Statin therapy might have a beneficial prognostic effect in patients with COVID-19, given its immunomodulative, anti-inflammatory and anti-atherosclerotic properties. Our purpose was to test this hypothesis by using the COVID-19 registry of a Spanish university hospital.
MethodsWe conducted a single-center, observational and retrospective study in which hospitalized patients with COVID-19 diagnosed by PCR between March 2020 and October 2020 were included. By means of logistic regression, we designed a propensity score to estimate the likelihood that a patient would receive statin treatment prior to admission. We compared the survival of COVID-19 patients with and without statin treatment by means of Cox regression with inverse probability of treatment weighting (IPTW). The median follow-up was 406 days.
ResultsWe studied 1122 hospitalized patients with COVID-19, whose median age was 71 years and of which 488 (43.5%) were women. 451 (40.2%) patients received statins before admission. In the IPTW survival analysis, prior statin treatment was associated with a significant reduction in mortality (HR: 0.76; 95% CI: 0.59–0.97). The greatest benefit of previous statin therapy was seen in subgroups of patients with coronary artery disease (HR: 0.32; 95% CI: 0.18–0.56) and extracardiac arterial disease (HR: 0.45; 95% CI: 0.28–0.73).
ConclusionsOur study showed a significant association between previous treatment with statins and lower mortality in hospitalized patients with COVID-19. The observed prognostic benefit was greater in patients with previous coronary or extracardiac atherosclerotic disease.
El tratamiento con estatinas podría presentar un efecto pronóstico beneficioso en pacientes con COVID-19, dadas sus propiedades inmunomoduladoras, antiinflamatorias y estabilizadoras de la placa de ateroma. Nuestro propósito fue analizar esta hipótesis tomando como base el registro de COVID-19 de un hospital universitario español.
MétodosRealizamos un estudio observacional y retrospectivo en el que se incluyeron los pacientes hospitalizados con COVID-19 diagnosticado mediante PCR entre marzo de 2020 y octubre de 2020 en un centro. Mediante regresión logística, diseñamos una puntuación de propensión para estimar la probabilidad de que un paciente recibiese tratamiento con estatinas antes del ingreso. Comparamos la supervivencia de los pacientes con y sin tratamiento con estatinas mediante la regresión de Cox ponderada por la inversa de la probabilidad de recibir el tratamiento (IPT). La mediana de seguimiento fue de 406 días.
ResultadosEstudiamos 1.122 pacientes hospitalizados con COVID-19, cuya mediana de edad era de 71 años y de los cuales 488 (43,5%) eran mujeres. 451 (40,2%) pacientes recibían estatinas antes del ingreso. En el análisis de supervivencia ponderado por la IPT, el tratamiento previo con estatinas se asoció a una reducción significativa de la mortalidad (HR: 0,76; IC 95%: 0,59–0,97). El mayor beneficio del tratamiento previo con estatinas se observó en los subgrupos de pacientes con enfermedad arterial coronaria (HR: 0,32; IC 95%: 0,18–0,56) y enfermedad arterial extracardiaca (HR: 0,45; IC 95%: 0,28–0,73).
ConclusionesNuestro estudio mostró una asociación significativa entre el tratamiento previo con estatinas y una menor mortalidad en pacientes hospitalizados con COVID-19. El beneficio pronóstico observado fue mayor en los pacientes con enfermedad aterosclerótica coronaria o extracardiaca previa.
The disease caused by the new type 2 coronavirus responsible for severe acute respiratory syndrome (SARS-CoV-2), known as COVID-19, is a major challenge for the health system. Despite the advances made in its prevention and treatment, COVID-19 continues to cause significant morbidity and mortality. There is, therefore, a need to look for new therapies to improve the prognosis of patients affected by this disease.
Statin therapy has been proposed as a potential source of significant clinical benefit in patients with COVID-19.1 The rationale behind this working hypothesis is based on its known immunomodulatory, anti-inflammatory and antioxidant properties.2 It has been suggested that statin therapy may limit cytokine release and disease-associated lung damage3; in addition, due to its stabilizing effect on atherosclerotic plaque4 it could also reduce the significant risk of cardiovascular complications in these patients.5
Information from several observational studies and their corresponding meta-analyses suggests that patients with COVID-19 who are pre-treated with statins have a lower risk of mortality and serious disease-related complications.6–12 Other authors, however, have not been able to confirm this alleged clinical benefit.13 It should be noted that there is significant heterogeneity among the published studies, which limits the validity of pooled analyses to draw reliable conclusions. Moreover, the vast majority of studies have focused only on clinical outcome during the hospital phase, and there is little data on the possible impact of statin therapy on longer-term prognosis.
In view of previous information pointing to a possible benefit of statin therapy in patients with COVID-19 and, at the same time, in view of the fact that there are still gaps in knowledge regarding this working hypothesis, we set out to explore this hypothesis further, using as a basis the information contained in a clinical registry of patients with COVID-19 from a Spanish tertiary level university hospital.
MethodsStudy descriptionThe information presented in this manuscript was obtained through an anonymised download from the database of the clinical registry of patients with COVID-19 of the Complejo Hospitalario Universitario de A Coruña (CHUAC), belonging to the Servicio Galego de Saúde (SERGAS). This is an observational and retrospective registry that included all patients diagnosed with COVID-19 using any of the validated microbiological methods (PCR, serology, rapid antigen test) in our health area from March 2020 onwards. The study protocol was approved by the Clinical Research Ethics Committee of the Autonomous Community of Galicia and the of Spanish Agency of Medicines and Medical Devices. Informed consent was obtained from patients, either in writing or verbally, for their inclusion in the study.
The study described in this manuscript only considered patients aged ≥18 years who were hospitalised with a PCR-confirmed diagnosis of COVID-19 for SARS-CoV-2 in a respiratory tract biological specimen between 1 March 2020 and 31 October 2020.
Study objectivesThe main objective of the present study was to evaluate a possible positive effect of statin pre-treatment on the survival of patients hospitalized for COVID-19.
