Previous works seem to agree in the higher mortality of cancer patients with COVID-19. Identifying potential prognostic factors upon admission could help identify patients with a poor prognosis.
MethodsWe aimed to explore the characteristics and evolution of COVID-19 cancer patients admitted to hospital in a multicenter international registry (HOPE COVID-19).
Our primary objective is to define those characteristics that allow us to identify cancer patients with a worse prognosis (mortality within 30 days after the diagnosis of COVID-19).
Results5838 patients have been collected in this registry, of whom 770 had cancer among their antecedents. In hospital mortality reached 258 patients (33.51%). The median was 75 years (65–82). Regarding the distribution by sex, 34.55% of the patients (266/770) were women.
The distribution by type of cancer: genitourinary 238/745 (31.95%), digestive 124/745 (16.54%), hematologic 95/745 (12.75%).
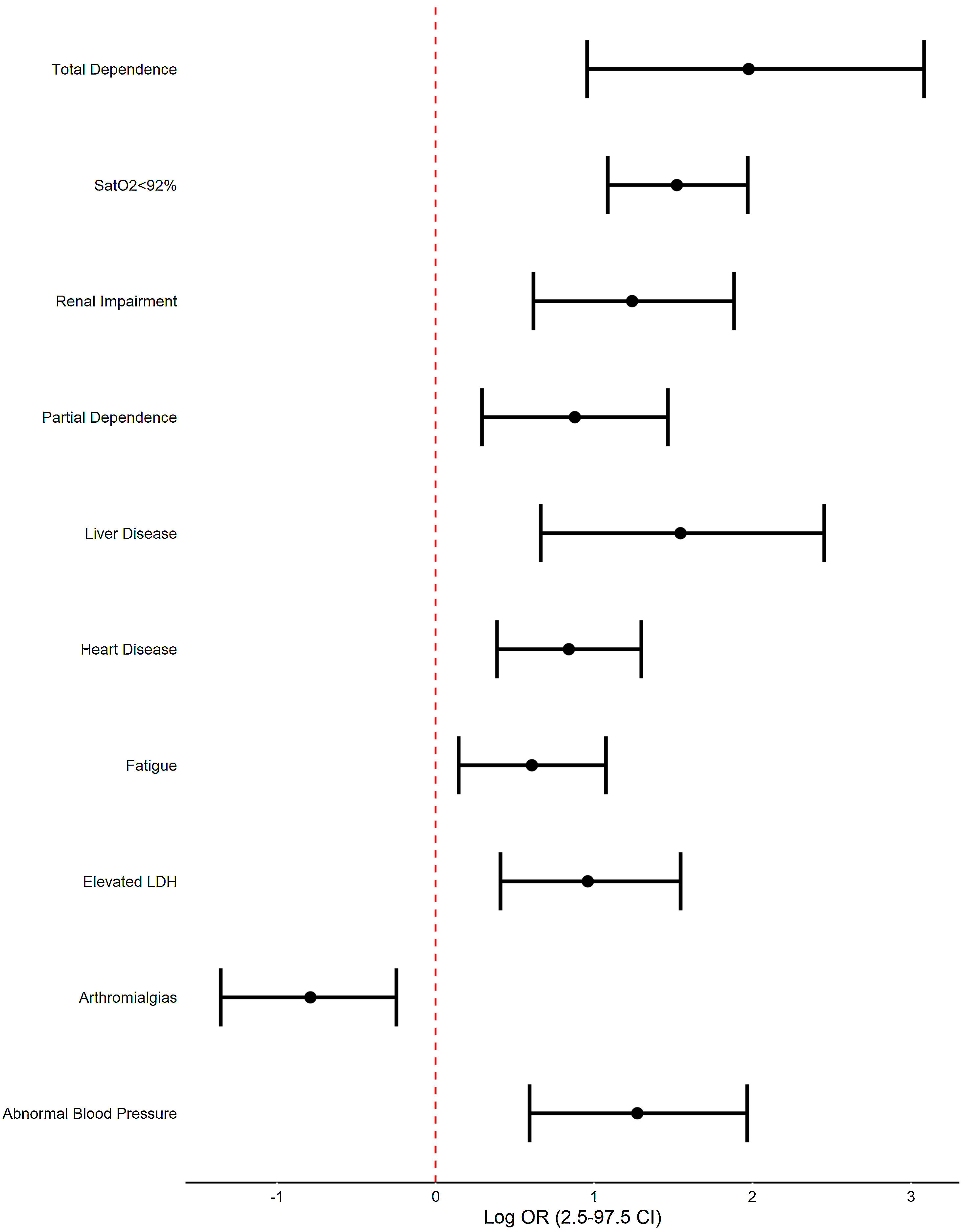
In multivariate regression analysis, factors that are independently associated with mortality at admission are: renal impairment (OR 3.45, CI 97.5% 1.85–6.58), heart disease (2.32, 1.47–3.66), liver disease (4.69, 1.94–11.62), partial dependence (2.41, 1.34–4.33), total dependence (7.21, 2.60–21.82), fatigue (1.84, 1.16–2.93), arthromialgias (0.45, 0.26–0.78), SatO2<92% (4.58, 2.97–7.17), elevated LDH (2.61, 1.51–4.69) and abnormal decreased Blood Pressure (3.57, 1.81–7.15). Analitical parameters are also significant altered.
