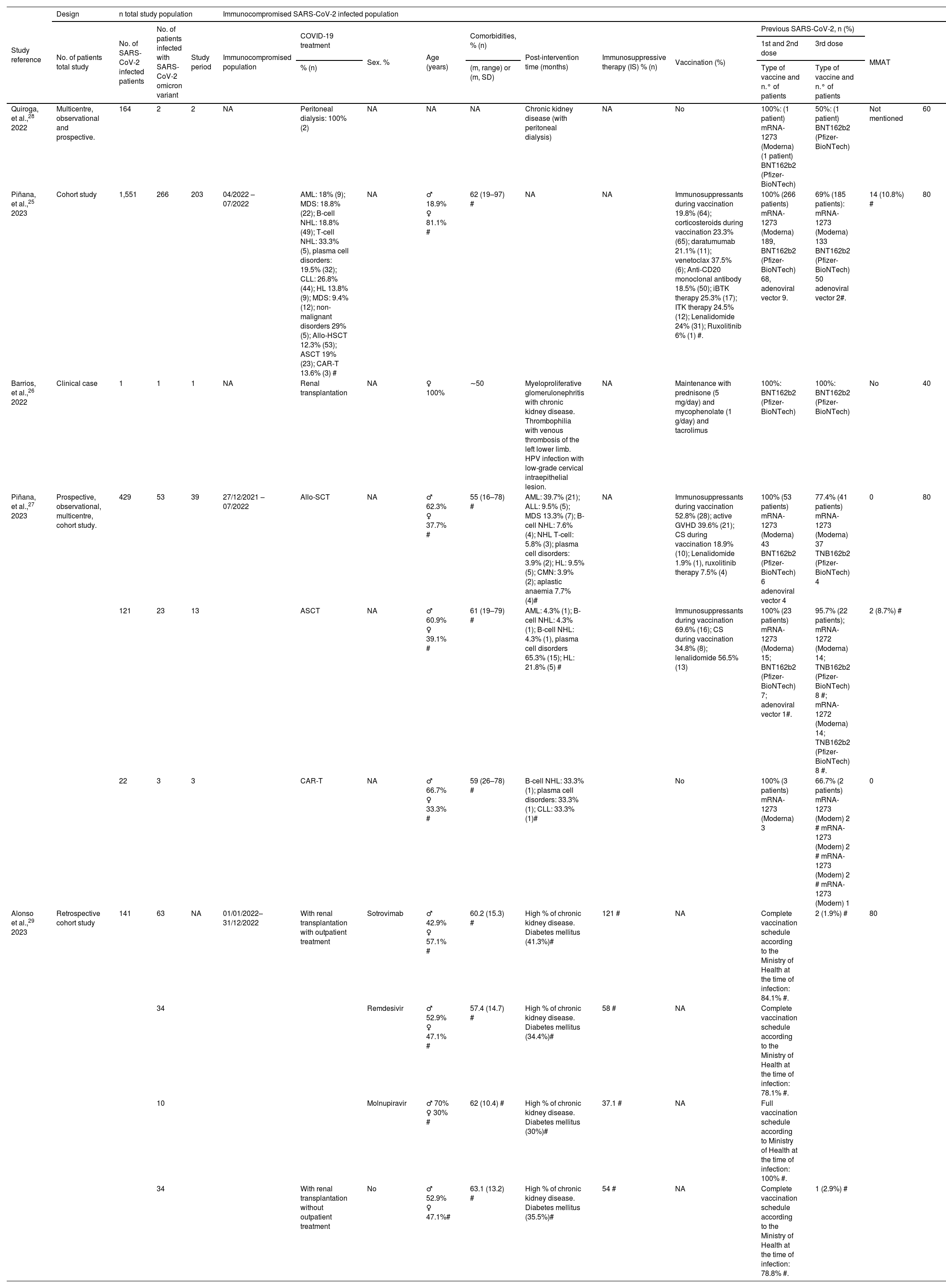
The characteristics of the included studies and population are shown in Table 2.
Characteristics of the included studies and their populations. Description of the immunocompromised population.
