A recent outbreak of coronavirus disease 2019 (COVID-19) occurs in the worldwide. Angiotensin-converting enzyme 2 (ACE2) can mediate coronavirus entry into host cells. Therefore, renin–angiotensin system inhibitors (RASI) were suspected of contributing to the increase of coronavirus infection. We aimed to analyze the effects of RASI in COVID-19 patients with hypertension.
Patients and methodIn this retrospective, single-center study, 27 COVID-19 patients with hypertension, who were admitted to the Shanghai Public Health Clinical Center from January 25, 2020 to January 31, 2020, were analyzed for clinical features, laboratory parameters, medications and the length of stay. All the patients were given antiviral and antihypertension treatment, of which 14 patients were treated with RASI and 13 patients without RASI.
ResultsComparing the two groups, we did not found statistically significant differences in clinical symptoms and laboratory tests. Furthermore, cough was not aggravated.
ConclusionsThrough the analysis of this small sample, RASI could be deemed safe and effective to control high blood pressure of COVID-19 patients. Further analysis with a larger sampling size is required to explore the underlying mechanisms.
Un reciente brote de la enfermedad coronavirus 2019 (COVID-19) se produce en todo el mundo. La enzima convertidora de angiotensina 2 (ACE2) puede mediar la entrada del coronavirus en las células huésped. Por lo tanto, se sospechaba que los inhibidores del sistema renina-angiotensina (SRA) contribuían al aumento de la infección por coronavirus. Nos propusimos analizar los efectos de los SRA en los pacientes COVID-19 con hipertensión.
Pacientes y métodoEn este estudio retrospectivo, de un solo centro, se analizaron 27 pacientes de COVID-19 con hipertensión, que fueron admitidos en el Centro Clínico de Salud Pública de Shangai desde el 25 de enero de 2020 hasta el 31 de enero de 2020, para determinar las características clínicas, los parámetros de laboratorio, los medicamentos y la duración de la estancia. A todos los pacientes se les administró un tratamiento antiviral y antihipertensivo, de los cuales 14 pacientes fueron tratados con SRA y 13 sin SRA.
ResultadosComparando los dos grupos, no encontramos diferencias estadísticamente significativas en los síntomas clínicos y las pruebas de laboratorio. Además, la tos no se agravó.
ConclusionesA través del análisis de esta pequeña muestra, el SRA podría considerarse seguro y eficaz para controlar la presión arterial alta de los pacientes con COVID-19. Es necesario realizar más análisis con una muestra de mayor tamaño para explorar los mecanismos subyacentes.
Coronavirus disease 2019 (COVID-19) is a newly identified strain of coronavirus that causes illness ranging from effects similar to the common cold to fatal diseases in people across the world. As of 2:00 am CEST, 3 May 2020, 215 countries around the world have seen cases of coronavirus disease 2019 (COVID-19) and there have been 3,356,205 confirmed including 238,730 deaths, reported to WHO. On February 11, 2020, the World Health Organization (WHO) officially named the disease caused by the 2019-nCoV as coronavirus disease (COVID-19). There is evidence that COVID-19 can be transmitted from person to person through respiratory droplets and close contact, which poses a huge challenge to public health.1 According to the latest research on single-cell RNA-seq, researchers believe that the SARS-CoV-2 RNA expression and replication are related to angiotensin-converting enzyme 2 (ACE2).2 Importantly, ACE2 was confirmed as the SARS-CoV-2 cell entry receptor, which is the same as SARS-CoV.3 Renin–angiotensin system inhibitor (RASI) is a commonly used antihypertensive drug. However, it is not clear whether RASI can increase the expression of ACE2, leading to the higher risk of SARS-CoV-2 infection. Should patients with hypertension stop using RASI? We explored the question about the effects of administering RASI to patients with COVID-19 and hypertension by analyzing the data from the Shanghai Public Health Clinical Center.
