Peritoneal carcinomatosis refers to a shedding or tumor that spreads to the peritoneal serosa and structures of the abdominal cavity. It is an entity with a poor prognosis. Several conditions can cause this, the most common being colon, rectum, ovary, stomach or appendix cancers, including peritoneal pseudomyxoma, among others. The abdominal cavity invasion is considered a clinical stage IV. For a long time life expectancy of this entity was very short. With the advent of meticulous techniques in cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) the prognosis of patients has changed. In some conditions, these procedures are standard treatments. CRS is a very important prognostic factor; leaving a less residual disease in the patient, the evolution will be better. The HIPEC starts immediately after the surgical event. The hyperthermia increases the cytotoxic effect of antineoplastic drugs. Numerous studies have appeared in medical literature wherein the clear improvement in survival of the affected population is demonstrated. It is essential that a multidisciplinary team participates in the decision for the best treatment option and the maximum clinical benefit of the patients.
Peritoneal carcinomatosis (PC) refers to the shedding, implantation and dissemination of a tumor, either localized or massive, to the peritoneal serosa, as well as the adjacent structures of the abdominal cavity. Its presence indicates a clinical stage IV. It is usually associated with gynecological tumors and tumors of the digestive tract.1–3 The exact incidence of PC as a primary site and as a recurrent site is not known with any certainty, since most analytic and imaging studies used to monitor different pathologies do not allow for the detection of said dissemination in initial studies. Numbers vary according to the pathology; the most representative is colon cancer. Estimations suggest that initial recurrence in the peritoneum after a surgery with curative intentions is 10–20%. Peritoneal dissemination occurs in 40–70% of total recurrences and only 5–8% present a disease strictly confined in the peritoneum. Considering all patients with the inclusion of all original pathologies, medical literature shows that 15% of patients arrive with PC at first and 35% die of intraperitoneal recurrence.4 Up to a few years ago, this entity had had an adverse prognosis with a fatal outcome within months.5 However, the evolution of the disease can be changed with an excellent full cytoreductive surgery (CRS) and the emergence of intraperitoneal chemotherapy (IPCT). Life expectancy used to be very limited and dependant on the base pathology: between 3 and 6 months for gastric base PC,4,6 11–21 months for colon/rectal PC and 14–24 months for ovarian PC, on average. The variant linked to peritoneal pseudomyxoma has shown better survival rates, due to the tumor's biology and its response to multimodal treatment. In all the previously mentioned cases, CRS and IPCT have increased these numbers.
Today, peritoneal affection is being considered as a locoregional dissemination, thus generating the idea of performing metastasectomies in said entity with the purpose of leaving patients disease-free. In the late 80s, Dr. Sugarbaker developed a treatment with a radical approach, consisting of a combination of CRS and IPCT, the latter in its early post-operative modality (EPIC early postoperative intraperitoneal chemotherapy), and in cases requiring hyperthermia (HIPEC hypertermic intraperitoneal chemotherapy). The key objective of the radical approach is to completely eliminate the visible disease through CRS and EPIC or HIPEC, and to eradicate non-visible tumor residues. CRS ought to be thorough in order to release adherences, in addition to retreating tumor implantations, so that chemotherapy, once administered, is distributed homogeneously amongst the intra-abdominal organ surfaces.7,8
During the last decades, CTIP and CRS have been significantly revolutionized, thus resulting in favorable results in patient survival rates, which had not been achievable in previous years.