| Study reference | Design | n total study population | Immunocompromised SARS-CoV-2 infected population | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients total study | No. of SARS-CoV-2 infected patients | No. of patients infected with SARS-CoV-2 omicron variant | Study period | Immunocompromised population | COVID-19 treatment | Sex. % | Age (years) | Comorbidities, % (n) | Post-intervention time (months) | Immunosuppressive therapy (IS) % (n) | Vaccination (%) | Previous SARS-CoV-2, n (%) | MMAT | |||
| 1st and 2nd dose | 3rd dose | |||||||||||||||
| % (n) | (m, range) or (m, SD) | Type of vaccine and n.° of patients | Type of vaccine and n.° of patients | |||||||||||||
| Quiroga, et al.,28 2022 | Multicentre, observational and prospective. | 164 | 2 | 2 | NA | Peritoneal dialysis: 100% (2) | NA | NA | NA | Chronic kidney disease (with peritoneal dialysis) | NA | No | 100%: (1 patient) mRNA-1273 (Moderna) (1 patient) BNT162b2 (Pfizer-BioNTech) | 50%: (1 patient) BNT162b2 (Pfizer-BioNTech) | Not mentioned | 60 |
| Piñana, et al.,25 2023 | Cohort study | 1,551 | 266 | 203 | 04/2022 – 07/2022 | AML: 18% (9); MDS: 18.8% (22); B-cell NHL: 18.8% (49); T-cell NHL: 33.3% (5), plasma cell disorders: 19.5% (32); CLL: 26.8% (44); HL 13.8% (9); MDS: 9.4% (12); non-malignant disorders 29% (5); Allo-HSCT 12.3% (53); ASCT 19% (23); CAR-T 13.6% (3) # | NA | ♂ 18.9% ♀ 81.1% # | 62 (19–97) # | NA | NA | Immunosuppressants during vaccination 19.8% (64); corticosteroids during vaccination 23.3% (65); daratumumab 21.1% (11); venetoclax 37.5% (6); Anti-CD20 monoclonal antibody 18.5% (50); iBTK therapy 25.3% (17); ITK therapy 24.5% (12); Lenalidomide 24% (31); Ruxolitinib 6% (1) #. | 100% (266 patients) mRNA-1273 (Moderna) 189, BNT162b2 (Pfizer-BioNTech) 68, adenoviral vector 9. | 69% (185 patients): mRNA-1273 (Moderna) 133 BNT162b2 (Pfizer-BioNTech) 50 adenoviral vector 2#. | 14 (10.8%) # | 80 |
| Barrios, et al.,26 2022 | Clinical case | 1 | 1 | 1 | NA | Renal transplantation | NA | ♀ 100% | ∼50 | Myeloproliferative glomerulonephritis with chronic kidney disease. Thrombophilia with venous thrombosis of the left lower limb. HPV infection with low-grade cervical intraepithelial lesion. | NA | Maintenance with prednisone (5 mg/day) and mycophenolate (1 g/day) and tacrolimus | 100%: BNT162b2 (Pfizer-BioNTech) | 100%: BNT162b2 (Pfizer-BioNTech) | No | 40 |
| Piñana, et al.,27 2023 | Prospective, observational, multicentre, cohort study. | 429 | 53 | 39 | 27/12/2021 – 07/2022 | Allo-SCT | NA | ♂ 62.3% ♀ 37.7% # | 55 (16–78) # | AML: 39.7% (21); ALL: 9.5% (5); MDS 13.3% (7); B-cell NHL: 7.6% (4); NHL T-cell: 5.8% (3); plasma cell disorders: 3.9% (2); HL: 9.5% (5); CMN: 3.9% (2); aplastic anaemia 7.7% (4)# | NA | Immunosuppressants during vaccination 52.8% (28); active GVHD 39.6% (21); CS during vaccination 18.9% (10); Lenalidomide 1.9% (1), ruxolitinib therapy 7.5% (4) | 100% (53 patients) mRNA-1273 (Moderna) 43 BNT162b2 (Pfizer-BioNTech) 6 adenoviral vector 4 | 77.4% (41 patients) mRNA-1273 (Moderna) 37 TNB162b2 (Pfizer-BioNTech) 4 | 0 | 80 |