Additionally, we aimed to assess the potential impact of maintenance or discontinuation of prior statin therapy on survival of patients hospitalised for COVID-19, as well as to explore the potential prognostic benefit of statin therapy in different clinical subgroups of patients hospitalised for COVID-19.
Statin treatmentThe primary independent variable in this study was statin treatment prior to hospitalization with COVID-19. Any statin among those marketed in our country was accepted. High-potency statin therapy was defined as regimens equal to or greater than 40mg daily of atorvastatin or 20mg daily of rosuvastatin.14 Patients who were not receiving statins prior to hospitalisation were considered the control group, regardless of whether or not a statin therapy was initiated during admission.
Outcome variablesPatients included in the study underwent follow-up until the date of their death or, alternatively, until October 2021. All-cause mortality was the primary outcome variable.
In addition to mortality, the incidence of adverse clinical outcomes during the hospital phase was also analysed, such as acute respiratory distress, need for mechanical ventilation (invasive and/or non-invasive), need for admission to critical care units, acute coronary syndrome, acute heart failure, acute kidney injury, deep vein thrombosis, and pulmonary thromboembolism.
Statistical analysisIn this manuscript, categorical variables are shown as number of patients and proportions, while quantitative variables are expressed as mean±standard deviation (SD).
In some laboratory variables there is a significant number of missing values, which are specified in the corresponding tables. No missing value imputation method has been applied in this study, so the data shown are for the subgroup of patients with known values.
Using multivariate logistic regression analysis, we constructed a propensity score that allowed us to estimate the likelihood of a patient receiving statin therapy prior to hospitalisation with a diagnosis of COVID-19 based on their baseline clinical characteristics. The model included 14 clinical variables – categories of age, sex, smoking history, hypertension, diabetes mellitus, atrial fibrillation, heart failure, coronary artery disease, peripheral arterial disease, cerebrovascular disease, chronic kidney disease, asthma, chronic obstructive pulmonary disease, neoplasms –, 5 variables related to pre-admission prescriptions – anti-platelets, anticoagulants, beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin 2 receptor blockers – and one interaction variable – sex * beta-blocker prescription –. The inclusion of variables in the propensity model was based primarily on clinical and literature criteria, seeking to select relevant clinical characteristics that might influence the likelihood of a patient receiving statins prior to hospital admission. The selection of the interaction variable sex * beta-blocker prescription was based on a statistical criterion, as its inclusion in the model was found to improve the balance between the groups.
The decision to incorporate age as a categorised variable and not as a continuous variable was taken after finding that it did not meet the linearity assumption necessary for its inclusion in the logistic regression model, due to the low frequency of prescription of these drugs at both age ends of the population. Variables relating to additional therapeutic measures taken during admission – e.g., i.v. corticosteroid prescription, admission to critical care – were not taken into account for the estimation of the propensity score, as they reflected clinical decisions taken after statin prescription. For a similar reason, laboratory variables were not included in the model, as these were determined at the time of hospitalisation and thus after exposure to the pharmacological group under study.
The possible influence of statin exposure prior to hospitalization on the risk of death of patients was studied using an inverse probability of treatment weighted (IPTW) Cox regression analysis.15 To do this, we attributed a specific weight to each case that was determined on the basis of their propensity score for receiving statins. Thus, for patients who actually received statin treatment, the individual case weight was calculated as “1/propensity score”, while in the control group the individual case weight was calculated as “1/(1–propensity score)”. To avoid a disproportionate increase in the sample size, the individual weights of each case were stabilized, multiplying their value by the marginal probability of receiving the treatment.16
The balance of baseline characteristics between the statin treatment group and the control group was assessed taking into account the standardized mean difference (SDM). According to Austin’s rule17 and taking into account the sample size of the study, variables with a SDM<0.10 were considered to be well balanced between the two groups under study.
To strengthen the consistency of the main result of the IPTW survival analysis, we performed two sensitivity analyses with multivariate adjustment models that included age as a continuous variable (model 1) and variables related to therapeutic measures taken during admission that were unbalanced between groups – use of tocilizumab, use of remdesivir, use of lopinavir–ritonavir, use of hydroxychloroquine, admission to the critical care unit, use of invasive mechanical ventilation – (model 2).
Survival curves, both unweighted and IPTW, for patients with and without statin prior treatment were constructed using the Kaplan–Meier method and compared with the log-rank test.
Finally, we performed an exploratory analysis of the effect of prior statin treatment in several subgroups of hospitalised patients with clinically relevant COVID-19, based on age (<70 years vs. 12≥70 years) and sex, as well as the presence of hypertension, diabetes mellitus or previous history of coronary artery disease or extracardiac arterial disease, by introducing the corresponding interaction terms into the statistical model.
Statistical significance for the hypothesis tests was defined as a p-value<0.05. Statistical analysis was performed with SPSS 25 and Stata 14.
