Stroke represents a public health enemy. Currently, and in spite of multiple clinical trials, thrombolysis remains as the only approved therapy. Most preclinical trials and animal trials employing stem cell-based therapies have shown very promising evidence of benefits. The aim of this review is to provide a landscape of what has been done in human clinical trials, and what are the possible ways that stem cell therapy may enhance functional recovery in stroke patients.
Acute stroke is the sixth most common cause of death among Mexicans and the third cause in elderly people; just in 2014 it was responsible for 30,000 deaths.1 Though these numbers may even be sub-registered.2 More startling is the fact that stroke is the leading cause of disability in this country and worldwide.3 This is because in spite of the high mortality, 75% of patients survive a stroke with some kind of sequel; this is 258 patients daily.4
The main mid-term and long-term sequels of stroke are dysphagia, fatigue, muscle weakness, paralysis, visual problems, incontinence, chronic pain, seizures, insomnia, depression, dementia, aphasia, amnesia among many others.5
The annual incidence of stroke is 232/100,000 people, with a prevalence of 8/1000.6 With a population of 112 million Mexicans, this means almost 900,000 people survive with some type of disability or sequel. Worse, after a year almost half of them survive with significant sequels that affect their quality of life and their capacity to perform daily life activities (Modified Rankin Scale [mRS] between 2 and 5).7
As if that were not enough, the economic impact is notorious as well. Among hospital expenses, studies, treatments, doctors’ fees, work incapacities, early retirement, rehabilitation programs and more, each patient surviving stroke yearly spends around $25,000 dollars.4 This is of course without taking into consideration the social and emotional expenses.
Stroke prevalence is on the rise worldwide,8 and our country is not the exception.9 In Mexico the median age of a first stroke event is of 68 years (ranges 52–84).7 Epidemiology studies project that by 2050, the group conformed by people aging between 55 and 80 will almost triple, to 28%.10 Therefore, by that year there will be more than 2 million stroke survivors; 1 in every 60 Mexicans.
Even though there have been many attempts to develop new stroke cures, a thrombolytic approach with tissue plasminogen activator for an acute ischemic stroke remains as the only approved therapy.11 It is imperative to find or develop a new treatment to enhance recovery and restore, at least partially, the lost neurological functions.
Physiopathology of strokeEtiologyStroke can be divided into ischemic stroke, constituting 85% of cases (atherothrombotic, cardioembolic, small vessel disease), and hemorrhagic stroke (subarachnoid hemorrhage, intraparenchymal hemorrhage) which accounts for the remainder 15%.12 The later one has an overall worse prognosis.13 The primary risk factors for stroke include, but are not limited to, hypertension, diabetes mellitus, smoking, dyslipidemia and atrial fibrillation.14
Cerebral ischemia is graded depending on the cerebral blood flow (CBF; which would normally be of 50–55ml/100g/min). Anatomically, stroke lesions can be divided into an ischemic penumbra (CBF of 15ml/100g/ml), with functionally impaired neurons, though still reversible with acute stroke therapy, and an ischemic central core (CBF of 6ml/100g/ml) with irreversible neuronal death.15
Natural historyLack of energy substrates quickly leads to dysfunction of energy-dependant ion transport pumps and depolarization of neurons and glia.16 This depolarization releases excitatory neurotransmitters, primary glutamate, which amplify the damage by the release of free radicals and interruption of the electron chain transport.17 The following oxidative stress contributes to neuronal death by disruption of the cell membrane.18 Apoptosis also mediates many of the lost neurons, predominantly in the penumbra region if no acute treatment is installed.19
Afterwards, astrocytes concentrate along ischemic lesions, and produce proteoglycans to form a glial scar, which act as both a physical and biochemical barrier to axonal regeneration and sprouting, limiting the reconnection of neural circuits and contributing to many of the long-term sequels of stroke.20,21
Most important for the matter of this review it is the robust inflammatory reaction following cerebral ischemia. Inflammatory molecules (e.g. interleukin-1 [IL-1], interleukin-6 [IL-6] and tumoral nectrotic factor-alpha [TNF-α]) are predominantly deleterious in the early phase after an ischemic stroke22 and paradoxically promote brain regeneration and neurovascular remodeling in the later or chronic phases.23
Finally, stroke releases many chemotactic molecules (e.g. interleukin 8 [IL-8], monocyte chemoattractant protein-1 [MCP-1]) both for leucocytes and stem cells.24 Particularly stromal-derived factor 1a (SDF1a) is released by activated endothelial cells after hypoxic injuries, and its receptor (CXC chemokine receptor-4 [CXCR4]) is up-regulated as well.25 They both acts as chemoattractants that mediate neural26 and bone marrow27 stem cell migration to injured areas, which is critical for stem cell-based therapies.