| 121 | 23 | 13 | ASCT | NA | ♂ 60.9% ♀ 39.1% # | 61 (19–79) # | AML: 4.3% (1); B-cell NHL: 4.3% (1); B-cell NHL: 4.3% (1), plasma cell disorders 65.3% (15); HL: 21.8% (5) # | Immunosuppressants during vaccination 69.6% (16); CS during vaccination 34.8% (8); lenalidomide 56.5% (13) | 100% (23 patients) mRNA-1273 (Moderna) 15; BNT162b2 (Pfizer-BioNTech) 7; adenoviral vector 1#. | 95.7% (22 patients); mRNA-1272 (Moderna) 14; TNB162b2 (Pfizer-BioNTech) 8 #; mRNA-1272 (Moderna) 14; TNB162b2 (Pfizer-BioNTech) 8 #. | 2 (8.7%) # | |||||
| 22 | 3 | 3 | CAR-T | NA | ♂ 66.7% ♀ 33.3% # | 59 (26–78) # | B-cell NHL: 33.3% (1); plasma cell disorders: 33.3% (1); CLL: 33.3% (1)# | No | 100% (3 patients) mRNA-1273 (Moderna) 3 | 66.7% (2 patients) mRNA-1273 (Modern) 2 # mRNA-1273 (Modern) 2 # mRNA-1273 (Modern) 2 # mRNA-1273 (Modern) 1 | 0 | |||||
| Alonso et al.,29 2023 | Retrospective cohort study | 141 | 63 | NA | 01/01/2022–31/12/2022 | With renal transplantation with outpatient treatment | Sotrovimab | ♂ 42.9% ♀ 57.1% # | 60.2 (15.3) # | High % of chronic kidney disease. Diabetes mellitus (41.3%)# | 121 # | NA | Complete vaccination schedule according to the Ministry of Health at the time of infection: 84.1% #. | 2 (1.9%) # | 80 | |
| 34 | Remdesivir | ♂ 52.9% ♀ 47.1% # | 57.4 (14.7) # | High % of chronic kidney disease. Diabetes mellitus (34.4%)# | 58 # | NA | Complete vaccination schedule according to the Ministry of Health at the time of infection: 78.1% #. | |||||||||
| 10 | Molnupiravir | ♂ 70% ♀ 30% # | 62 (10.4) # | High % of chronic kidney disease. Diabetes mellitus (30%)# | 37.1 # | NA | Full vaccination schedule according to Ministry of Health at the time of infection: 100% #. | |||||||||
| 34 | With renal transplantation without outpatient treatment | No | ♂ 52.9% ♀ 47.1%# | 63.1 (13.2) # | High % of chronic kidney disease. Diabetes mellitus (35.5%)# | 54 # | NA | Complete vaccination schedule according to the Ministry of Health at the time of infection: 78.8% #. | 1 (2.9%) # | |||||||
ALL: acute lymphocytic leukaemia; Allo-HSCT: allogeneic stem cell transplantation; AML: acute myeloid leukaemia; ASCT: autologous stem cell transplantation; CAR-T: chimeric antigen receptor T-cell; CD20: cluster of differentiation 20; CLL: chronic lymphocytic leukaemia; CMN: chronic myeloproliferative neoplasm; CMS: chronic myeloproliferative syndrome; COVID-19: coronavirus disease 2019; CS: corticosteroids; GVHD: graft-versus-host disease; HL: Hodgkin lymphoma; HPV: human papillomavirus; iBTKs: Bruton’s tyrosine kinase inhibitors; IS: immunosuppressant; MDS: myelodysplastic syndrome; MMAT: mixed methods assessment tool; m: mean; mRNA: messenger ribonucleic acid; n: number; NA: not available; NHL: non-Hodgkin lymphoma; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; SD: standard deviation; TKIs: tyrosine kinase inhibitors.