ConclusionIn patients with cancer from the HOPE registry, 30-day mortality from any cause is high and is associated with easily identifiable clinical factors upon arrival at the hospital. Identifying these patients can help initiate more intensive treatments from the start and evaluate the prognosis of these patients.
Trabajos previos parecen coincidir en la mayor mortalidad de los pacientes con cáncer y COVID-19. La identificación de posibles factores pronósticos en el momento del ingreso podría ayudar a identificar a los pacientes con mal pronóstico.
MétodosNos propusimos explorar las características y la evolución de los pacientes con cáncer y COVID-19 ingresados en un registro internacional multicéntrico (HOPE COVID-19).
Nuestro objetivo principal es definir aquellas características que nos permitan identificar a los pacientes con cáncer de peor pronóstico (mortalidad en los 30 días siguientes al diagnóstico de COVID-19).
ResultadosEn este registro se ha recogido a 5.838 pacientes, de los cuales 770 tenían cáncer entre sus antecedentes. La mortalidad hospitalaria alcanzó a 258 pacientes (33,51%). La mediana fue de 75 años (65-82). En cuanto a la distribución por sexo, el 34,55% de los pacientes eran mujeres (266/770).
La distribución por tipo de cáncer: genitourinario 238/745 (31,95%), digestivo 124/745 (16,54%) y hematológico 95/745 (12,75%).
En el análisis de regresión multivariante, los factores que se asocian de forma independiente con la mortalidad al ingreso son: insuficiencia renal (OR 3,45; IC 97,5%: 1,85-6,58), cardiopatía (2,32; 1,47-3,66), hepatopatía (4,69; 1,94-11,62), dependencia parcial (2,41; 1,34-4,33), dependencia total (7,21; 2,60-21,82), fatiga (1,84, 1;16-2,93), artromialgias (0,45; 0,26-0,78), SatO2 <92% (4,58; 2,97-7,17), LDH elevada (2,61; 1,51-4,69) y disminución anormal de la presión arterial (3,57; 1,81-7,15). Los parámetros analíticos también están significativamente alterados.
ConclusiónEn los pacientes con cáncer del registro HOPE, la mortalidad a los 30 días por cualquier causa es elevada y se asocia a factores clínicos fácilmente identificables a su llegada al hospital. La identificación de estos pacientes puede ayudar a iniciar tratamientos más intensivos desde el principio y evaluar el pronóstico de estos pacientes.
Covid-19 infection has affected a large number of patients and caused deaths throughout the world in recent months, mainly due to its lung involvement.1 Some populations are especially vulnerable to this infection, including cancer patients. Initial publications suggested that cancer patients may have an increased risk of contracting the infection as well as develop complications more frequently and severely.2–4 This could be related to the multifactorial immunosuppression situation that these patients present in relation to the tumour, the treatments and other intercurrent processes.5–6
On the other hand, cancer patients tend to be older, affected by other comorbidities, which would also explain this worse evolution.
The objective of this study is to analyze the epidemiological, clinical and evolutionary characteristics of cancer patients reported in the international HOPE registry with the intention of identifying poor prognostic factors at the time of admission to a hospital centre that allow us to intensify the measures of support from the beginning and better understand the evolution of this infection in cancer patients.
MethodsStudy design and populationThe Health Outcome Predictive Evaluation for COVID-19 (HOPE-COVID-19) registry (clinicaltrials.gov NCT04334291) is a multicenter, international study, designed as a retrospective cohort real-life registry, with voluntary participation and without any financial remuneration for neither researchers nor patients. All patients admitted to hospital for COVID-19 or those deceased were suitable for the study. There were no exclusion criteria, except for patients or families’ explicit refusal to participate. Patients from 41 centres in 30 cities and 6 countries (Canada, China, Cuba, Ecuador, Germany, Italy and Spain) were included.
Data sourceDemographic, clinical, and outcome data were extracted from electronic medical records in all participating centres. Confidentiality was guaranteed by typing all patient information anonymously and stored in a password-protected secure online database (www.HopeProjectMD.com).