Physiopathology and the plasmatic peritoneal barrierCancers in the abdomen spread via three different routes: haematogeneous, lymphatic and celomic. The latter led to the hypothesis that in eliminating this type of dissemination, the risk of extension of the disease would decrease and free-of-recurrence survival rate would increase. Peritoneal liquid goes from the pelvis to the diaphragm and is defined by the reflections of the peritoneum. Intraperitoneal seeding through ascites is one of the most significant forms of peritoneal metastasis and the leading cause of PC. Regardless of the dissemination mechanism, tumor cells spreading to the peritoneal cavity do so in different ways: through gravity, peristalsis and/or negative pressure of the diaphragmatic muscles.2,9 Once the tumor cells adhere, they penetrate the mesothelial monolayer and initiate the PC process. The peritoneal tissue provides a source that is rich in nutrients, growth factors and chemokines, leading to a favorable environment for tumor cell proliferation.9 The plasmatic peritoneal barrier maintains a positive gradient of chemotherapy, causing medications with a high molecular weight to remain in the abdominal cavity for a longer period of time, allowing for a greater exposure of tumor cells to the medications, compared to the intravenous route.1,4,10
DiagnosisDifferent techniques are used in diagnosis, such as imaging studies like ultrasounds, CAT scans, NMR scans and PET/CT positron emission tomographies with fluorodeoxyglucose 18F. Nevertheless, these studies have their limitations. They are usually used more in staging and for non-resectable disease assessment.4 CAT scan sensitivity for PC diagnosis ranges between 41 and 93% with a specificity between 79 and 96%. CAT scans can prove previously established imaging patterns, including the “omental cake” which represents fat implants, thickening and heterogeneity, subcapsular implants, nodular lesions, associates and mesenteric fat tissue tumor infiltration.2
There are different systems to measure PC. The most utilized is the peritoneal carcinomatosis index (PCI), which is based on the peritoneal nodules’ size and quantitative distribution. The abdominal cavity is divided into 13 regions and the volume of the disease is determined in every region (Fig. 1). After a thorough surgical inspection, the extension of the disease is measured in relation to every region, assigning them a number (score from 0 to 39). PCI has a prognosis value in addition to estimating the possibility of full cytoreduction. A series published a survival rate at 5 years of 50% for PCI<10, 20% for PCI 10–20 and 0% for PCI>20.5,11,12 Sugarbaker recommends a palliative management with a PCI greater than 20.13 Some groups use a PCI>26 as a reference.
Surgical treatmentThe decision of the oncological surgical treatment type depends on the anatomic location of the malignancy as well as its biological behavior. Cytoreductor management may require 6 types of peritonectomy procedures, used to resect cancer from all abdominal surfaces. These are the types: (1) Pelvic peritonectomy, with or without the excision of the sigmoid colon or rectum, mesorectum, uterus and annexes. (2) Major omentectomy, with or without a splenectomy, and with or without a right colectomy. (3) Left hemidiaphragm peritonectomy. (4) Right hemidiaphragm peritonectomy, with or without a Glisson capsule, and with or without a sub-hepatic peritonectomy. (5) Minor omentectomy with a cholecystectomy, and (6) Gastrectomy and other intestinal resections.7 Major omentectomy, oophorectomy (in post-menopausal) as well as cholecystectomy will always be carried out, the latter with the purpose of avoiding post-operative complications related to chemotherapy. Peritonectomy procedures allow us to accomplish the objective of removing all visible disease, with acceptable post-surgical complications, reporting morbidities in 25% and mortality in 1.5%.12 CRS can be evaluated using the Sugarbaker technique, according to the residual disease classification after cytoreductive surgery: CC0 defined as non-visible, CC1, persistent nodules under 0.25, CC2, nodules between 0.25 and 2.5cm and CC3, nodules over 2.5cm.14 The important thing is to accomplish full cytoreductive surgery, determined as CC0 or CC1.15,16 CRS residual disease plays a prognosis role regarding survival rate. At 5 years, it is 35% with CC0 and CC1 versus 0% with CC2 and CC3.12 Recently Esquivel, et al. reported the role of laparoscopy for the completion of CRE with HIPEC in 14 patients. CC0 was accomplished in 13 patients, 10 (77%) via laparoscopy and 3 (23%) via open surgery. However, they were well-selected patients, with a low tumor load and without intestinal involvement.17