#These data are for the SARS-CoV-2 infected population, no variants specified. The study does not provide data for the omicron variant.
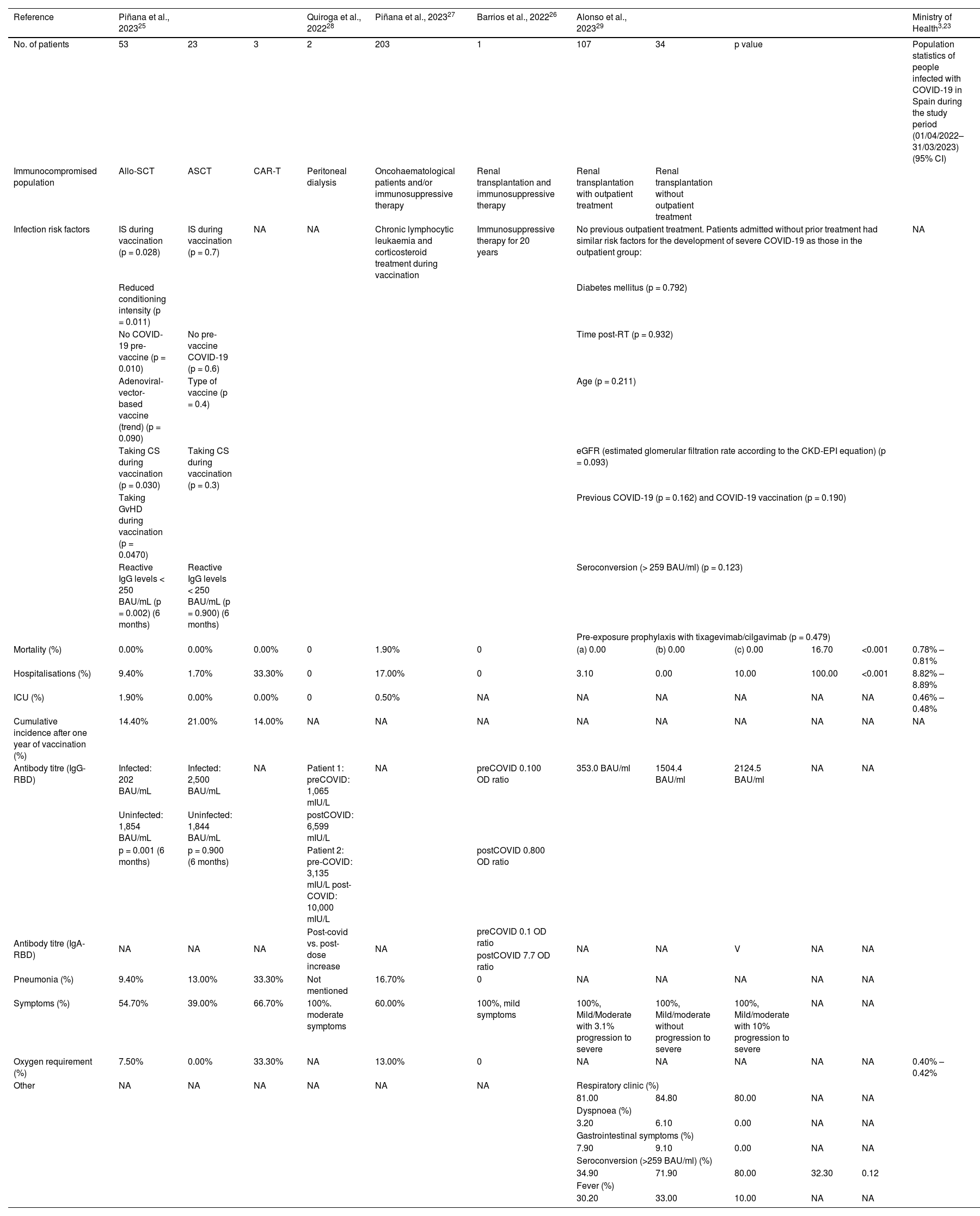
Table 3 shows the health outcomes of the immunocompromised population with severe or persistent COVID-19 described in the selected studies and the statistics of the general COVID-19 infected population in Spain during the study period (01/04/2022–31/03/2023). All immunocompromised patients affected by COVID-19 variants during the omicron predominant period had milder or moderate symptoms25–29 compared with earlier variants; however, these studies also showed that infection with this variant was associated with a significant clinical burden with higher rates of hospitalisation, ICU admission and mortality than in the general population. Thus, hospitalisation data showed significantly higher rates in the immunocompromised population than in the general population (between 9.4–100% vs. 8.82–8.89%).25,27,29
Risk factors and health outcomes in the immunocompromised population with COVID-19.
| Reference | Piñana et al., 202325 | Quiroga et al., 202228 | Piñana et al., 202327 | Barrios et al., 202226 | Alonso et al., 202329 | Ministry of Health3,23 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | 53 | 23 | 3 | 2 | 203 | 1 | 107 | 34 | p value | Population statistics of people infected with COVID-19 in Spain during the study period (01/04/2022–31/03/2023) (95% CI) | ||
| Immunocompromised population | Allo-SCT | ASCT | CAR-T | Peritoneal dialysis | Oncohaematological patients and/or immunosuppressive therapy | Renal transplantation and immunosuppressive therapy | Renal transplantation with outpatient treatment | Renal transplantation without outpatient treatment | ||||
| Infection risk factors | IS during vaccination (p = 0.028) | IS during vaccination (p = 0.7) | NA | NA | Chronic lymphocytic leukaemia and corticosteroid treatment during vaccination | Immunosuppressive therapy for 20 years | No previous outpatient treatment. Patients admitted without prior treatment had similar risk factors for the development of severe COVID-19 as those in the outpatient group: | NA | ||||
| Reduced conditioning intensity (p = 0.011) | Diabetes mellitus (p = 0.792) | |||||||||||
| No COVID-19 pre-vaccine (p = 0.010) | No pre-vaccine COVID-19 (p = 0.6) | Time post-RT (p = 0.932) | ||||||||||
| Adenoviral-vector-based vaccine (trend) (p = 0.090) | Type of vaccine (p = 0.4) | Age (p = 0.211) | ||||||||||
| Taking CS during vaccination (p = 0.030) | Taking CS during vaccination (p = 0.3) | eGFR (estimated glomerular filtration rate according to the CKD-EPI equation) (p = 0.093) | ||||||||||
| Taking GvHD during vaccination (p = 0.0470) | Previous COVID-19 (p = 0.162) and COVID-19 vaccination (p = 0.190) | |||||||||||