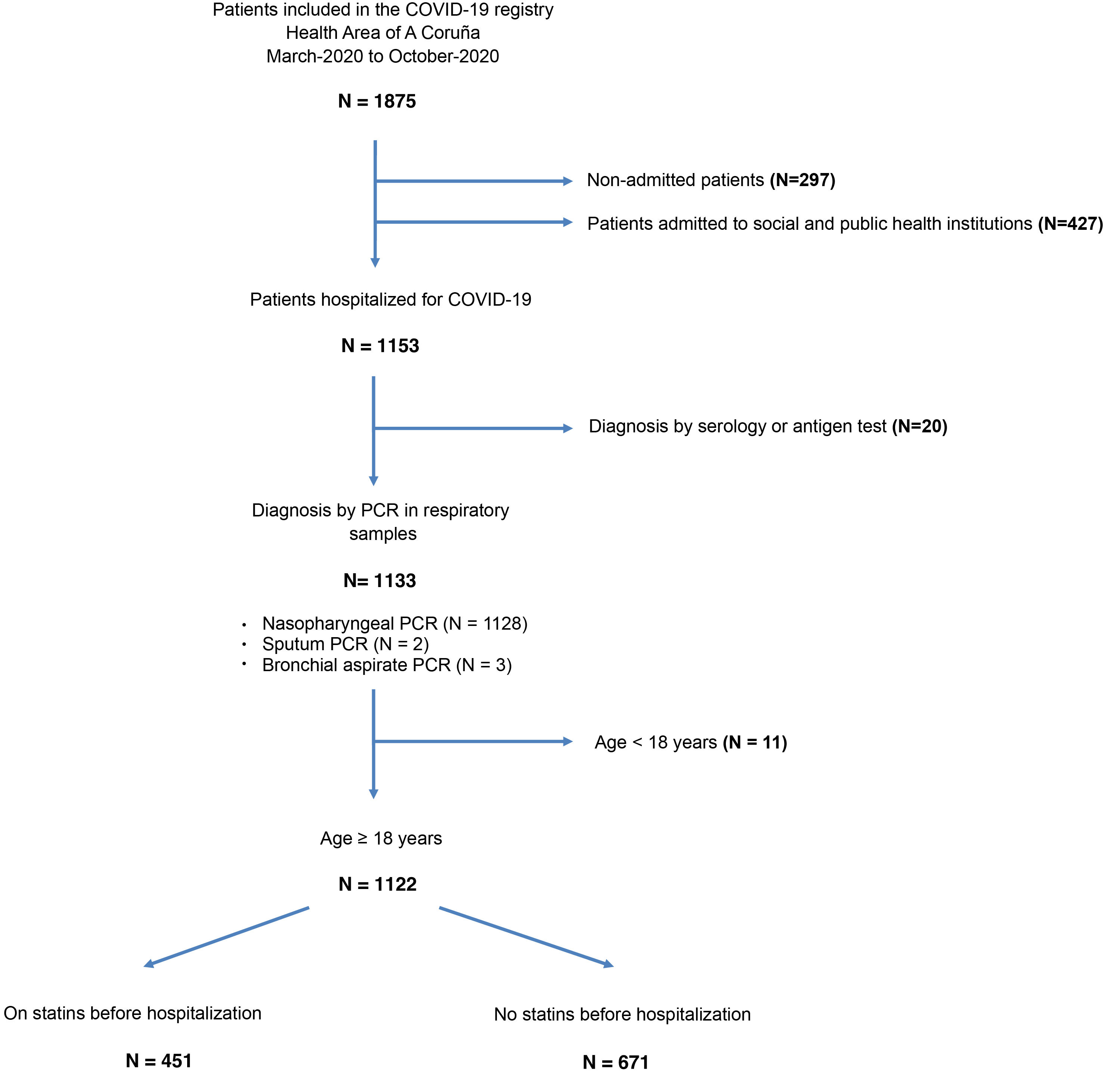
ResultsPatientsThe present study population included 1122 patients aged ≥18 years who were hospitalised with COVID-19 at our centre between March 2020 and October 2020. The process of selecting patients for the study is outlined in Fig. 1.
Of the 1122 patients studied, 451 (40.2%) were receiving statin therapy prior to admission. Specifically, 243 (21.7%) patients were receiving atorvastatin, 113 (10.1%) simvastatin, 36 (3.2%) pravastatin, 33 (2.9%) rosuvastatin, 18 (1.6%) pitavastatin, 6 (0.5%) fluvastatin and 1 (0.1%) lovastatin. In total, 241 (21.5%) patients were receiving high-potency statin therapy and 205 (18.3%) were receiving low- or medium-potency statin therapy. In 1 (0.1%) patient the type of statin prescribed was not recorded and in 5 (0.4%) patients the dose was not recorded.
Statin pre-treatment was maintained during hospitalisation for COVID-19 in 182 (40.4%) patients, while 269 (59.6%) patients discontinued the treatment. In addition, during admission, statin treatment was initiated in 17 (2.5%) of the patients who were not previously receiving them.
Table 1 presents the baseline characteristics of patients hospitalised with COVID-19 in our study, based on the presence or absence of prior statin therapy. Numerous baseline clinical variables can be seen to have a significant imbalance between the two groups of patients, detected by the defined criterion of a SDM>0.10. Thus, patients treated with statins were more frequently male, had a higher mean age, and a higher prevalence of cardiovascular risk factors and comorbidities, such as coronary, cerebrovascular, or peripheral atherosclerotic disease, heart failure, chronic kidney disease, atrial fibrillation, heart disease, chronic obstructive pulmonary disease and neoplasms.
Clinical characteristics of 1122 hospitalized patients with COVID-19, based on whether or not they received prior statin treatment.