Patients and methodsStudy design and participantsFrom January 25, 2020 to January 31, 2020, we received a total of 27 patients with hypertension combined with COVID-19 at the Shanghai Public Health Clinical Center. All cases were confirmed COVID-19 cases with hypertension history more than 3 months. The clinical criteria for diagnosis and discharge refer to the “Diagnosis and Treatment of New Coronavirus Infectious Pneumonia” (sixth edition) standards. Most of the cases had a history of relevant epidemiology, such as fever or respiratory symptoms. All patients were diagnosed after examination of SARS-CoV-2 RNA by RT-PCR and chest CT scanning. By February 29, 26 of the 27 cases were discharged from hospital. This study was approved by the Ethics Committee of the Shanghai Public Health Clinical Center (2019-S047-02, Review date: January 13, 2020)4 and was exempted from the need for informed consent from patients.
Data collectionThe research team collected and analyzed 27 patients’ medical records, epidemiological, clinical, and laboratory characteristics. Information of treatment and outcome of the patients were obtained from the inpatient management system. Blood pressure and medication were recorded during hospitalization.
Therapeutic strategiesAll patients rested in bed, and were monitored for vital signs and finger oxygen saturation, and given effective oxygen therapy in time. The basic principles of treatment are nutritional support and symptomatic treatment to maintain water and electrolyte balance and a stable internal environment. Antiviral therapy was tried with interferon, lopinavir/litonavir, dnrunavir and abidol. Antibiotics were used if necessary. Other therapy includes γ-globulin, thymosin alpha, vitamin C and traditional Chinese medicine. Existing antihypertensive drugs continued to be used, unless the blood pressure continued to exceed 150/100mmHg, then increased the amount or added new drugs (diuretic or CCB).