| Reactive IgG levels < 250 BAU/mL (p = 0.002) (6 months) | Reactive IgG levels < 250 BAU/mL (p = 0.900) (6 months) | Seroconversion (> 259 BAU/ml) (p = 0.123) | ||||||||||
| Pre-exposure prophylaxis with tixagevimab/cilgavimab (p = 0.479) | ||||||||||||
| Mortality (%) | 0.00% | 0.00% | 0.00% | 0 | 1.90% | 0 | (a) 0.00 | (b) 0.00 | (c) 0.00 | 16.70 | <0.001 | 0.78% – 0.81% |
| Hospitalisations (%) | 9.40% | 1.70% | 33.30% | 0 | 17.00% | 0 | 3.10 | 0.00 | 10.00 | 100.00 | <0.001 | 8.82% – 8.89% |
| ICU (%) | 1.90% | 0.00% | 0.00% | 0 | 0.50% | NA | NA | NA | NA | NA | NA | 0.46% – 0.48% |
| Cumulative incidence after one year of vaccination (%) | 14.40% | 21.00% | 14.00% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Antibody titre (IgG-RBD) | Infected: 202 BAU/mL | Infected: 2,500 BAU/mL | NA | Patient 1: preCOVID: 1,065 mIU/L | NA | preCOVID 0.100 OD ratio | 353.0 BAU/ml | 1504.4 BAU/ml | 2124.5 BAU/ml | NA | NA | |
| Uninfected: 1,854 BAU/mL | Uninfected: 1,844 BAU/mL | postCOVID: 6,599 mIU/L | ||||||||||
| p = 0.001 (6 months) | p = 0.900 (6 months) | Patient 2: pre-COVID: 3,135 mIU/L post-COVID: 10,000 mIU/L | postCOVID 0.800 OD ratio | |||||||||
| Antibody titre (IgA-RBD) | NA | NA | NA | Post-covid vs. post-dose increase | NA | preCOVID 0.1 OD ratio | NA | NA | V | NA | NA | |
| postCOVID 7.7 OD ratio | ||||||||||||
| Pneumonia (%) | 9.40% | 13.00% | 33.30% | Not mentioned | 16.70% | 0 | NA | NA | NA | NA | NA | |
| Symptoms (%) | 54.70% | 39.00% | 66.70% | 100%. moderate symptoms | 60.00% | 100%, mild symptoms | 100%, Mild/Moderate with 3.1% progression to severe | 100%, Mild/moderate without progression to severe | 100%, Mild/moderate with 10% progression to severe | NA | NA | |
| Oxygen requirement (%) | 7.50% | 0.00% | 33.30% | NA | 13.00% | 0 | NA | NA | NA | NA | NA | 0.40% –0.42% |
| Other | NA | NA | NA | NA | NA | NA | Respiratory clinic (%) | |||||
| 81.00 | 84.80 | 80.00 | NA | NA | ||||||||
| Dyspnoea (%) | ||||||||||||
| 3.20 | 6.10 | 0.00 | NA | NA | ||||||||
| Gastrointestinal symptoms (%) | ||||||||||||
| 7.90 | 9.10 | 0.00 | NA | NA | ||||||||
| Seroconversion (>259 BAU/ml) (%) | ||||||||||||
| 34.90 | 71.90 | 80.00 | 32.30 | 0.12 | ||||||||
| Fever (%) | ||||||||||||
| 30.20 | 33.00 | 10.00 | NA | NA | ||||||||
Allo-HSCT: allogeneic stem cell transplantation, ASCT: autologous stem cell transplantation, BAU: binding antibody units, CAR-T: chimeric antigen receptor T-cell, CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration, COVID-19: coronavirus disease 2019, CS: corticosteroids, eGFR: estimated glomerular filtration rate, ICU: Intensive Care Unit, IgA: immunoglobulin A, IgG: immunoglobulin G, IS: immunosuppressants, NA: not applicable, NA: not available, OD: optical density, RBD: receptor binding domain, RT: renal transplantation, (a)Sotrovimab, (b)Remdesivir, (c)Molnupiravir.
The oncohaematological patient suffers from immunocompromise due to the disease itself and the treatments administered to control it.