| Unweighted sample | IPTW sample | |||||
|---|---|---|---|---|---|---|
| Without statins (n=671) | With statins (n=451) | SDMa | Without statins | With statins | SDMa | |
| Previous medical history | ||||||
| Age (years), mean±SD | 63.6±18.9 | 74.7±10.4 | +0.730 | 68.6±17.7 | 70.5±12.7 | +0.127 |
| Age groups, n (%) | +0.668 | +0.017 | ||||
| 18–59 years | 274 (40.8%) | 39 (8.6%) | 185 (27.1%) | 111 (25.3%) | ||
| 60–69 years | 122 (18.2%) | 98 (21.7%) | 134 (19.6%) | 91 (20.8%) | ||
| 70–79 years | 127 (18.9%) | 164 (36.4%) | 175 (25.7%) | 118 (26.9%) | ||
| 80 years or older | 148 (22.1%) | 150 (33.3%) | 188 (27.6%) | 118 (26.9%) | ||
| Female, n (%) | 309 (46.1%) | 179 (39.7%) | −0.129 | 219 (42.7%) | 280 (41.1%) | −0.033 |
| Hypertension | 243 (36.2%) | 308 (68.3%) | +0.678 | 337 (49.5%) | 231 (52.6%) | +0.063 |
| Diabetes mellitus | 81 (12.1%) | 155 (34.4%) | +0.547 | 139 (20.4%) | 97 (22.1%) | +0.043 |
| Smoking history | 179 (26.7%) | 173 (38.4%) | +0.251 | 222 (32.6%) | 145 (33%) | +0.009 |
| Coronary artery disease | 23 (3.4%) | 80 (17.7%) | +0.478 | 62 (9.1%) | 40 (9.1%) | −0.003 |
| Heart failure | 56 (8.3%) | 91 (20.2%) | +0.343 | 88 (12.9%) | 58 (13.2%) | +0.011 |
| Atrial fibrillation | 63 (9.4%) | 75 (16.6%) | +0.216 | 79 (11.6%) | 54 (12.3%) | +0.023 |
| Cerebrovascular disease | 42 (6.3%) | 58 (12.9%) | +0.226 | 59 (8.7%) | 41 (9.3%) | +0.022 |
| Peripheral arterial disease | 26 (3.9%) | 43 (9.5%) | +0.228 | 48 (7%) | 28 (6.4%) | −0.029 |
| Chronic obstructive pulmonary disease | 39 (5.8%) | 42 (9.3%) | +0.133 | 53 (7.8%) | 32 (7.3%) | −0.018 |
| Bronchial asthma | 54 (8%) | 35 (7.8%) | −0.011 | 49 (7.2%) | 29 (6.6%) | −0.022 |
| Chronic kidney disease | 55 (8.2%) | 91 (20.2%) | +0.348 | 96 (14.1%) | 59 (13.4%) | −0.015 |
| Chronic liver disease | 23 (3.4%) | 21 (4.7%) | +0.068 | 33 (4.8%) | 16 (3.7%) | −0.054 |
| Neoplasm | 116 (17.3%) | 102 (22.6%) | +0.134 | 136 (20%) | 92 (21%) | +0.027 |
| Other drugs | ||||||
| Beta blockers | 52 (8%) | 120 (27%) | +0.516 | 118 (17.3%) | 70 (15.9%) | −0.040 |
| Angiotensin converting enzyme inhibitors | 78 (12%) | 85 (19%) | +0.202 | 89 (13.1%) | 68 (15.5%) | +0.070 |
| Angiotensin 2 receptor blockers | 103 (15%) | 153 (34%) | +0.436 | 160 (23.5%) | 103 (23.5%) | −0.001 |
| Antiplatelet agents | 56 (8%) | 151 (34%) | +0.649 | 134 (19.7%) | 84 (19.1%) | −0.015 |
| Anticoagulants | 57 (9%) | 73 (16%) | +0.235 | 73 (10.7%) | 51 (11.6%) | +0.028 |
SD: standard deviation; SDM: standardized deviation of the means; IPTW: inverse probability of treatment weighted.
Positive SDM value indicates that the mean of the variable is higher in the statin-treated group than in the control group, while a negative SDM value indicates that the mean of the variable is higher in the control group than in the statin-treated group. The absolute values |SDM|>0.10, highlighted in black, identify variables that show a significant imbalance between the two groups under study.
Table 2 shows the baseline clinical characteristics of patients with and without prior statin exposure after sample IPTW with stabilised individual weights. The vast majority of the relevant clinical variables in this analysis had an absolute value |SDM|<0.10, i.e., a good balance between the two groups under study. The only baseline clinical variable that showed a significant imbalance between the study groups was age, analysed as a continuous variable [see previous explanation in the Methodology section]; however, a balanced distribution of patients was achieved according to the defined age categories.
Clinical status of patients with COVID-19 and therapeutic measures undertaken during admission.
| Unweighted sample | IPTW sample | |||||
|---|---|---|---|---|---|---|
| Without statins (n=671) | With statins (n=451) | SDMa | Without statins (n=681) | With statins (n=439) | SDMa | |
| Clinical situation on admissionb | ||||||