Confirmed COVID-19 cases were those with a positive nasal and pharyngeal swab sample obtained at admission using real time reverse transcriptase-polymerase chain reaction (RT-PCR) as per WHO recommendations. Data included comorbidities (hypertension, diabetes, dyslipidemia, obesity, smoking, lung, heart, cerebrovascular, renal, liver and connective tissue disease, cancer, dementia, etc.); emergency room assessment variables, clinical assessments during hospitalization (radiology, laboratory findings, clinical signs and symptoms, severity as use of ventilatory support or admission to intensive care unit [ICU], etc.); and discharge status. All procedures and treatments were applied by the medical team in each centre, following clinical practice guides and protocols.
Study outcomesWe stratified cancer patients in the registry in two groups, survivor vs non-survivor at discharge.
Our primary objective is to define those clinical, analytical and radiological characteristics that allow us to identify those cancer patients with the worst prognosis at the time of hospital admission. Events were described according to local investigators’ criteria, upon HOPE-COVID-19 registry definitions.
Ethical issuesThe study was approved by the Ethics Research Committee from Hospital Clinico San Carlos (Madrid, Spain) (20/241-E) and the Spanish Drug Agency authorities (AEMPS classification: EPA-0D) and by local committees when needed. Written informed consent was waived owing to the severity of the situation and the use of deidentified retrospective data. However, verbal authorization from either patients or caregivers was required.
Statistical analysisCategorical variables were summarized with absolute numbers and the percentage of each group regarding the total population under investigation. Chi-Square-test (or Fisher's exact test) were used to determine statistical differences in categorical variables distribution between patients with or without in-hospital death. Due to the short-term evolution of the disease, we did not use the actuarial method to perform a survival analysis. Monte Carlo simulation was applied to Chi-Square-test when conditions were not met. Statistical significance was adjusted using Bonferroni-Hochberg multiple correction.
In order to identify the risk factors associated with the disease outcome we adjusted a logistic regression model, performing univariate regression analysis in those variables with significant between-group differences. In order to perform the multivariate analysis we included in the model those variables that showed significancy in either the distribution analysis or univariate models, and performed a backward-elimination strategy. Odd ratios with 95% confidence intervals were calculated to assess the relative risk of each variable.
All statistical tests were two-tailed, and a p value of <0.05 was considered statistically significant. Statistical analyses were performed using R v3.6.3 under R-Studio 1.1.383. (R Development Core Team Vienna, Austria; https://www.r-project.org).
ResultsIn the writing of this section, we have summarized those variables that will be important when comparing the group of living cancer patients vs. deceased patients.
All the variables were collected at the time of the patient in the Emergency Room.
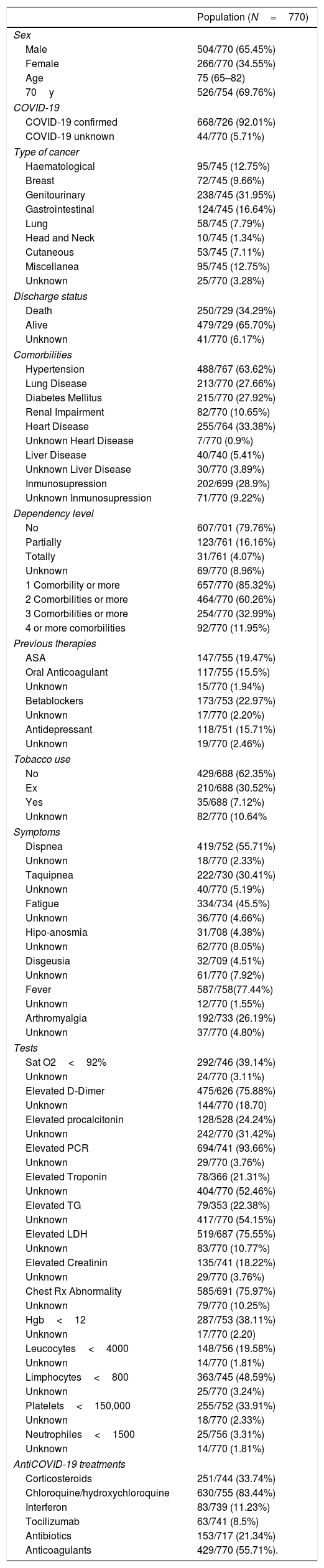
Demographics (Table 1)As 5 of June, 2020 a total of 5838 patients were included in the registry HOPE-COVID-19. Of these, 770 patients had cancer (13.19%).