Laboratory inspectionAll confirmed cases were examined by peripheral white blood cell count, absolute lymphocyte value, red blood cell sedimentation rate (ESR), procalcitonin (PCT), C-reactive protein (CRP), total triglyceride (TG), cardiac troponin I (cTNI), brain natriuretic peptide (pro-BNP), and renal function. The phenotypic analysis of lymphocytes (CD4, CD8, CD 3+ T cells) in peripheral blood was performed by a flow cytometer (BD Biosciences; San Diego, USA). Abnormal liver damage was defined as any parameter greater than the upper limit of normal value (ULN) of ALT (40U/L), AST (35U/L), ALP (100U), GGT (45U/L), TB (20.5μmol/L), and LDH (245U/L).
Statistical analysisContinuous measurements were compared by Student's T test or Mann–Whitney U test. Normal distributions were expressed with mean±standard deviation (SD). Categorical variables (shown as percentages) were compared using Fisher's exact test. Statistical analysis software SPSS22.0 was used for all analyses in this study.
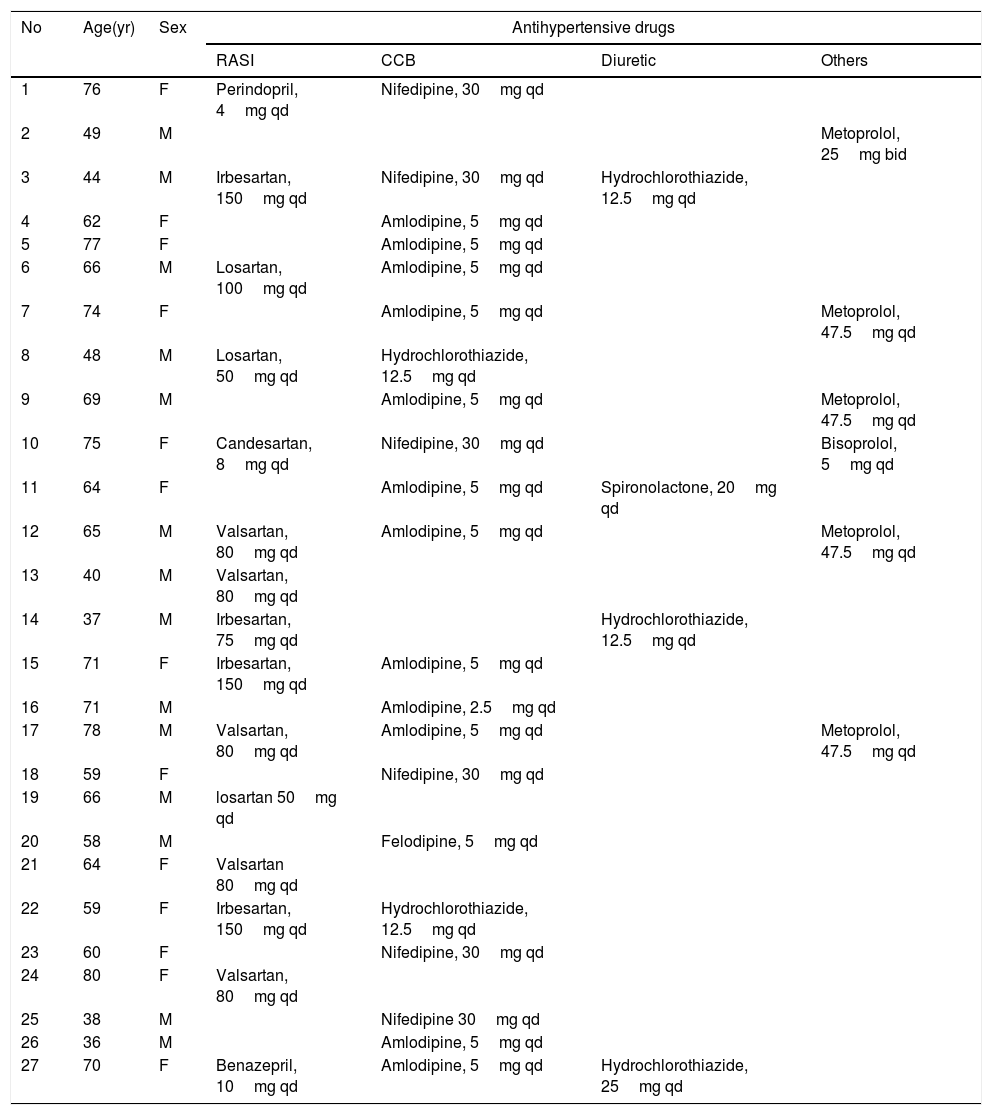
ResultsAs of January 31, 2020, 27 cases of COVID-19 with hypertension were admitted to the Shanghai Public Health Clinical Center, of which 13 (48.1%) were female and 14 (51.9%) were male. The age of the patients was 61.3±13.5 years, with a range from 37 years to 80 years. The patient's clinical manifestations were fever (70.4%), cough (33.3%), and phlegm (29.6%). The specific drugs for antihypertension treatment are shown in Table 1. All patients received 1–3 oral antihypertensive drugs, including diuretics, calcium antagonists, β-blockers, ACEI, and ARB. Most patients (85.2%) did not need to adjust antihypertensive drugs. The arterial pressure in the RASI group was 101.7±9.5mmHg, which was not statistically different (p=0.641) with that of the non-RASI group (101.1±9.9mmHg).
The antihypertension drugs of 27 cases of the COVID-19 patients with hypertension.