In Spain, the Spanish Group of Haematopoietic Transplantation and Cell Therapy (GETH-TC), conducted the VACUNHEM registry to determine the disease burden. The multicentre prospective cohort study demonstrated a hospitalisation rate of 18%, and a mortality rate of 1.9% during the omicron lineage circulation (n = 203).25 Multivariate analysis identified the highest incidence in patients with chronic lymphocytic leukaemia (29%) treated with corticosteroids (24.5%).25
An additional analysis of the VACUNHEM registry by Piñana et al. studied the ICU admission rate and observed significantly higher rates among allogeneic transplant patients compared to the general population (1.9% vs. 0.46–0.48%).27,29 In these patients, multivariate analysis identified both immunosuppressive therapy during vaccination (p = 0.028) and reduced conditioning intensity (p = 0.011) as significant risk factors. In addition, that analysis showed other risk factors for SARS-CoV-2 infection such as the absence of pre-vaccination SARS-CoV-2 infection (p = 0.01), glucocorticoid therapy coincident with vaccination (p = 0.03), active graft-versus-host disease (GVHD) coinciding with vaccination (p = 0.047) or IgG levels < 250 binding antibody units (BAU)/mL at six months post-vaccination (p = 0.002).27
Clinical burden in the renal transplant patientThe low humoral response in CKD patients and, in particular, in renal transplant (RT) recipients has led to the recommendation of successive booster doses of vaccine in these patients. Most of the studies have focused on the results in RT through their humoral immune response. The SENCOVAC study, the largest published to date, analysed the humoral response after successive doses of vaccine during the first year of vaccination in Spain (28 days [n = 301], three months [n = 3,439], six months [n = 1,018] and 12 months [n = 4,079]).28,30 The latest report, published in late 2022, showed that up to 9% of RT patients had a negative humoral response (antibody titre below 32 BAU/mL) after four doses of vaccine.30,31 The analysis showed that factors associated with developing a lower antibody titre included prescription of steroid and mycophenolate mofetil therapy.31 Of particular concern is the situation of persistently humoral negative patients who do not seroconvert, even after receiving booster doses, as they are mostly RT patients.30
The need to protect the RT patient, even during the circulation of omicron lineages, is demonstrated in another retrospective study on the burden of disease in 141 RT patients in a series collected between January and December 2022. These patients had severe COVID-19 at the time of hospital admission, with the exception of three, who had received prior outpatient treatment with monoclonal antibodies. Mortality reached 16.7% in those who had not received outpatient treatment,29 showing a rate 20 times higher than that reported in the general population (0.78–0.81%).3,29 More than 80% of patients with RT treated as outpatients experienced respiratory symptoms.29
Alonso et al., investigating the impact of outpatient treatment with monoclonal antibodies, observed that a small number of patients (n = 107) required hospitalisation, and none of them died, in stark contrast to the 100% hospitalisation and mortality rate recorded in those who did not receive outpatient treatment. Notably, patients admitted without prior treatment (n = 34) had similar risk factors for the development of severe COVID-19 as those in the outpatient group: DM (p = 0.792), time post-transplant (p = 0.932), age (p = 0.211), Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) estimated glomerular filtration rate (eGFR) (p = 0.093), previous COVID-19 (p = 0.162), COVID-19 vaccination (p = 0.19), seroconversion (> 259 BAU/mL) (p = 0.123) and pre-exposure prophylaxis with tixagevimab/cilgavimab (p = 0.479).29
Quality assessmentThe results of the quality assessment according to the MMAT model are summarised in Table 2. For all selected studies, the screening questions were answered positively. The quality assessment found that most studies met the criteria for representativeness, appropriate measurement and completeness of data. However, confounding factors were not considered in some studies. The overall quality score of the studies was “high” in three of them (80%), “medium” in one (60%) and “low” in the remaining one (40%). All studies were included regardless of their methodological quality.
DiscussionThis is the first published systematic review of Spanish data analysing the impact of the omicron variant on immunocompromised patients. The analysis shows that, although omicron is a less virulent variant, the consequences of COVID-19 in immunocompromised patients are particularly relevant.
Immunocompromised patients constitute a heterogeneous population that includes diverse clinical situations such as haematological malignancies, solid organ transplant recipients, patients with chimeric antigen receptor T-cell therapy (CAR-T), patients with primary or secondary immunodeficiencies (e.g. human immunodeficiency virus [HIV] infection), and those receiving immunosuppressive drug therapy.7 These populations with an altered immune response are more vulnerable than the general population and generate a significant burden of disease in patients who also consume, or have consumed, large amounts of healthcare resources.
Factors that increase the risk of developing severe COVID-19 in the general population (advanced age, underlying diseases: CKD, cardiovascular disease and DM) are also risk factors with significant presence in the immunocompromised population.4 In fact, in the present study, many of the patients had additional comorbidities (DM, CKD or multimorbidity) that per se, further increase the intrinsic risk derived from their impaired immune response.28,29 Many of the included patients were on active treatment with immunosuppressive drugs, and treatments such as T-cell depleting or suppressive therapies (calcineurin inhibitors -cyclosporine, tacrolimus, mycophenolate mofetil, belatacept) or B-cell depleting therapies (rituximab, ocrelizumab, obinutuzumab) have been reported to be associated with an increased likelihood of death from COVID-19.32,33
All patients included in this review mostly experienced COVID-19 infection with mild symptoms.25–29 This lower severity of disease may be due in part to the extensive vaccination of the population at the time of the emergence of the omicron variant. However, although more than 80% of the total population of autologous or allogeneic haematopoietic stem cell transplant recipients and CAR-T had received three doses of vaccine, up to 12.4%, 19% and 13.6%, respectively, were infected with SARS-CoV-2.27 In addition, up to 10% of patients with RT and outpatient treatment with molnupiravir for COVID-19 vaccinated with the full regimen showed progression to severe disease29 and higher rates of hospitalisation, ICU admission and mortality than the general population. On the other hand, immunocompromised patients also show a significant decrease in antibody titres three to six months after initial vaccination and booster doses.34 The study by Piñana et al. described that SARS-CoV-2 infections are more common in patients with antibody titres < 250 BAU/mL than in those with titres ≥ 250 BAU/mL.27 Moreover, this difference was significant six months post-vaccination. In the study, there were cases of infection in patients with titres > 250 BAU/mL, but significantly less than in the < 250 BAU/mL group six months post-vaccination.