CTIPAs its name states, it is about administrating chemotherapy agents via intraperitoneal. There is evidence that some drugs administered in large amounts via intraperitoneal maintain a significantly higher concentration in the peritoneal area compared to plasmatic concentration. A large concentration of drugs offers a biochemical advantage in the treatment of patients with microscopic neoplastic disease in the peritoneal cavity.18 Very positive results with the use of chemotherapy in patients with peritoneal carcinomatosis, sarcomatosis and mesothelioma have been reported. The most commonly used drugs in the CTIP scenario are oxaliplatin, irinotecan, adriamycin, cisplatin, mitomycin, paclitaxel and gemcitabine. CTIP causes local and systemic toxicity, since the drugs will eventually enter the blood stream.1 Once the drugs are administered via IP, they destroy tumor cells directly, as well as decrease cells of the inflammatory process, altering the ability to withstand an infectious process. Thus, sterility and asepsis during the full procedure are imperative. As previously mentioned, CTIP can be administered in two different ways, EPIC and HIPEC, both preceded by CRS. The difference between them is that catheters are left for the administration of CT in the first one, which is usually performed in 5 days, and the second one is a single postoperative procedure. Today, the most utilized is HIPEC. There are studies where both HIPEC and EPIC treatment modalities are combined, which has not generated benefits in survival rates, though it has generated greater toxicity.19
HIPECThe connection between chemotherapy and hyperthermia is what's known as HIPEC. It is limited to a single treatment session. Simultaneous use of HIPEC with intravenous chemotherapy (bidirectional chemotherapy) improves survival rate results in some types of PC. The interest on hyperthermia has focused on three fundamental aspects: (1) increased temperatures themselves have a cytotoxic effect, (2) hyperthermia increases the inactivation by radiation rate and more importantly (3) the cytotoxic effect of chemotherapy agents is increased with the elevated local temperature.20 Hyperthermia of 40–42°C, along with chemotherapy, help drugs penetrate malignant tissue (from 3 to 6mm), thus increasing the cytotoxic effect.2,4 From the first studies within the use of CTIP literature, is phase I, which researched the role of 5-fluorouracil. Said drug was administered through dialysis catheters to individuals with tumors limited to the peritoneal cavity, the patients developed the same adverse effects caused by that of intravenous administration, two patients displayed a clinical response, concentrations of peritoneal liquid were measured and they decreased in the first order with an average lifespan of 1.6h. In 4h 82% of the IP drug was absorbed, and the medication's plasmatic levels began to rise after 30–45min. There was a significant difference between intraperitoneal and plasmatic levels; concentrations in peritoneal liquid were 298 times greater at 4h compared to the levels in blood.21 In the late 80s, Sugarbaker proved that it was possible to keep patients with pseudomyxoma peritonei free of disease with cytoreductive surgery and the administration of chemotherapy with 5-fluorouracil and mitomycin C.22 Other studies have used other drugs which have proven to be compatible with hyperthermia, these being oxaliplatin, mitomycin C, cisplatin, doxorubicin, paclitaxel and irinotecan.23,24 Intra-abdominal temperature should not exceed 43° in order to avoid adverse effects harmful to the tissue, such as intestinal perforation.
Patient selection for CTIPIn the past, many patients were treated with HIPEC despite being CC2 and CC3, resulting in the absence of expected benefits. Residual disease is an important criterion in patient selection for this management protocol. The extent of the disease at the time the patient begins treatment will correlate to the eventual results. Patient selection should be done early, and it is important to have a multidisciplinary team participating in the process. Today, there are 4 major points to take into account when selecting patients: the invasive nature of the disease, previous CATs, ICPs and the full CRS score.2,25
Indications for CRS and CTIP are shown in Table 1. Patients with a good functional state, a low volume peritoneal disease and an absence of extra-abdominal metastasis are more commonly benefited by the treatment.1,26 Patients admitted to CRS and HIPEC must have a leukocyte count of >3000/mm3, polymorphonuclears of >1500, platelet count of >100,000/mm3, normal creatinine or calculated depuration at >50ml/min and a signed consent form.27 Contraindications for HIPEC are age >70 years, major comorbidities, a reaction to chemotherapeutics, malnutrition, extra-abdominal metastasis, non-rescuable hepatic metastasis, massive retroperitoneal disease or voluminous lymph node involvement and signs of intestinal occlusion.4 Concerning sarcomatosis treatment by GIST and round cell tumors, studies are limited and treatment with HIPEC is not indicated.13
Indications for the combined treatment of CRS and CTIP.