Patient epidemiological and clinical characteristics.
| Population (N=770) | |
|---|---|
| Sex | |
| Male | 504/770 (65.45%) |
| Female | 266/770 (34.55%) |
| Age | 75 (65–82) |
| 70y | 526/754 (69.76%) |
| COVID-19 | |
| COVID-19 confirmed | 668/726 (92.01%) |
| COVID-19 unknown | 44/770 (5.71%) |
| Type of cancer | |
| Haematological | 95/745 (12.75%) |
| Breast | 72/745 (9.66%) |
| Genitourinary | 238/745 (31.95%) |
| Gastrointestinal | 124/745 (16.64%) |
| Lung | 58/745 (7.79%) |
| Head and Neck | 10/745 (1.34%) |
| Cutaneous | 53/745 (7.11%) |
| Miscellanea | 95/745 (12.75%) |
| Unknown | 25/770 (3.28%) |
| Discharge status | |
| Death | 250/729 (34.29%) |
| Alive | 479/729 (65.70%) |
| Unknown | 41/770 (6.17%) |
| Comorbilities | |
| Hypertension | 488/767 (63.62%) |
| Lung Disease | 213/770 (27.66%) |
| Diabetes Mellitus | 215/770 (27.92%) |
| Renal Impairment | 82/770 (10.65%) |
| Heart Disease | 255/764 (33.38%) |
| Unknown Heart Disease | 7/770 (0.9%) |
| Liver Disease | 40/740 (5.41%) |
| Unknown Liver Disease | 30/770 (3.89%) |
| Inmunosupression | 202/699 (28.9%) |
| Unknown Inmunosupression | 71/770 (9.22%) |
| Dependency level | |
| No | 607/701 (79.76%) |
| Partially | 123/761 (16.16%) |
| Totally | 31/761 (4.07%) |
| Unknown | 69/770 (8.96%) |
| 1 Comorbility or more | 657/770 (85.32%) |
| 2 Comorbilities or more | 464/770 (60.26%) |
| 3 Comorbilities or more | 254/770 (32.99%) |
| 4 or more comorbilities | 92/770 (11.95%) |
| Previous therapies | |
| ASA | 147/755 (19.47%) |
| Oral Anticoagulant | 117/755 (15.5%) |
| Unknown | 15/770 (1.94%) |
| Betablockers | 173/753 (22.97%) |
| Unknown | 17/770 (2.20%) |
| Antidepressant | 118/751 (15.71%) |
| Unknown | 19/770 (2.46%) |
| Tobacco use | |
| No | 429/688 (62.35%) |
| Ex | 210/688 (30.52%) |
| Yes | 35/688 (7.12%) |
| Unknown | 82/770 (10.64% |
| Symptoms | |
| Dispnea | 419/752 (55.71%) |
| Unknown | 18/770 (2.33%) |
| Taquipnea | 222/730 (30.41%) |
| Unknown | 40/770 (5.19%) |
| Fatigue | 334/734 (45.5%) |
| Unknown | 36/770 (4.66%) |
| Hipo-anosmia | 31/708 (4.38%) |
| Unknown | 62/770 (8.05%) |
| Disgeusia | 32/709 (4.51%) |
| Unknown | 61/770 (7.92%) |
| Fever | 587/758(77.44%) |
| Unknown | 12/770 (1.55%) |
| Arthromyalgia | 192/733 (26.19%) |
| Unknown | 37/770 (4.80%) |
| Tests | |
| Sat O2<92% | 292/746 (39.14%) |
| Unknown | 24/770 (3.11%) |
| Elevated D-Dimer | 475/626 (75.88%) |
| Unknown | 144/770 (18.70) |
| Elevated procalcitonin | 128/528 (24.24%) |
| Unknown | 242/770 (31.42%) |
| Elevated PCR | 694/741 (93.66%) |
| Unknown | 29/770 (3.76%) |
| Elevated Troponin | 78/366 (21.31%) |
| Unknown | 404/770 (52.46%) |
| Elevated TG | 79/353 (22.38%) |
| Unknown | 417/770 (54.15%) |
| Elevated LDH | 519/687 (75.55%) |
| Unknown | 83/770 (10.77%) |
| Elevated Creatinin | 135/741 (18.22%) |
| Unknown | 29/770 (3.76%) |
| Chest Rx Abnormality | 585/691 (75.97%) |
| Unknown | 79/770 (10.25%) |
| Hgb<12 | 287/753 (38.11%) |
| Unknown | 17/770 (2.20) |
| Leucocytes<4000 | 148/756 (19.58%) |
| Unknown | 14/770 (1.81%) |
| Limphocytes<800 | 363/745 (48.59%) |
| Unknown | 25/770 (3.24%) |
| Platelets<150,000 | 255/752 (33.91%) |
| Unknown | 18/770 (2.33%) |
| Neutrophiles<1500 | 25/756 (3.31%) |
| Unknown | 14/770 (1.81%) |
| AntiCOVID-19 treatments | |
| Corticosteroids | 251/744 (33.74%) |
| Chloroquine/hydroxychloroquine | 630/755 (83.44%) |
| Interferon | 83/739 (11.23%) |
| Tocilizumab | 63/741 (8.5%) |
| Antibiotics | 153/717 (21.34%) |
| Anticoagulants | 429/770 (55.71%). |
The median age is 75 years (65–82). 69.76% had more than 70 years at admission. Regarding the distribution by sex, 34.55% (266/770) were women.