Immunocompromised patients were not included in most pivotal trials evaluating the safety and efficacy of SARS-CoV-2 vaccines.35–37 However, data from subsequent observational studies have shown that a significant proportion of immunocompromised patients require a higher number of doses to obtain antibody titres consistent with humoral protection. Despite this, up to 46% of solid organ recipients fail to respond to a second dose.38,39 In patients with haematological malignancies, the number of severe or critical COVID-19 cases is significantly lower after the fourth dose of vaccination.40 Therefore, national health recommendations suggest four doses of vaccine in immunocompromised patients.31 Even so, RT patients have suboptimal responses to the fourth dose of vaccination, with up to 10% of non-responders.30,31,41 Therefore, it appears that simply vaccinating patients with low antibody levels or those who lose antibodies early may not be sufficient, so it may be important to consider more frequent follow-up between recommended doses.42
In line with these data in the CKD spectrum, RT patients have shown increased vulnerability to SARS-CoV-2 infection and a suboptimal response to vaccination. In Spain, the COVID-19 Registry of the Spanish Society of Nephrology (SEN) showed that despite routine vaccination of CKD patients (including haemodialysis, peritoneal dialysis and RT) and a decrease in the virulence of the omicron lineage, mortality remains higher than in the general population, particularly in the kidney transplant cohort.43 Factors associated with this vulnerability include the inherent immunosuppressed state of CKD, the extensive comorbidity these patients exhibit, and the need to prescribe immunosuppressive drugs.31,44
On the other hand, Cazorla et al. have recently published the effectiveness of administering a fifth dose of the vaccine to RT patients, demonstrating that, although it increases the rate of antibodies and reduces the percentage of seronegative patients, it does not protect all.41
Similarly, studies that have assessed cell-mediated immunity demonstrate a decrease in response after successive doses of SARS-CoV-2 vaccination.45
In addition to vaccination and monoclonal antibodies as prevention strategies, other therapeutic strategies such as antiviral treatment with nirmatrelvir/ritonavir have been tested in patients with COVID-19. Out of a total of 1,859 patients included, 47.9% (479) had omicron variant disease. In this study, patients with haematological malignancies were more likely to receive nirmatrelvir/ritonavir when they had extrapulmonary symptomatology or a second booster dose of vaccine at the start of COVID-19, as opposed to chronic lung disease and obesity. The mortality rate in patients treated with nirmatrelvir/ritonavir was lower than in those treated with other targeted therapies.46 It is important to note in this regard that RT patients cannot be treated with nirmatrelvir/ritonavir due to their CKD and polypharmacy, leaving them physiologically and therapeutically vulnerable.47
Other antivirals administered in the same setting, such as molnupiravir, have not shown significant differences in terms of hospitalisation, survival and mortality, although this antiviral has a more favourable drug-drug interaction profile.48
Regarding pre-exposure therapies, several studies describe the effectiveness of these therapies in different populations of immunocompromised patients who do not have an adequate immune response to vaccination, demonstrating that the prophylactic use of combined monoclonal antibodies to COVID-19 can halt progression to severe disease.49
This review shows that while all patients without monoclonal antibody treatment were hospitalised for COVID-19, only a low percentage of those who received monoclonal antibodies required hospitalisation. In addition, the mortality rate was significantly higher in those who did not receive monoclonal antibodies compared to the zero-rate reported in patients who did receive monoclonal antibodies.29 Until a few months ago, there were several monoclonal antibody treatments approved for prophylactic and therapeutic use in the immunocompromised population. However, in recent months, almost all of these drugs have been reported to have lost efficacy.50
Patients with immunosuppression are at risk of prolonged SARS-CoV-2 infection. Specifically, if we talk about patients with oncohematological malignancies, the term “chronic persistent infection” arises, in which the virus develops immune evasion mechanisms with increased viral excretion, producing gradual but continuous damage to the lower respiratory tract. This type of disease arises in patients who are generally immunocompromised, either because of their underlying disease (mostly lymphoproliferative syndromes) or because of their treatment (mainly anti-cluster differentiation 20 [CD20] monoclonal antibodies). Mutations in the ORF-1ab and PL2pro pathways and other enzyme substrates have been identified in the aforementioned immune evasion, which may explain the lack of immunogenicity of this patient profile to current active immunisation techniques49 and the emergence of new variants. Another fundamental aspect in patients with oncohaematological malignancies is the so-called “long term” COVID, with a partially unknown pathophysiology. This concept, in contrast to the previous one, refers to the infection of a patient who does not develop symptoms, but with a persistent positive viral load. Its incidence is high with the omicron variant, although lower than with other variants.51