| 1) Malignant ascites |
| 2) Peritoneal pseudomyxoma after a CRS |
| 3) Peritoneal mesothelioma after a CRS |
| 4) Primary colon and rectal cancer |
| - Peritoneal seeding of limited distribution and small volume |
| - Perforated colon cancer |
| - Colon cancer involving adjacent organs |
| - Colon cancer disseminated to the ovaries |
| - Colon cancer with a positive cytology IP |
| - Tumor rupture at a primary resection |
| 5) Recurrent colon and rectal cancer with carcinomatosis |
| - Peritoneal seeding of limited distribution and small volume |
| - Krukenberg |
| - Tumor rupture at a resection of a recurrence |
| - Completed ebulking of a recurring disease at more than one site |
| 6) Recurring ovarian cancer with limited dissemination to the peritoneum |
| - Prolonged interval free of disease between initial treatment and recurrence |
| - Limited or no options for chemotherapy via IV |
| 7) Primary gastric cancer with limited peritoneal implants after a complete resection of both |
| 8) Peritoneal sarcomatosis |
| - Sarcomatosis following CRS |
| - Primary abdomino pelvic sarcomatosis with doubtful resection margins |
| - Primary abdomino pelvic sarcomatosis with tumor rupture at resection |
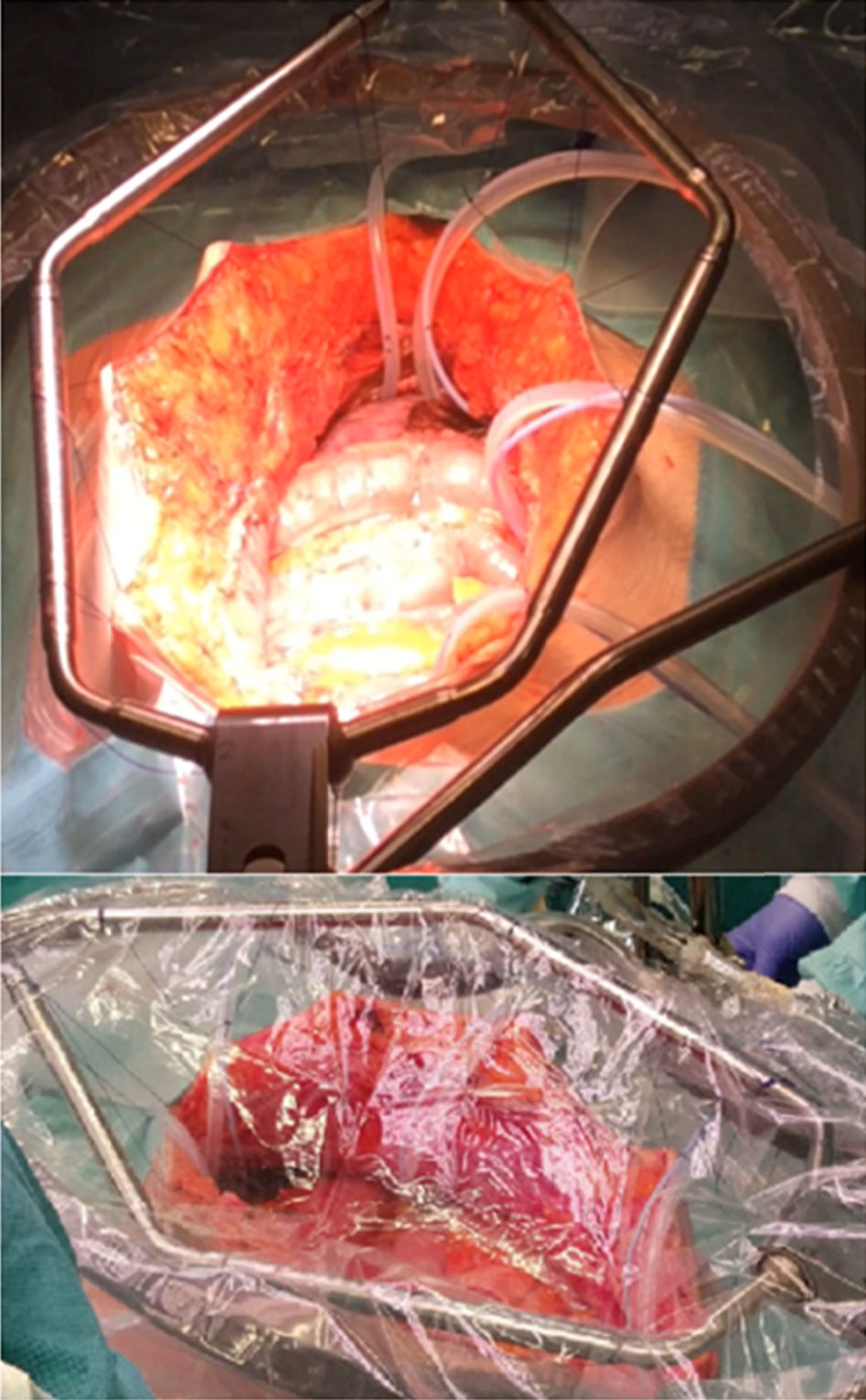
A strict monitoring of the patient's vital signs is required (temperature ought to be measured with an esophageal temperature probe) and a proper monitoring of diuresis. After a full resection of tumor implants and the liberation of the loops removing fibrin bridges and adherences, placement of administration tubes (at least a perfusion tube) and drainage tubes takes place prior to HIPEC. In addition, three temperature sensors are placed in the abdominal cavity (superior, middle and inferior). Before initiating HIPEC, the chemotherapy complement being used is administered intravenously (bidirectional chemotherapy). There are two techniques to perform HIPEC; open (coliseum) and closed. The most utilized technique is coliseum (Fig. 2). There are no studies proving that one is better than the other. An extracorporeal circulation machine is used for HIPEC treatment (Fig. 3),28 which previously heats an isotonic dialysis solution or a glucose solution at 1.5%, minimum of 2l, at 40–42°C. Once the temperature is reached, the abdominal cavity is filled with the previously preheated solution. This solution is distributed evenly, trying to reach the desired temperature homogenously in the abdominal cavity, and once this is accomplished the administration of chemotherapy takes place, manipulating the influx tube as well as the tissue and intestinal loops in order to continue with the uniformity of intra-abdominal temperature. After the time, which can be between 30 and 120min, is completed, the cavity is emptied, the abdomen is reassessed in order to find tissue lesions or bleeding and when the procedure is finished, the circuit is retracted, drainages are placed and closure by planes is performed.13,27