The distribution by type of cancer is as follows: genitourinary 238/745 (31.95%), digestive 124/745 (16.54%), hematologic 95/745 (12.75%), breast 72/245 (9.66%), lung 58/745 (7.79%), cutaneous 53/745 (7.11%), head and neck 10/745 (1.34%), miscellanea 95/745 (12.75%), unknown 25/745 (3.24%).
92.01% of the patients (668/770) presented confirmation of Covid-19 infection at the microbiological level.
Regarding the medical history of these patients, 488/767 (63.62%) had hypertension, 213/770 (27.66%) lung disease, 215/770 (27.92%) diabetes mellitus, renal impairment 82/770 (10.65%), heart disease 255/764 (33.38%), liver disease 40/740 (5.41%), immunosuppression 202/699 (28.9%). 657/770 (85.32%) of the patients presented at least 1 comorbidity, 464/770 (60.26%) 2 or more, 254/770 (32.99%) 3 or more, and 92/770 (11.95%) 4 or more comorbidities.
Regarding the level of dependency, which would reflect the patient's baseline functional situation, 607/701 (79.76%) had no level of dependency, 123/761 (16.16%) had partial dependency, and 31/761 (4.07%) they were totally dependent.
Symptoms of presentation in emergency room (Table 1)At admission 587/758 (77.44%) had fever, 419/752 (55.72%) dyspnoea, 334/734 (45.5%) fatigue, 222/730 (30.41%) tachypnea, 192/733 (26.19%) arthromyalgia, 32/709 (4.51%) dysgeusia and 31/708 (4.38%) hipo/anosmia, as the most frequent symptoms.
Radiographic and laboratory findings upon admission. (Table 1)Radiological findings were abnormal in 585/691 (75.97%) of patients, with the bilateral pattern being the most common (65.56%).
At the gasometric level, 291/746 (39.14%) of the patients had a saturation below 92%.
As for the most relevant analytical findings, the elevation of D-dimer affected 475/626 (75.88%), elevation of procalcitonin in 128/528 (24.24%), elevation of PCR in 694/741 (93.66%), elevation of troponin in 78/366 (21.31%), elevation of triglycerides in 79/353 (22.38%), elevation of LDH in 519/687 (75.55%), creatinine elevation above 1.5 times the normal value in 135/741 (18.22%), leukocytes below 4000 in 148/756 (19.58%), Hg<12 in 287/753 (38.11%), lymphocytes<800 in 362/745 (48.59%), neutrophils<1500 in 25/756 (3.31%), neutrophils>7500 in 299/756 (39.55%9 and platelets<150,000 in 255/752 (33.91%).
Previous therapies (Table 1)The most common drugs included in the patients’ usual prescription were: Acetil salicic acid 147/755 (19.47%), oral anticoagulants 117/755 (15.5%), betablockers 173/753 (22.97%) and antidepressant 118/751 (15.71%).
Treatment during entry and evolution (Table 1)The treatments against COVID19 were periodically modified according to scientific knowledge and the protocols were being modified.
251/744 (33.74%) received corticosteroids, 630/755 (83.44%) chloroquine/hydroxychloroquine, 83/739 (11.23%) interferon, 63/741 (8.5%) tocilizumab, 153/717 (21.34%) antibiotics, and 429/770 (55.71%) anticoagulants.
258 of 770 patients (33.51%) died during admission.