| No | Age(yr) | Sex | Antihypertensive drugs | |||
|---|---|---|---|---|---|---|
| RASI | CCB | Diuretic | Others | |||
| 1 | 76 | F | Perindopril, 4mg qd | Nifedipine, 30mg qd | ||
| 2 | 49 | M | Metoprolol, 25mg bid | |||
| 3 | 44 | M | Irbesartan, 150mg qd | Nifedipine, 30mg qd | Hydrochlorothiazide, 12.5mg qd | |
| 4 | 62 | F | Amlodipine, 5mg qd | |||
| 5 | 77 | F | Amlodipine, 5mg qd | |||
| 6 | 66 | M | Losartan, 100mg qd | Amlodipine, 5mg qd | ||
| 7 | 74 | F | Amlodipine, 5mg qd | Metoprolol, 47.5mg qd | ||
| 8 | 48 | M | Losartan, 50mg qd | Hydrochlorothiazide, 12.5mg qd | ||
| 9 | 69 | M | Amlodipine, 5mg qd | Metoprolol, 47.5mg qd | ||
| 10 | 75 | F | Candesartan, 8mg qd | Nifedipine, 30mg qd | Bisoprolol, 5mg qd | |
| 11 | 64 | F | Amlodipine, 5mg qd | Spironolactone, 20mg qd | ||
| 12 | 65 | M | Valsartan, 80mg qd | Amlodipine, 5mg qd | Metoprolol, 47.5mg qd | |
| 13 | 40 | M | Valsartan, 80mg qd | |||
| 14 | 37 | M | Irbesartan, 75mg qd | Hydrochlorothiazide, 12.5mg qd | ||
| 15 | 71 | F | Irbesartan, 150mg qd | Amlodipine, 5mg qd | ||
| 16 | 71 | M | Amlodipine, 2.5mg qd | |||
| 17 | 78 | M | Valsartan, 80mg qd | Amlodipine, 5mg qd | Metoprolol, 47.5mg qd | |
| 18 | 59 | F | Nifedipine, 30mg qd | |||
| 19 | 66 | M | losartan 50mg qd | |||
| 20 | 58 | M | Felodipine, 5mg qd | |||
| 21 | 64 | F | Valsartan 80mg qd | |||
| 22 | 59 | F | Irbesartan, 150mg qd | Hydrochlorothiazide, 12.5mg qd | ||
| 23 | 60 | F | Nifedipine, 30mg qd | |||
| 24 | 80 | F | Valsartan, 80mg qd | |||
| 25 | 38 | M | Nifedipine 30mg qd | |||
| 26 | 36 | M | Amlodipine, 5mg qd | |||
| 27 | 70 | F | Benazepril, 10mg qd | Amlodipine, 5mg qd | Hydrochlorothiazide, 25mg qd | |
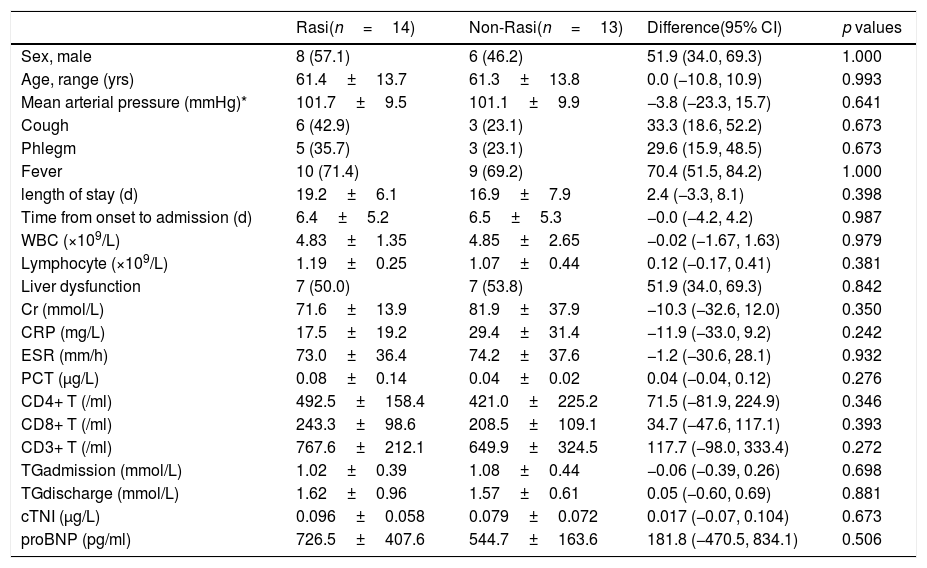
There are 14 (51.9%) cases in the RASI group including 8 males, and 13 (48.1%) cases in the non-RASI group including 6 males (Table 2). There was no statistically significant difference in age and sex ratio between the two groups. No difference was found in clinical and symptoms (fever, cough, phlegm), as well as in time from onset to admission, length of stay, and laboratory tests between the two groups.