Over the last two decades, combined treatment with CRS and HIPEC has modified therapeutic treatment for patients with malignancies on the peritoneal surface, becoming the gold standard for the management of some of them. Accepted pathologies for this are: peritoneal pseudomyxoma, mesothelioma, colon cancer, ovarian cancer and gastric cancer. The benefit of CTIP is shown in Table 2. Cardi et al., recently published the use of CRS and HIPEC in non-conventional pathologies from a series of 253 patients, 28 of them with a differential diagnosis of sarcoma, GIST, and different types of cancer such as of the small intestine, pancreas, breast, bladder, lung and uterus, which showed a mean overall survival (OS) of 56 months and OS at 5 years of 40.3% with a difference amongst patients with CC0 and CC1 (52.3 versus 25.7%).29 The two key components in the treatment of this pathology are CRS and CTIP. A systematic review of the efficiency of cytoreductive surgery and the use of CTIP in peritoneal pseudomixoma was published by Yan et al., In this review, survival rate means of 51–156 months are shown, OS at 1, 2, 3 and 5 years was 80–100%, 76–96%, 59–96% and 56–92% respectively, with a global mortality rate of 0–18%.30 Yonemura et al., studied patients with gastric cancer who underwent CRS and HIPEC, reporting survival rates at 5 years of 61% compared to 42% from surgery alone.31 Regarding colon cancer, an improvement has also been proven in OS (P<0.0001).32 Verwaal et al., randomized patients with colon and rectal cancer to receive systemic chemotherapy with or without palliative surgery versus CRS with HIPEC, proving a benefit in mean survival rates in favor of CRS with HIPEC. In a follow-up at 21.6 months, OS mean was 22.3 versus 12.6 months (p=0.032), in a subgroup analysis, researchers showed that patients with 0–5 affected regions have better results compared to patients with 6 or 7 affected regions (OS mean >29 versus 5.4 months {p<0.0001}, respectively). Reported toxicities of 3rd and 4th degree are low; amongst the most common are leucopenia (15%), fever (6%), bleeding (8%), and gastrointestinal fistulas (15%).33 In 2008, Elias et al., reported the benefit of chemo-hyperthermia with oxaliplatin in patients with PC of colorectal origin, reporting a survival rate of 63 months, and a survival rate at 5 years of 51%.34 Hompes et al., reported morbidity and mortality rates with the use of CRS and HIPEC; mortality rates at 30 days was 0%, the rates of complications of any degree was 52%, anastomosis leakage 10.4%, and bleeding 6.3%, with an average hospital stay of 20 days. With a follow-up at 22.7 months, he reported a OS of 97.9 and 88.7% at 1 and 2 years, respectively. Disease free survival (DFS) of 65.8 and 45.5% at 1 and 2 years, respectly.35 Mortality rate for this treatment has been reported at 8%.33,36 Glehen et al., conducted a multi-institutional, retrospective study, which included 506 patients with colon and rectal cancer. Morbidity rates were 22.9% and mortality rates were 4%, patients who underwent CRS reached OS means of 32.4 months compared to 8.4 months for those patients for whom CRS was not possible.37 A meta-analysis published by Huo et al., analyzed treatment with CRS and HIPEC versus CRS and intravenous chemotherapy in patients with primary and recurrent epithelial ovarian cancer. In this study, an improvement in survival rate in favor of CRS and HIPEC is proven.38 Barrios, et al. reported 618 patients with CRS and HIPEC, 561 (91%) with CC0-1 and 57 non-optimal surgeries (9%). Out of the patients with CC0-1, 44% had colon cancer, 20% had peritoneal pseudomixoma, 15% had recurrent ovarian cancer, 5% had gastric cancer, 4% had cancer in the appendix, 3% had mesothelioma, 2.3% had rectal cancer, and 6.7% had some other type of cancer. Survival rate mean was 60.2 months for the group in general, 51.2 months for the colon cancer