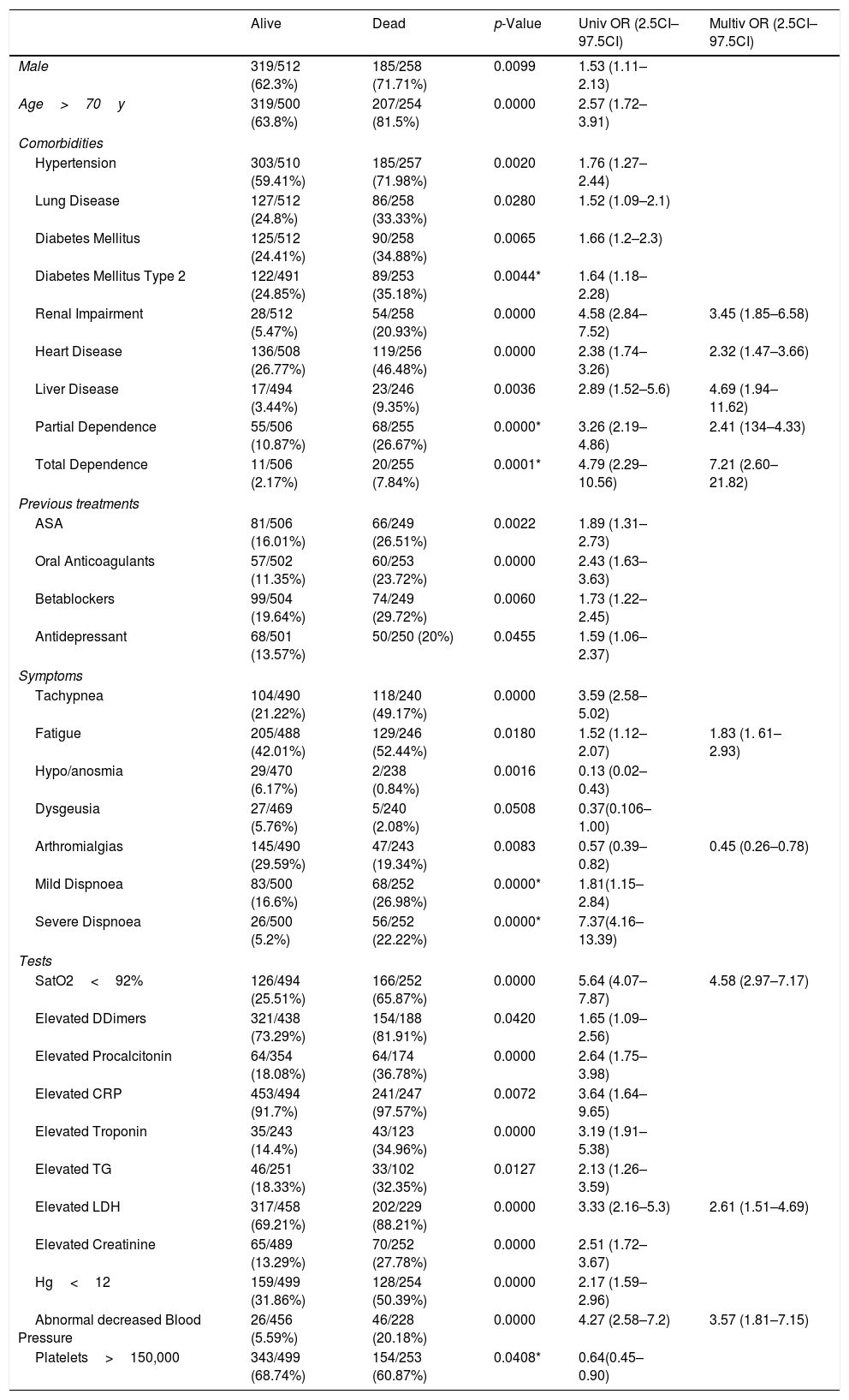
Comparison of variables between the living and the dead (Table 2)In the univariate analysis between the group of living at discharge and deceased, we can find the following results (only those variables that have statistical significance are referred to): regarding sex, male gender is a factor of poor prognosis (p 0.0099), as well as the age over 70 years (p 0.0000).
Uni and multivariable regression models of prognostic variables associated with mortality.
| Alive | Dead | p-Value | Univ OR (2.5CI–97.5CI) | Multiv OR (2.5CI–97.5CI) | |
|---|---|---|---|---|---|
| Male | 319/512 (62.3%) | 185/258 (71.71%) | 0.0099 | 1.53 (1.11–2.13) | |
| Age>70y | 319/500 (63.8%) | 207/254 (81.5%) | 0.0000 | 2.57 (1.72–3.91) | |
| Comorbidities | |||||
| Hypertension | 303/510 (59.41%) | 185/257 (71.98%) | 0.0020 | 1.76 (1.27–2.44) | |
| Lung Disease | 127/512 (24.8%) | 86/258 (33.33%) | 0.0280 | 1.52 (1.09–2.1) | |
| Diabetes Mellitus | 125/512 (24.41%) | 90/258 (34.88%) | 0.0065 | 1.66 (1.2–2.3) | |
| Diabetes Mellitus Type 2 | 122/491 (24.85%) | 89/253 (35.18%) | 0.0044* | 1.64 (1.18–2.28) | |
| Renal Impairment | 28/512 (5.47%) | 54/258 (20.93%) | 0.0000 | 4.58 (2.84–7.52) | 3.45 (1.85–6.58) |
| Heart Disease | 136/508 (26.77%) | 119/256 (46.48%) | 0.0000 | 2.38 (1.74–3.26) | 2.32 (1.47–3.66) |
| Liver Disease | 17/494 (3.44%) | 23/246 (9.35%) | 0.0036 | 2.89 (1.52–5.6) | 4.69 (1.94–11.62) |
| Partial Dependence | 55/506 (10.87%) | 68/255 (26.67%) | 0.0000* | 3.26 (2.19–4.86) | 2.41 (134–4.33) |
| Total Dependence | 11/506 (2.17%) | 20/255 (7.84%) | 0.0001* | 4.79 (2.29–10.56) | 7.21 (2.60–21.82) |
| Previous treatments | |||||
| ASA | 81/506 (16.01%) | 66/249 (26.51%) | 0.0022 | 1.89 (1.31–2.73) | |
| Oral Anticoagulants | 57/502 (11.35%) | 60/253 (23.72%) | 0.0000 | 2.43 (1.63–3.63) | |
| Betablockers | 99/504 (19.64%) | 74/249 (29.72%) | 0.0060 | 1.73 (1.22–2.45) | |
| Antidepressant | 68/501 (13.57%) | 50/250 (20%) | 0.0455 | 1.59 (1.06–2.37) | |
| Symptoms | |||||
| Tachypnea | 104/490 (21.22%) | 118/240 (49.17%) | 0.0000 | 3.59 (2.58–5.02) | |
| Fatigue | 205/488 (42.01%) | 129/246 (52.44%) | 0.0180 | 1.52 (1.12–2.07) | 1.83 (1. 61–2.93) |
| Hypo/anosmia | 29/470 (6.17%) | 2/238 (0.84%) | 0.0016 | 0.13 (0.02–0.43) | |
