The risk of HIV-1 mother-to-child transmission (MTCT) is associated mainly with gestational age at which antiretroviral therapy begins and the HIV-1 RNA plasma viral load at delivery. Regimens with integrase inhibitors (INI) are increasing in high-risk pregnant women. The objective was to review the experience with INI in a Madrid Cohort of mother-infant pairs.
Patients and methodsRetrospective, multicentric, observational study, of HIV-infected pregnant women exposed to INI. Patients of 9 hospitals were included (2000–2017).
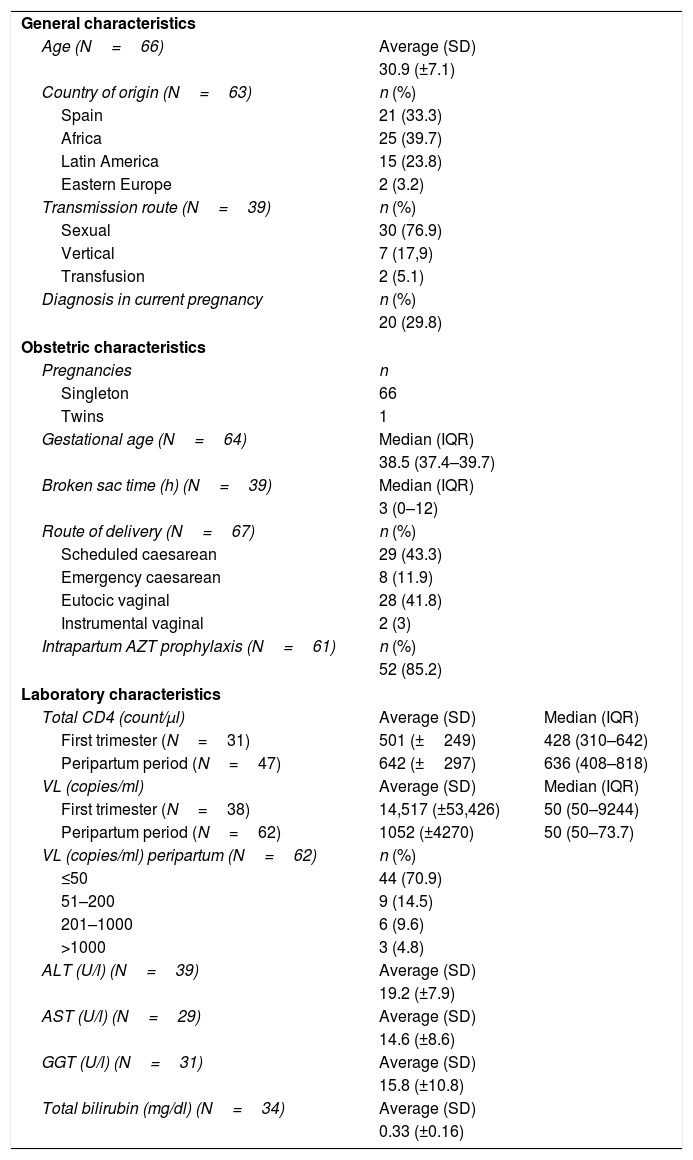
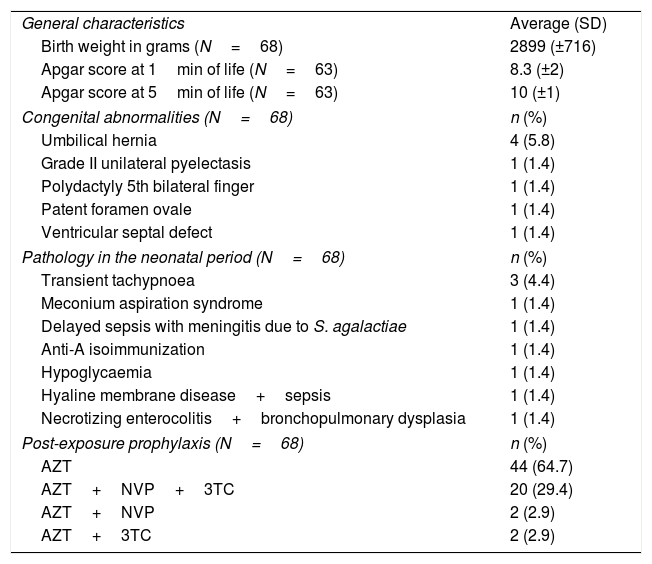
ResultsSixty-seven pregnant women exposed to INI (cohort: 1423) and 68 children (17.6% premature babies, 34.3% with combined postexposure prophylaxis). There were no cases of MTCT. Of 24 women with no previous antiretroviral therapy, 20 were diagnosed in current pregnancy. Of 43 women with antiretroviral therapy before pregnancy, 65% received INI before conception. Raltegravir was the most used (80.5%). There was a statistically significant increase (p=0.02) of mothers with undetectable viral load at delivery. INI were well tolerated. In 11.7% of exposed children minor congenital anomalies were detected.
ConclusionsINI seem safe and effective in the prevention of MTCT. Our findings support their use as intensification regimens in pregnant women with high risk of MTCT.
El riesgo de transmisión vertical (TV) del VIH depende fundamentalmente de la edad gestacional de inicio del tratamiento antirretroviral y la carga viral materna al parto. Son crecientes las pautas con inhibidores de integrasa (INI) en embarazadas con situaciones de riesgo. Nuestro objetivo fue revisar la experiencia con INI en la Cohorte de Madrid de madres-niños.
Pacientes y métodosEstudio retrospectivo, multicéntrico, observacional, de gestantes con infección por VIH-1 expuestas a INI de 9 hospitales públicos durante 2000-2017.
ResultadosHubo 67 gestantes (cohorte: 1.423) y 68 neonatos (el 17,6% prematuros, el 34,3% con profilaxis combinada). No hubo casos de TV. Veinte mujeres se diagnosticaron en la gestación actual. De 43 con tratamiento antirretroviral previo a gestación, el 65% recibía INI preconcepcional. El más empleado fue raltegravir (80,5%). Aumentó significativamente (p=0,02) la proporción de madres con carga viral indetectable al parto. La tolerancia a INI fue adecuada. Hubo anomalías congénitas menores en el 11,7% de los niños.
ConclusionesLos INI parecen seguros y eficaces como prevención de TV. Nuestros hallazgos refuerzan su utilidad como intensificación en gestantes que llegan al tercer trimestre con pauta no supresora.