the clinical characteristics of patients with or without RASI treatment.
| Rasi(n=14) | Non-Rasi(n=13) | Difference(95% CI) | p values | |
|---|---|---|---|---|
| Sex, male | 8 (57.1) | 6 (46.2) | 51.9 (34.0, 69.3) | 1.000 |
| Age, range (yrs) | 61.4±13.7 | 61.3±13.8 | 0.0 (−10.8, 10.9) | 0.993 |
| Mean arterial pressure (mmHg)* | 101.7±9.5 | 101.1±9.9 | −3.8 (−23.3, 15.7) | 0.641 |
| Cough | 6 (42.9) | 3 (23.1) | 33.3 (18.6, 52.2) | 0.673 |
| Phlegm | 5 (35.7) | 3 (23.1) | 29.6 (15.9, 48.5) | 0.673 |
| Fever | 10 (71.4) | 9 (69.2) | 70.4 (51.5, 84.2) | 1.000 |
| length of stay (d) | 19.2±6.1 | 16.9±7.9 | 2.4 (−3.3, 8.1) | 0.398 |
| Time from onset to admission (d) | 6.4±5.2 | 6.5±5.3 | −0.0 (−4.2, 4.2) | 0.987 |
| WBC (×109/L) | 4.83±1.35 | 4.85±2.65 | −0.02 (−1.67, 1.63) | 0.979 |
| Lymphocyte (×109/L) | 1.19±0.25 | 1.07±0.44 | 0.12 (−0.17, 0.41) | 0.381 |
| Liver dysfunction | 7 (50.0) | 7 (53.8) | 51.9 (34.0, 69.3) | 0.842 |
| Cr (mmol/L) | 71.6±13.9 | 81.9±37.9 | −10.3 (−32.6, 12.0) | 0.350 |
| CRP (mg/L) | 17.5±19.2 | 29.4±31.4 | −11.9 (−33.0, 9.2) | 0.242 |
| ESR (mm/h) | 73.0±36.4 | 74.2±37.6 | −1.2 (−30.6, 28.1) | 0.932 |
| PCT (μg/L) | 0.08±0.14 | 0.04±0.02 | 0.04 (−0.04, 0.12) | 0.276 |
| CD4+ T (/ml) | 492.5±158.4 | 421.0±225.2 | 71.5 (−81.9, 224.9) | 0.346 |
| CD8+ T (/ml) | 243.3±98.6 | 208.5±109.1 | 34.7 (−47.6, 117.1) | 0.393 |
| CD3+ T (/ml) | 767.6±212.1 | 649.9±324.5 | 117.7 (−98.0, 333.4) | 0.272 |
| TGadmission (mmol/L) | 1.02±0.39 | 1.08±0.44 | −0.06 (−0.39, 0.26) | 0.698 |
| TGdischarge (mmol/L) | 1.62±0.96 | 1.57±0.61 | 0.05 (−0.60, 0.69) | 0.881 |
| cTNI (μg/L) | 0.096±0.058 | 0.079±0.072 | 0.017 (−0.07, 0.104) | 0.673 |
| proBNP (pg/ml) | 726.5±407.6 | 544.7±163.6 | 181.8 (−470.5, 834.1) | 0.506 |
RASI, the inhibitor of Renin–angiotensin system. Data are n (%) or mean±SD.
The classic RAS regulation pathway is that renin acts on angiotensinogen to produce angiotensin (Ang) I, and Ang I produces Ang II, which is under the action of ACE. On the other hand, the non-classical RAS regulatory pathway is also known as the negative ACE2-angiotensin 1–7 [Ang (1–7)] axis, which refers to the generation of Ang (1–7) by Ang II under the action of ACE2. Renin–angiotensin system inhibitors (RASI), such as ACEI or ARB drugs, may indirectly improve ACE2 activity through restraining ACE level.