| Dysgeusia | 27/469 (5.76%) | 5/240 (2.08%) | 0.0508 | 0.37(0.106–1.00) | |
| Arthromialgias | 145/490 (29.59%) | 47/243 (19.34%) | 0.0083 | 0.57 (0.39–0.82) | 0.45 (0.26–0.78) |
| Mild Dispnoea | 83/500 (16.6%) | 68/252 (26.98%) | 0.0000* | 1.81(1.15–2.84) | |
| Severe Dispnoea | 26/500 (5.2%) | 56/252 (22.22%) | 0.0000* | 7.37(4.16–13.39) | |
| Tests | |||||
| SatO2<92% | 126/494 (25.51%) | 166/252 (65.87%) | 0.0000 | 5.64 (4.07–7.87) | 4.58 (2.97–7.17) |
| Elevated DDimers | 321/438 (73.29%) | 154/188 (81.91%) | 0.0420 | 1.65 (1.09–2.56) | |
| Elevated Procalcitonin | 64/354 (18.08%) | 64/174 (36.78%) | 0.0000 | 2.64 (1.75–3.98) | |
| Elevated CRP | 453/494 (91.7%) | 241/247 (97.57%) | 0.0072 | 3.64 (1.64–9.65) | |
| Elevated Troponin | 35/243 (14.4%) | 43/123 (34.96%) | 0.0000 | 3.19 (1.91–5.38) | |
| Elevated TG | 46/251 (18.33%) | 33/102 (32.35%) | 0.0127 | 2.13 (1.26–3.59) | |
| Elevated LDH | 317/458 (69.21%) | 202/229 (88.21%) | 0.0000 | 3.33 (2.16–5.3) | 2.61 (1.51–4.69) |
| Elevated Creatinine | 65/489 (13.29%) | 70/252 (27.78%) | 0.0000 | 2.51 (1.72–3.67) | |
| Hg<12 | 159/499 (31.86%) | 128/254 (50.39%) | 0.0000 | 2.17 (1.59–2.96) | |
| Abnormal decreased Blood Pressure | 26/456 (5.59%) | 46/228 (20.18%) | 0.0000 | 4.27 (2.58–7.2) | 3.57 (1.81–7.15) |
| Platelets>150,000 | 343/499 (68.74%) | 154/253 (60.87%) | 0.0408* | 0.64(0.45–0.90) | |
p Values corresponds with those obtained in the chi-square/fisher test distribution analysis. * p Values obtained in the univariable regression model.
Among the personal history, hypertension (p 0.0020), pulmonary disease (p 0.0280), DM (0.0065), kidney failure (p 0.0000), heart disease (p 0.0000), liver disease (p 0.0036), taking acetil salicilic acid (p 0.0022), oral anticoagulants (p 0.0000), beta-blockers (p 0.0060) and antidepressants (p 0.0455) are factors of poor prognosis. Factors such as the existence of total (p 0.0001) or partial dependence (p 0.0000), mild dyspnoea (p 0.0000) or severe (p 0.0000) are clearly related to a poor prognosis.
The analysis of the symptoms and signs presented upon arrival showed that tachypnea (p 0.0000) and fatigue (p 0.0180) were factors of poor prognosis, while the presence of anosmia (p 0.0016) and arthromyalgia (p 0.0083) arised as good prognostic factors.