group, 45.4 months for the recurrent ovarian cancer group, 29 months for the gastric cancer group, 36 months for the appendix cancer (non-pseudomixoma) group, 46 months for the mesothelioma group, and 24 months for the rectal cancer group. Moreover, the global complication rate was 27.5%, amongst the most frequent were central line infections (3.8%), non-focal fevers (3.8%), urinary tract infections (2.7%), and haemoperitoneums (2.1%). Re-interventions were necessary in 3.5% and the mortality rate was 0.1%.39 Today, the COLOPEC protocol is in the works. This protocol will evaluate PC prevention with HIPEC in patients with high-risk colon cancer.27
Scientific evidence of the use of CTIP and HIPEC.
| Reference | Base pathology | Treatment | GSR |
|---|---|---|---|
| Cardi et al.29 | Sarcomas, GIST, cancer of the small intestine, pancreas, breast, bladder, lung and uterus | CRS+HIPEC | Mean, 56 months At 5 years, 40.3% CC0 52.3% CC1 25.7% |
| Yan et al.30 | Peritoneal pseudomyxoma | CRS and CTIP | Mean, 51–156 months |
| Yonemura et al.31 | Gastric cancer | CRS+HIPEC | At 5 years, 61% |
| Verwaal et al.33 | Colon and rectal cancer | CRS+HIPEC vs. Systemic chemotherapywith/out surgery | Mean, 22.3 vs. 12.6 months |
| Elias et al.34 | Colon and rectal cancer | CRS+HIPEC | Mean, 63 months |
| Hompes et al.35 | NR | CRS+HIPEC | At 2 years, 88.7% |
| Glehen et al.37 | Colon and rectal cancer | CRS+HIPEC vs no CRS | Mean, 32.4 vs. 8.4 month |
| Huo et al.38 | Ovarian cancer | CRS+HIPEC vs Systemic CT+surgery | NR |
| Barrios et al.39 | Colon cancer, peritoneal pseudomyxoma, recurrent ovarian cancer, stomach, appendix, mesothelioma and colon cancer | CRS+HIPEC | Mean, 60.2 months |
Some positive prognosis indicators are CRS, lymph node invasion, limited extension of PC, age under 65 and the use of adjuvant chemotherapy.4,37
Quality of life prior and subsequent to the CRS and HIPEC procedures was assessed by a study. The study showed a physical activity and functionality decrease in post-surgery; however, the patients returned to normal after 3 months of treatment.40
Neoadjuvance in CRS with HIPECThe optimal sequence of systemic chemotherapy in CRS radical treatment with HIPEC is not fully defined. There are few studies for neoadjuvance and evidence is discordant. Kuijpers et al., proved that there is no difference for OS and DFS between adjuvance and neoadjuvance.41 Some advantages that neoadjuvant chemotherapy may have are: undetectable systemic disease treatment, biological tumor behavior assessment and reduction in the tumor load. Some probable disadvantages are the possible toxicity in post-surgery, progression of the disease, difficulty to stage the disease and difficulty to assess the response to chemotherapy. CRS and HIPEC are conducted 4 weeks after the last chemotherapy dose and 6 weeks if bevacizumab was used.37
ConclusionCRS and HIPEC have revolutionized the treatment of patients with PC, reaching better results in global survival and free-of-disease rates. Patient selection is crucial and should be conducted by a multidisciplinary team in order to achieve better results. It is imperative to accomplish full cytoreductive surgery in the management of these patients, thus assuring the best prognosis. CRS and HIPEC can be considered the new golden standard in the management of patients with PC.
Conflict of interestThere is no conflict of interest reported by any of the participants.