| Systolic blood pressure (mmHg), mean±SD | 129.4±21.9 | 133.1±22.8 | +0.166 | 129±21.8 | 133±21.9 | +0.186 |
| PaO2 (mmHg) | 71.9±27.1 | 67.7±19.1 | −0.182 | 72.1±26.2 | 69.5±21 | −0.108 |
| PaCO2 (mmHg) | 35.8±7 | 36±7.2 | +0.027 | 35.9±7.4 | 35.8±6.6 | −0.014 |
| pH | 7.45±0.10 | 7.44±0.06 | −0.099 | 7.45±0.06 | 7.45±0.05 | −0.051 |
| PaO2/FiO2 | 281±126.1 | 260±102.5 | −0.183 | 277.3±117.6 | 272.4±100.6 | −0.045 |
| Laboratory on admissionb | ||||||
| Platelets (×109/l) | 214.9±107 | 202.3±85.3 | −0.131 | 207.4±99.7 | 201.3±83.3 | −0.067 |
| Leukocytes (×109/l) | 7.1±4.2 | 7.5±6.3 | +0.068 | 7.2±4.2 | 7.2±6 | −0.002 |
| Lymphocytes (×109/l) | 1.14±0.79 | 1.18±2.88 | +0.018 | 1.1±0.8 | 1.2±2.7 | +0.054 |
| Neutrophils (×109/l) | 5.5±3.7 | 5.9±3.8 | +0.083 | 5.7±3.6 | 5.8±3.8 | +0.030 |
| Haemoglobin (g/dl) | 13.3±1.9 | 13.1±2.0 | −0.132 | 13.2±2.0 | 13.3±1.9 | +0.096 |
| Creatinine (mg/dl) | 0.99±0.54 | 1.26±1.09 | +0.309 | 1.11±0.71 | 1.11±0.87 | −0.008 |
| GOT (IU/l) | 44.0±40.2 | 47.2±40.8 | +0.079 | 44.6±40 | 47.2±38.2 | +0.067 |
| GPT (IU/l) | 46.6±50.0 | 41.9±34.4 | −0.111 | 44.3±47.5 | 43.8±32.6 | −0.014 |
| ESR (mm) | 49.0±27.3 | 54.7±28.3 | +0.204 | 50.7±29.2 | 51.1±28.3 | +0.012 |
| C-reactive protein (mg/l) | 7.9±7.2 | 8.1±6.8 | +0.038 | 8.0±6.9 | 8.0±6.9 | +0.007 |
| Ferritin (ng/ml) | 623.2±702.7 | 675.7±749.0 | +0.072 | 603.4±658.1 | 694.5±717 | +0.132 |
| D-dimers | 1.382.4±2.815.7 | 2.015.8±7.921.3 | +0.107 | 1.758.6±3.069 | 1.627.2±7.280.8 | −0.023 |
| Interleukin 6 | 34.8±79.1 | 33.2±56.2 | −0.023 | 38.8±77 | 29±43.7 | −0.156 |
| Therapeutic measures during admission | ||||||
| Lopinavir–ritonavir, n (%) | 275 (41%) | 193 (42.8%) | +0.037 | 274 (40.2%) | 213 (48.5%) | +0.167 |
| Hydroxychloroquine | 307 (45.8%) | 227 (50.3%) | +0.092 | 322 (47.2%) | 239 (54.4%) | +0.144 |
| Tocilizumab | 59 (8.8%) | 63 (14%) | +0.163 | 59 (8.7%) | 71 (16.2%) | +0.228 |
| Remdesivir | 65 (9.7%) | 31 (6.9%) | −0.102 | 61 (9%) | 27 (6.2%) | −0.109 |
| Low molecular weight heparin | 609 (90.8%) | 410 (90.9%) | +0.005 | 625 (91.8%) | 404 (92%) | +0.013 |
| IV corticosteroids | 353 (52.6%) | 258 (57.2%) | +0.092 | 367 (53.9%) | 238 (54.2%) | +0.005 |
| Admission to critical care unit | 64 (9.5%) | 62 (13.7%) | +0.131 | 66 (9.7%) | 61 (13.9%) | +0.130 |
| Non-invasive mechanical ventilation | 80 (11.9%) | 50 (11.1%) | −0.026 | 85 (12.5%) | 47 (10.7%) | −0.053 |
| Invasive mechanical ventilation | 51 (7.6%) | 49 (10.9%) | +0.113 | 48 (7%) | 49 (11.2%) | +0.146 |
SD: standard deviation; SDM: standardized difference of means; GOT: glutamic oxaloacetic transaminase; GPT: glutamate-pyruvate-transaminase; IPTW: inverse probability of treatment weighted; ESR: erythrocyte sedimentation rate.
A positive SDM value indicates that the mean of the variable is higher in the statin-treated group than in the control group, while a negative SDM value indicates that the mean of the variable is higher in the control group than in the statin-treated group. The absolute values |SDM|>0.10, highlighted in bold font, identify variables that show a significant imbalance between the two groups under study.
Missing values: systolic blood pressure (n=779), PaO2 (n=287), PaCO2 (n=289), pH (n=292), PaO2/FiO2 (n=718), platelets (n=15), leukocytes (n=15), lymphocytes (n=15), neutrophils (n=15), haemoglobin (n= 14), creatinine (n=27), GOT (n=50), GPT (n=96), ESR (n=744), C-reactive protein (n=368), ferritin (n=615), D-dimers (n=466), interleukin 6 (n=343).
Additional Material Appendix B shows the distribution of propensity scores in the study patients, both in the unweighted population and in the IPTW population.
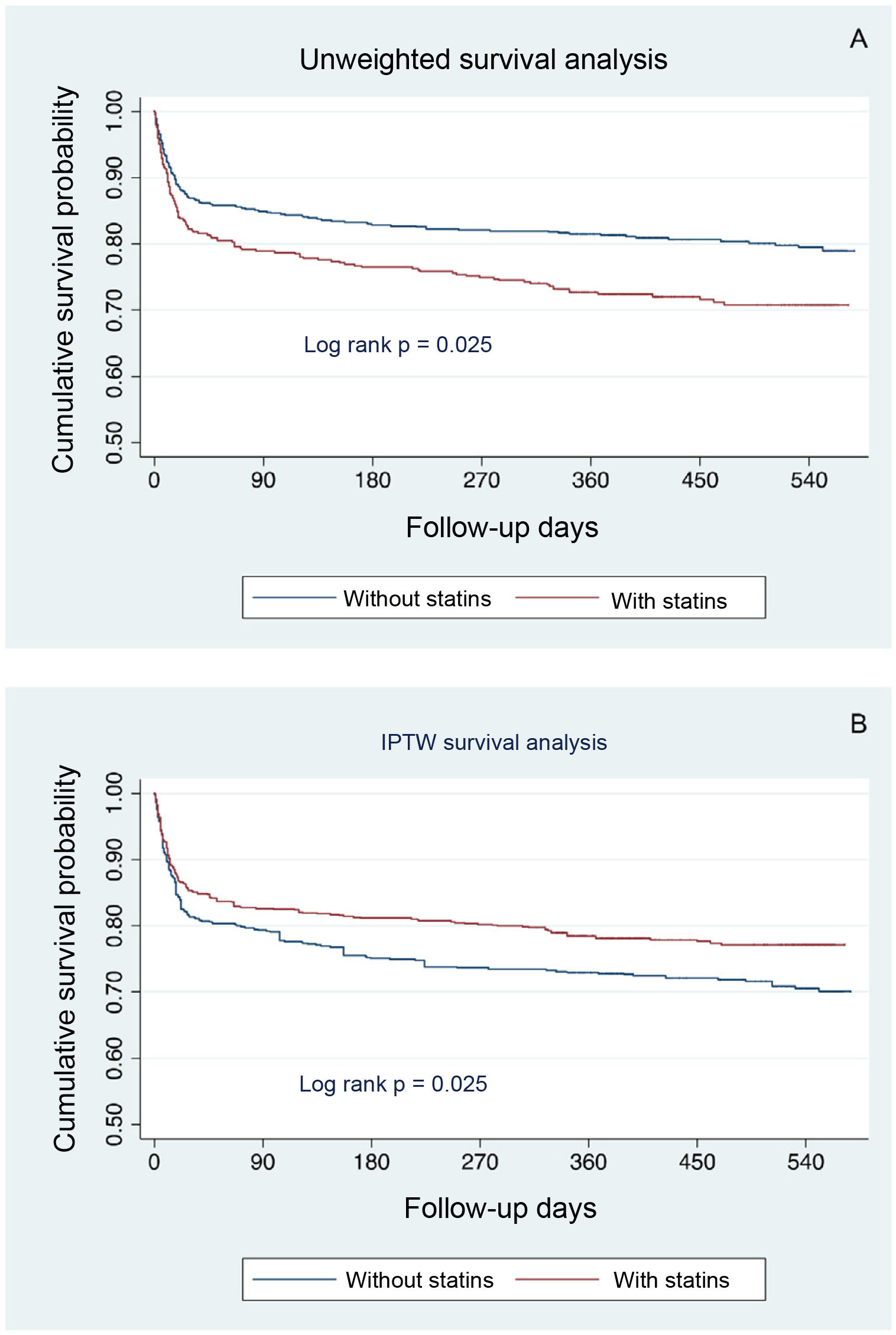
SurvivalThe median follow-up period of the patients was 406days (interquartile range 338–548 days). During this period, there were 130 (27.8%) deaths in the group of patients prescribed statins prior to admission and 131 (20%) deaths in the control group.