Regarding the explorations carried out at that initial moment, the following alterations have been correlated with a worse prognosis: O2 saturation <92% (p 0.0000), elevated Ddimers (p 0.0402), elevation of procalcitonin (p 0.0000), elevation of PCR (p 0.0072), troponin elevation ((p 0.0000), triglyceride elevation (p 0.0127), LDH elevation (p 0.0000), creatinine elevation above 1.5 times the maximum normal value (p 0.0000), Hg >12mg/dL (p 0.0000) and abnormal blood pressure (p 0.0000) again lead to a higher risk of in-hospital death.
The protective effect of hipo/anosmia (p 0.0016) and arthromyalgias (p 0.0083) is striking. This could be explained by the population information campaign that has been carried out on the special correlation between these symptoms and Covid-19 infection that motivates the population to go to medical services earlier or to a special neurotropism in less virulent strains of Covid19, with a better prognosis.
Regarding the multivariate analysis (Fig. 1) we found renal impairment (OR 3.45, CI 97.5% 1.85–6.58), heart disease (2.32, 1.47–3.66), liver disease (4.69, 1.94–11.62), partial dependence (2.41, 1.34–4.33), total dependence (7.21, 2.60–21.82), fatigue (1.84, 1.16–2.93), arthromialgias (0.45, 0.26–0.78), SatO2<92% (4.58, 2.97–7.17), elevated LDH (2.61, 1.51–4.69) and abnormal decreased blood pressure (3.57, 1.81–7.15) were associated with increased odds of fatal outcome.
DiscussionThe COVID-19 pandemic has triggered a health problem not seen in decades and which is affecting the global population.
Some subgroups of people are being more affected by this disease due to their age and comorbidities. Within this group are cancer patients; Among the reasons that, a priori, make this subgroup of patients more vulnerable are general factors such as age and associated comorbidities, but also aspects related to the multifactorial immunosuppression that affects them.
The management that cancer patients have had during the pandemic upon arrival at hospital emergency departments has been very similar to that of non-cancer patients. However, his immunosuppression situation has not been taken into account when articulating special circuits.
Our data shows that the mortality of cancer patients is high and this is reflected in other recently published works.3,4,7–9 In our series, the percentage of patients who died during admission was 33.51%, somewhat higher in the series above mentioned, which ranged from 11% to 28%.
A possible explanation for the higher mortality data in our series is that the group of patients could be at higher risk due to age and associated comorbidities, factors already known in more general series.
Regarding the factors that significantly mark a worse prognosis, in our series, they have a clear relationship with patients who present a complex medical history with prior comorbidities in functionally important systems as well as, at least, partial health and social dependence.
As for the symptoms, those already reported by other series are repeated, which mainly affect the respiratory system, clearly related to the importance of lung involvement in the evolution of these patients.
Another aspect of interest is the fact that the symptoms of hypo/anosmia and arthromyalgias seem to “protect” the patients; As we have previously commented, this better evolution could be explained by a matter of social awareness of the relationship between these symptoms and the possible infection with COVID-19 that makes patients consult before or because of the relationship with a different form of infection that will have to be done. Evaluate in other studies.
From the analytical point of view, our data clearly correlates a worse prognosis with altered analytical data in immunological reaction markers such as PCR, procalcitonin, LDH, Dimers, and others such as creatinine elevation in relation to initial multisystem failure. In this regard, the presence of neutrophil counts within normality is related to a better evolution, in relation, again, to a probable correct immune function. At the radiological level, the presence of signs of bilateral lung involvement also marks a worse evolution, in relation to other published works in this regard.10
Our work has a series of strengths such as being a multicenter, international registry, with an important sample of cases, with limited loss of information in data collection and collected by professionals from different specialties who have been at the forefront of fighting against COVID-19. However, it also has a number of limitations to consider. In the first place, it is not a study that analyzes specific data on cancer management, so there is a lack of data on therapies, stages, etc. Secondly, it is an analysis only on hospitalized patients, so we do not have information on outpatients. Third, it is not a prospective analysis which would limit biases and reinforce the statistical strength of the findings.
Although all these limitations reduce the power of the study when drawing conclusions, they can serve to generate working hypotheses in this area or, at least, compare with other similar series.
ConclusionsIn this large, international registry, 33.51% of cancer patients hospitalized for COVID-19 died of different causes. Cancer patients have higher mortality and should be treated in a more intensive manner when suspected of COVID-19 infection. The early identification of factors predicting a worse prognosis, such as those presented here, can help us to better manage this process and try to reduce mortality from COVID-19 in the cancer patient.
Conflict of interestsThe authors declare that they have no conflict of interest.