An indirect problem of persistent COVID-19 infection added to the above clinical considerations is that such a clinical condition is considered as an exclusion criterion for participation in CTs of new therapies, forcing a delay in their administration and negatively impacting the prognosis of these patients.52
Therefore, prevention of COVID-19 in immunocompromised patients should be a key objective of prevention policies in the post-pandemic era. During the omicron stage, the combination tixagevimab-cilgavimab was the only non-vaccination prophylactic treatment licensed for COVID-19 pre-exposure that showed efficacy for a period against certain omicron variants in immunocompromised patients.53,54 In Spain, experience with tixagevimab-cilgavimab in patients with no humoral response after successive doses of the vaccine proved to be safe and effective in achieving an acceptable protective antibody rate.55 Early approval of such treatment in other countries allowed evaluation of its usefulness during the omicron phase. A meta-analysis of 18 trials involving 25,345 immunocompromised patients treated with these drugs, 5,438 of whom were oncohematological patients, reported a reduction in hospitalisation, ICU and mortality rates of 66.19%, 82.13% and 92.39% respectively.56 In addition, another study published in France including 322 patients receiving tixagevimab-cilgavimab demonstrated its effectiveness against omicron, but not against the BQ.1.1 and XBB variants.57 In line with these results, the Public Health Commission approved the recommendation for the use of tixagevimab-cilgavimab in highly immunosuppressed individuals and others who could not be vaccinated against COVID-19. However, the subsequent emergence of new variants against which tixagevimab-cilgavimab lacks neutralising activity led to the recommendation for its use to be withdrawn in March 2023.58,59 Therefore, although SARS-CoV-2 vaccines are highly effective, it is still necessary to use additional passive immunisation prophylaxis strategies in immunocompromised individuals who do not respond adequately to vaccination, in addition to conventional, non-pharmacological, measures (face masks, ventilation, etc.).
An important limitation of this review is the analysis period of April 2022 to April 2023, which may omit data that has not yet been published. Another important limitation is that people with HIV were not considered separately. However, the data available for this study with the criteria outlined above did not allow us to include HIV-specific outcomes due to the clinical variability among them. The inclusion of exclusively Spanish studies results in the admission of five studies, which may limit the generalisability of conclusions due to the limited amount of data analysed. However, the focus on the Spanish population can be seen as an advantage in providing relevant and specific information to contribute to the current health strategy in Spain. Furthermore, this study focuses on the omicron variant of COVID-19, so the results cannot be directly extrapolated to future variants, although the general conclusions are valid. In spite of these limitations, the data collected show that the Spanish immunocompromised population will be one of the last to be adequately protected from the emergence of this new virus.
This review highlights that although COVID-19 omicron variant infection appears less severe in the general population, it remains a concern for immunocompromised patients. Rates of hospitalisation, ICU admissions and mortality remain high in this group, underlining the importance of targeted prophylactic measures. There is a need for continued evidence gathering and clinical-epidemiological surveillance, with further identification of prognostic factors associated with COVID-19 disease burden in immunocompromised patients. Although the acute phase of the pandemic has ended, the risk of COVID-19 for the immunocompromised remains high.
Ethical considerationsNot applicable.
FundingThis publication has been funded by AstraZeneca Farmacéutica Spain S.A. Authors did not and will not receive honoraria for their participation and authorship.
Conflict of interestRaúl Ortiz-de-Lejarazu has received financial compensation for academic activities, presentations or scientific reports from Abbott, AstraZeneca, bioMerieux, CSL, GSK, Moderna, MSD, Novavax, Pfizer, Roche and Sanofi.
Borja Quiroga is a member of SEN and has received fees for lectures and funding to attend courses and conferences from Vifor-Pharma, Astellas, Amgen, Bial, Ferrer, Novartis, AstraZeneca, Sandoz, Laboratorios Bial, Esteve, Sanofi-Genzyme and Otsuka in the last 36 months.
Alberto López García has received fees for lectures and funding to attend courses and conferences from Janssen, Abbvie, Abbot, Roche, Astrazeneca and Beigene in the last 36 months. He has received consultancy fees from Janssen, Astrazeneca and Beigene.
We would like to thank Dr. María Fernández-Prada, from the Preventive Medicine and Public Health Service of the Hospital Vital Álvarez Buylla (Asturias) and Dr. Patricia Aparicio-Domingo from AstraZeneca for their contribution to the study and critical reading. Patricia Aparicio-Domingo of AstraZeneca for their contribution to the study and critical reading of the study, not included in the authorship due to editorial limitation, and to Ana Corcuera Sánchez of AstraZeneca and Inmaculada Molina, Gloria González, Maite Artés and María Ruart of Adelphi Targis, SL for their contribution to the collection and interpretation of data, as well as the preparation of this manuscript.