Fig. 1 shows the unweighted Kaplan–Meier survival curve for both groups. Unweighted Cox regression analysis showed a significant increase in the risk of death among patients receiving pre-hospitalisation statin therapy (hazard ratio [HR]: 1.50; 95% CI: 1.18–1.91).
The IPTW Kaplan–Meier survival curves are shown in Fig. 2, panel A). Pre-admission statin therapy was associated with a statistically significant reduction in all-cause mortality during follow-up (HR: 0.76; 95%CI: 0.59–0.97) in the IPTW Cox regression analysis.
The protective effect of statin pre-treatment was maintained when the results of the IPTW Cox regression were further adjusted by a first multivariate model including age (HR: 0.75; 95% CI: 0.59–0.96) and by a second multivariate model which, in addition to age, also included variables related to therapeutic measures taken during hospitalisation that showed a significant imbalance between the two study groups – tocilizumab use, remdesivir use, lopinavir–ritonavir use, hydroxychloroquine use, admission to the critical care unit, invasive mechanical ventilation use – (HR: 0.76; 95% CI: 0.59–0.97).
The protective effect of treatment with statins was observed mainly in patients in whom the drug was maintained during admission (HR: 0.64; CI 95%: 0.43–0.94); however, the association between prior statin exposure and lower mortality did not reach statistical significance in the subgroup of patients in whom the drug was discontinued at the time of hospitalization (HR: 0.80; CI 95%: 0.61–1.06). Appendix B Supplementary Table S1 shows the baseline clinical characteristics of both subgroups of patients.
The association between prior statin treatment and a lower risk of death was obtained at the expense of the subgroup of patients treated with low or moderate potency statins (HR: 0.63; CI 95%: 0.45–0.88); however, we did not observe a significant association between prior high-potency statin therapy and the risk of death from any cause (HR: 0.88; CI 95%: 0.65–1.18).
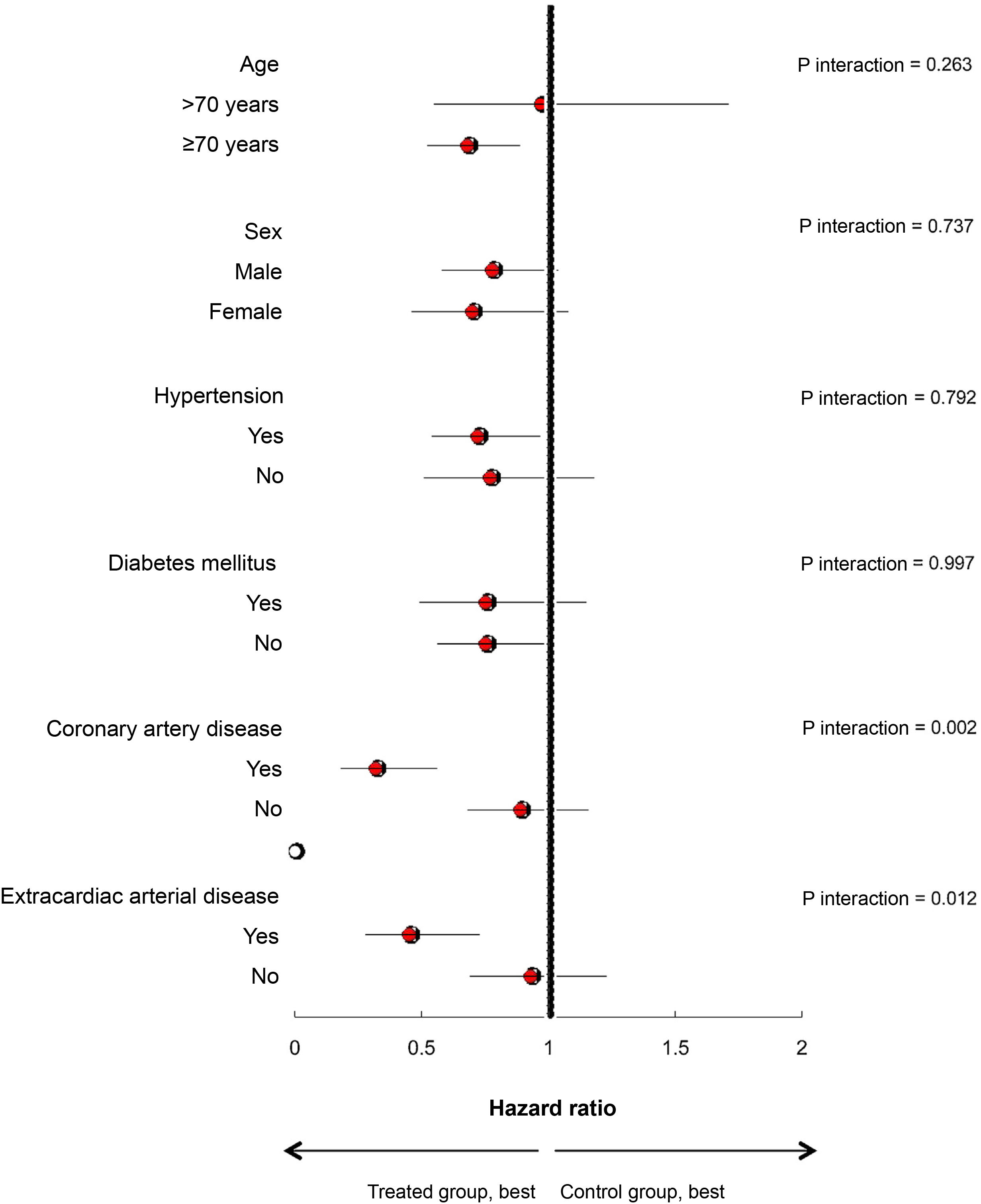
Sub-group analysisFig. 2, panel B, shows the observed statistical association between prior statin exposure and risk of all-cause death in different subgroups of hospitalised patients with COVID-19, estimated by IPTW Cox regression analysis.