It is worth noting that SARS-CoV-2 invades the human body in the same way as SARS-CoV,2 that is, the spike protein on the surface of the virus and the receptor ACE2 on the surface of respiratory epithelial cells combine with each other, then enter the cell, causing a series of pathological changes. One of the very important changes is that the human ACE2 expression is significantly down-regulated, and the classic RAS regulatory pathway is activated.5 The imbalance in the positive and negative axis of RAS, relative or absolute increase in Ang II levels, overstimulation of AT1, and the result is increased pulmonary capillary permeability and subsequent lung emergence edema, severe lung injury, and acute lung failure.6 However, it is controversial whether as one of the classical antihypertensive drugs RASI may increase the risk of coronavirus infection, or be beneficial for coronavirus infection control.
Cough is the main adverse effect of ACEI, and the mechanism is the increased bradykinin. Cough is also the most common symptom of coronavirus respiratory infections. Patients taking ACEI for a long period of time usually do not need to stop ACEI during respiratory infections, because coronavirus infection down-regulates ACE2 level, and does not affect ACE.7 ACE2 levels do not change bradykinin levels.8 Even if the RASI could change the level or activity of ACE2 in the target tissue, clinical data are insufficient to indicate whether this in turn promotes the binding and entry of SARS-CoV-2 spikes.7 Our data shows that there is no increase in severity of coughing in patients treated with RASI. Theoretically, ACE2 level is down-regulated after coronavirus binds ACE2, leading to the imbalance of positive and negative regulation of RAS, and abnormal increase in Ang II level and activity.9 RASI may be a suitable antihypertensive agent in these cases. Using ACEI or ARB drugs has no effects on patients’ symptoms, laboratory tests, and prognosis. Both ACEI and ARB have exact antihypertensive effects, as well as clinical effects such as protecting the cardiovascular and cerebrovascular, improving renal function, reducing left ventricular hypertrophy, preventing heart failure and preventing atrial fibrillation. Existing studies suggest that ACEIs, such as captopril or Lisinopril, do not affect the activity of ACE2,10 and there is no evidence that ACEI or ARB may bring special effects to patients with COVID-19 infection or new coronary pneumonia damage. So, we recommend that the overall control of patients with new coronary pneumonia is persistent, and most of them do not need to adjust hypertension drugs.
ConclusionsFrom the small sample data, patients with COVID-19 and hypertension who continued taking the original antihypertensive drugs had good blood pressure control and no obvious adverse reactions. ACEI or ARB drugs had no obvious effects on patients’ symptoms, laboratory tests, and prognosis. Concurrently, we did not found that ACEI or ARB caused additional side-effects in patients with COVID-19 infection or promoted new coronary pneumonia damage. These results need to be verified using larger cohort sample.
LimitationsThere are also deficiencies in our research. This study was retrospective, and some cases lacking documentation for the history of present illness. Moreover, all data were obtained from a single center at a certain time point. The sample size (n=27) is also limited. These inevitably lead to limited statistical power. However, our results were also verified by some related studies.7 Further studies should be designed to confirm whether RASI could increase ACE2 level, and the pharmacological action of ACE2 could affect the infectivity of SARS-cov-2.
Availability of dataNot applicable.
Authors’ contributionsHaiming Cui, Feng Wu and Zhenyu Fan contributed equally to this work.
FundingThis study was supported by National Natural Science Foundation of China (NSFC) Grant 81403258 (Haiming Cui) and NSFC Grant 81503371 (Feng Wu), and the Science and Technology Committee Shanghai Municipal Foundation Grant 19DZ1930404 (Haiming Cui).
Conflict of interestsThe authors declare that there is no conflict of interests.
We appreciate all the people who are fighting the COVID-19 epidemic around the world. Professor Fan Min fight against COVID-19, as the director of unit C7 at Raytheon Hospital, Wuhan, Hubei province. Professor Jilin Cheng and Dr. Zhenyu Fan make a large contribution to treat COVID-19 patients admitted to the Shanghai Public Health Clinical Center. We thank Cao Yang from Clinical Epidemiology and Biostatistics Department at Örebro University Hospital for native English modification.