Emicizumab is the first non-replacement therapy for prophylaxis in severe Hemophilia A.
AimThe principal aim of this study is to describe the results of our patients in prophylaxis with emicizumab, according to the usual clinical practice.
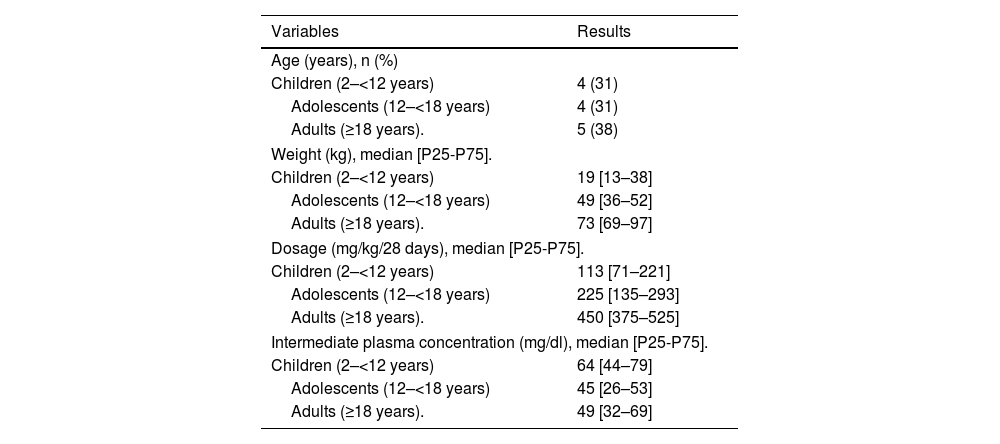
MethodsFollow-up of 13 patients from the start of prophylaxis, recording of bleeding, surgeries, adverse reactions and the need or not for factor therapy. Plasma levels were measured at follow-up visits, the technique was coagulative in one stage, modified by 1:20 dilution.
ResultsMedian plasma levels were 52.2mg [30.7–71.9]. Prophylaxis was safe and effective, only one spontaneous hemorrhage was recorded over time and no treatment was required. There were no thromboembolic events or serious hypersensitivity, anaphylaxis and anaphylactoid reactions. The incidence of injection site reactions was 8%. Perioperative management in minor interventions was carried out without adjuvant factorial therapy, in two major surgeries a dose of plasmatic FVIII concentrate (pFVIII) was required in the patient with HA without inhibitor and FVII in the patient with inhibitor and it was sufficient to stop the bleeding.
ConclusionThis study demonstrated emicizumab pharmacokinetics and its half life ensure optimal levels with prophylaxis treatment at doses established in the technical data sheet.
Emicizumab es la primera terapia no sustitutiva para profilaxis en Hemofilia A grave.
ObjetivosDescribir los resultados de nuestros pacientes en profilaxis con emicizumab, según la práctica clínica habitual.
Material y métodosSeguimiento de 13 pacientes desde el inicio de la profilaxis, registro de hemorragias, cirugías, reacciones adversas y necesidad o no de terapia factorial. Se midieron los niveles plasmáticos en las visitas de seguimiento, la técnica ha sido coagulativa en una etapa, modificada mediante dilución 1:20.
ResultadosLa mediana de niveles plasmáticos fue de 52,2mg [30,7–71,9]. La profilaxis resultó segura y eficaz, solamente se contabilizó una hemorragia espontánea a lo largo del tiempo y no precisó tratamiento. No hubo eventos tromboembólicos ni reacciones graves de hipersensibilidad, anafilaxia y anafilactoides. La incidencia de reacciones en el lugar de la inyección fue del 8%. El manejo perioperatorio en las intervenciones menores se llevó a cabo sin terapia factorial coadyuvante, en dos cirugías mayores de precisó una dosis de concentrado de FVIII plasmático (pFVIII) en el paciente con HA sin inhibidor y FVII en el paciente con inhibidor y fue suficiente para parar la hemorragia.
ConclusiónEste estudio demostró que la farmacocinética de emicizumab y su vida media aseguran niveles óptimos con tratamiento profiláctico a las dosis establecidas en la ficha técnica.