A statistically significant interaction was detected between the existence of atherosclerotic disease, both coronary (p=0.002) and extracardiac (cerebral or peripheral) (p=0.012), and the observed association between statin treatment and survival. Pre-treatment with statins was associated with a marked reduction in the risk of death in the subgroups of patients with coronary artery disease (HR: 0.32; 95% CI: 0.18–0.56) and with extracardiac arterial disease (HR: 0.45; 95% CI: 0.28–0.73).
We did not observe a significant interaction between statin exposure and survival of patients hospitalised for COVID-19 based on age, sex or the presence or absence of hypertension or diabetes mellitus (Fig. 3).
Other adverse clinical outcomes during hospitalizationTable 3 shows the cumulative incidence of different adverse clinical outcomes recorded during the in-hospital phase according to the presence or absence of prior statin treatment.
Adverse clinical events during hospital admission for COVID-19 in patients previously treated with statins and in patients in the control group, both in the unweighted analysis and the IPTW.
| Unweighted sample | IPTW sample | |||||
|---|---|---|---|---|---|---|
| Without statins (n=671) | With statins (n=451) | OR (95% CI) | Without statins (n=681) | With statins (n=439) | OR (95% CI) | |
| Acute respiratory distress | 152 (22.7%) | 134 (29.7%) | 1.44 (1.10–1.89) | 163 (23.9%) | 119 (27.1%) | 1.18 (0.90–1.55) |
| Acute myocardial infarction | 2 (0.3%) | 2 (0.4%) | 1.49 (0.21–10.6) | 1 (0.1%) | 1 (0.2%) | 1.03 (0.81–13.24) |
| Acute heart failure | 13 (1.9%) | 19 (4.2%) | 2.23 (1.09–4.05) | 14 (2.1%) | 14 (3.2%) | 1.47 (0.69–3.10) |
| Stroke | 4 (0.6%) | 7 (1.6%) | 2.63 (0.77–9.03) | 4 (0.6%) | 4 (0.9%) | 1.53 (0.37–6.35) |
| Pulmonary embolism | 25 (3.7%) | 21 (4.7%) | 1.26 (0.70–2.28) | 3. 4. 5%) | 23 (5.2%) | 1.04 (0.60–1.79) |
| Deep vein thrombosis | 6 (0.9%) | 7 (1.6%) | 1.75 (0.28–5.73) | 10 (1.5%) | 11 (2.5%) | 1.76 (0.74–4.20) |
| Acute kidney damage | 69 (10.3%) | 83 (18.4%) | 1.97 (1.39–2.78) | 94 (13.8%) | 62 (14.1%) | 1.02 (0.72–1.44) |
IPTW: inverse probability of treatment weighted; OR: odds ratio.
In bold font, statistically significant results.
The unweighted univariate analysis showed that statin-treated patients had a higher cumulative incidence of different adverse clinical outcomes, including acute respiratory distress, acute heart failure and acute kidney damage. However, after the IPTW analysis, no statistically significant differences were observed between the groups for the main adverse clinical outcomes analysed.
DiscussionThe present observational study, based on a cohort of adult patients hospitalised with COVID-19 in a Spanish university hospital, suggests that pre-treatment with statins is associated with a decreased risk of all-cause death in this population. According to our results, the benefit of treatment with statins would be greater in patients in whom the drug was maintained during hospitalization and in patients with a history of coronary or extracardiac (cerebrovascular or peripheral) atherosclerotic disease.
First, we would like to highlight the notable differences in the baseline clinical characteristics of hospitalised patients with COVID-19 who received prior statin therapy compared to those who did not, a fact that has been consistent in other previously published studies.18–20 The statin-treated group included older patients, mainly males and with a higher prevalence of comorbidities in the cardiovascular, renal, bronchopulmonary and neoplastic domains. It is not surprising, therefore, that in the survival analysis without weighting the treated group presented a significantly higher mortality than the control group. It was precisely the large number of baseline clinical variables with a significant imbalance between the two study groups that we chose to use the IPTW method for statistical adjustment of the results, since this technique is known to be appropriate to simultaneously control for the possible confounding effect of multiple covariates in studies of intermediate sample size that provide limited statistical power for the use of other adjustment methods, such as propensity score adjustment or simple multivariable regression.15 Using the IPTW method with stabilised weights17 in our study allowed an adequate balance of the baseline clinical variables considered as potential confounders, with the exception of a discrete age imbalance. As discussed above, IPTW survival analysis models, with or without additional adjustment for age or use of other concomitant treatments, showed a statistically significant and clinically relevant protective effect of statin therapy in patients hospitalised with COVID-19, with a reduction in the risk of death of approximately 25% over a follow-up of slightly more than 1 year after diagnosis of infection.
To date, more than 40 observational studies and several meta-analyses have been published in an attempt to discern whether statin therapy can have a positive prognostic effect in patients with COVID-19.6,18–21 The published meta-analyses show significant heterogeneity in terms of the type of studies and patients included and their methodology, and it should be noted that the validity of some of them13 has been openly questioned.22 The recent updated meta-analysis by Vahedian-Azimi et al.9 is probably the most comprehensive meta-analysis published to date, as it included data from 47 observational studies with a total aggregate sample of more than 3 million patients — although more than 90% of the cases came from a single large UK population-based study of patients with COVID-19 and diabetes mellitus.23 In this meta-analysis,9 the authors observed no overall association between statin use and a reduced risk of death or admission to critical care units among patients with COVID-19, but a reduced risk of orotracheal intubation; additionally, the authors describe a significant 46% reduction in all-cause mortality among statin users in whom treatment was effectively maintained during hospitalisation for COVID-19. The meta-analysis by Chow et al.,6 based on 13 observational studies involving more than 110,000 patients with COVID-19, reached a similar conclusion, observing a significant reduction in mortality among statin users where treatment was maintained during hospitalisation, as opposed to all other patients. However, Chow et al.6 did not observe a significant benefit of statin treatment among patients with COVID-19 who required admission to an intensive care unit. Diaz-Arocutipa et al.10 analysed 25 pooled observational studies involving more than 147,000 patients with COVID-19, concluding that statin therapy is associated with a significant reduction in the risk of death in the adjusted analysis, which was observed mainly at the expense of chronic statin users. Other meta-analyses of observational studies7,8,11 have shown similar conclusions. The meta-analysis by Zeim et al.,12 based on 8 observational studies using propensity score matching techniques and including more than 14,000 patients with COVID-19, stands out for its methodological quality. The authors of this paper12 concluded that statin therapy appears to be associated with a significant prognostic benefit in patients with COVID-19, with a 28% reduction in the risk of death independent of age, sex or previous history of hypertension or diabetes. Finally, two Spanish multicentre studies19,20 have also suggested a significant prognostic benefit of statin therapy in patients with COVID-19.
The apparent prognostic benefit of statin therapy in hospitalised patients with COVID-19 in our cohort was observed primarily in the group in which statins were maintained during hospitalisation; however, the effect observed in patients in whom treatment was discontinued at the time of hospitalisation did not reach statistical significance. This observation is consistent with previously published information, since, as we have already discussed, maintenance of prior statin therapy during hospitalisation with COVID-19 has been associated with a significant prognostic impact in these patients more consistently than a history of statin prescription prior to admission.6,8,9
Our study revealed a greater prognostic benefit of statin therapy in patients with COVID-19 who had a history of established coronary or extracardiac atherosclerotic disease, i.e. in subgroups of patients with an indication for lipid-lowering therapy in secondary prevention. This result suggests that the hypothetical protective effect of statin therapy in patients with COVID-19 could be due to its stabilising effect on atherosclerotic plaques in patients who are theoretically at higher risk of cardiovascular complications associated with the disease. Similarly, other authors have observed a particularly significant clinical benefit of statin therapy in patients with COVID-19 and coronary artery disease.18 While some authors have suggested that the potential benefit of statin therapy in patients with COVID-19 may be mediated by its immunomodulatory properties, which may mitigate the consequences of the systemic inflammatory response and lung damage associated with the disease,3,24 we did not observe a reduction in the incidence of respiratory distress in these cases. On the contrary, even after IPTW analysis, patients receiving statins in our cohort had a higher need for mechanical ventilation and admission to critical care units than patients not treated with statins.
Finally, we would like to comment on a striking finding of the present study, which is the apparent differential effect of the intensity of lipid-lowering therapy on the prognosis of patients with COVID-19. Surprisingly, the greatest reduction in the risk of death was observed among patients receiving treatment with low or intermediate potency statins, while the reduction in the risk of death observed among patients receiving high potency statins was quantitatively smaller and did not reach statistical significance. Although this exploratory result should be taken with caution and requires confirmation, it may be related to the increased risk of adverse reactions and drug interactions and, perhaps, a greater tendency to discontinuation of treatment associated with high-intensity lipid-lowering regimens, which could be of particular clinical relevance in a particularly vulnerable clinical period such as the hospital phase of COVID-19. Contrary to our study, other authors18 have found no difference in benefit between high-intensity statin therapy and moderate- or low-intensity statin therapy in patients with COVID-19.
The present study has some limitations. Firstly, it is an observational and retrospective study, which is therefore exposed to inherent selection, information and confounding biases. There are a significant number of missing values in the database used for the study, mainly affecting baseline laboratory variables and, to a lesser extent, the doses and type of statin used. In addition, information on prior prescription of statins and their maintenance or discontinuation at the time of hospital admission was obtained from the medical records, but we do not have data on the actual dispensing and adherence to treatment. In particular, the prescription of statins during hospitalisation to 17 patients in the control group might have limited the ability of the study to demonstrate the proposed objective; however, it is our opinion that this fact probably did not result in a significant modification of the results of the study, given the small number of cases involved. The statistical methodology employed, with hypothesis contrasts weighting and survival analysis by IPTW, allowed a good number of baseline clinical variables to be balanced between the two study groups, with the aim of making them more comparable. However, this technique does not control for possible confounding biases due to unmeasured covariates, nor does it ensure a good balance of measured covariates in the subgroups analysed retrospectively. Finally, it should be acknowledged that the external validity of the study is not guaranteed, as it included patients from a single centre.
In summary, when using IPTW techniques, we observed a statistically significant association between prior statin treatment and a lower risk of death in a retrospective cohort of adult hospitalised patients with COVID-19 in a Spanish university hospital. The prognostic benefit of statin therapy was greater in patients with a history of coronary or extracardiac atherosclerotic disease – cerebrovascular or peripheral – and in those in whom these drugs were maintained during admission due to infection. Our results suggest that, in the absence of contraindication or relevant adverse reactions, pre-treatment with statins should be maintained as far as possible during hospitalisation in patients with COVID-19 who have a well-established indication for statin therapy.
FundingThis research project was funded by a grant from the Fundación Mutua Madrileña for biomedical research focused on coronavirus infection (COVID-19) in its extraordinary call PRI-2020-13 of April 2020. The project was co-funded by the Consellería de Sanidade, the Servizo Galego de Saúde and ACIS through the Programa de refuerzo de la investigación sanitaria de Galicia Traslaciona COVID-19 with a charge to social patronage within the campaign “Botemos unha manha” of the Xunta de Galicia (File No. CT850A - 8).
Conflict of interestsNo conflict of interest is declared.