Neurorestorative therapiesAfter neuroprotection (acute) therapies have failed, and scarring, inflammation and edema have installed, the approach must be shifted then to a nuerorestorative therapy rather than on preventing the extension of a damage that has already been well established. This type of therapy focuses on orchestrating through all type of parenchymal cells (i.e. neuroblasts, immune cells, astrocytes, oligodendrocytes and neurons), the enhancement of endogenous neurogenesis, angiogenesis, axonal sprouting and synaptogenesis in affected brain tissue.28
Neurorestorative therapies include, but are not limited to, stem cells. There are also ongoing pharmacological investigations29 and other type of treatments such as electromagnetic stimulation, device-based strategies, repetitive training and task-oriented strategies.30 Rehabilitation could exploit the combination of functional reorganization and adaptation after stroke.31 Of all, currently only constraint-induced therapy has evidenced some type of efficacy.32
Stem cellsTypes of stem cellsSince their first descriptions in 1963 by Till and McCulloch,33 stem cells have been used as an alternative to many diseases, especially those without any other treatment options. Neurologic conditions have not been the exception. In fact they represent the fifth most common cause for ongoing clinical trials based just on mesenchymal stem cell therapy (after bone, heart, gastrointestinal and autoimmune disorders).34
A stem cell is considered as such when two properties are met: capacity for long-term self-renewal without senescence and the ability to differentiate into one or more specialized cell types. Based on their transdifferentiation spectrum stem cells are classified as totipotent if they can form any cell in the body (including placental cells), as pluripotent if they are able to divide into any cell of the three germ-lines of early embryogenesis, and as multipotent if they are already committed to only one germ-line (e.g. most bone marrow stem cells can only differentiate into mesoderm derived cells).35
Stem cells are also classified based on their origin as embryonic or adult type. Embryonic stem cells (ESC) are pluripotent cells that are obtained by manipulating embryos before implantation.36 Adult (somatic) stem cells are multipotent (or pluripotent) cells obtained from mature differentiated tissues such as bone marrow, umbilical cord, human olfactory mucosa, fat tissue and brain.37
Finally, another type of stem cell is the induced pluripotent stem cells (iPSC). In this technique, differentiated mouse fibroblasts are reprogrammed to an embryonic-like state (pluripotency) by transfer of nuclear contents into oocytes or by fusion with embryonic stem cells.38
Neural stem cellContrary to old assumptions, evidence of neurogenesis in the adult human brain has been demonstrated.39 Neural stem cells (NSC) are a multipotent variant of stem cells present in the brain.40 These cells are located in the subventricular zone (SVZ) of the third ventricle41 and in the subgranular zone (SGZ) of dentate gyrus,42 and respond to brain insults that cause neuronal death such as stroke,43 Huntington's disease,44 and Alzheimer's disease.45 NSC not only proliferate but also migrate to areas of lesion even in elderly patients.46 NSC can be cultured in vitro for stem cell therapies, and even if administered intravenously, have the capacity to migrate into ischemic areas.47
It is been documented that after a stroke NSC expand and mature into well differentiated neurons and integrate functionally into neuronal circuits.48 After a stroke, the brain's environment holds a rise in many growth factors that induce changes in NSC's mitotic cell cycle such as reduction of G1 phase49 which boosts mitotic rate up to a 12-fold increase in number42 as well as activation of phosphatidylinositol 3-kinases-Akt signaling pathway which enhances cell survival, proliferation, differentiation and migration.50,51 Stroke also activates many genes involved in neurogenesis during embryonic development, especially those of transforming growth factor-beta [TGF-β] superfamily (bone morphogenic protein 8 [BMP2], bone morphogenetic protein type 1 receptors [BMPR1] and growth differentiation factor 2 [GDF2]).52 These newly formed neurons differentiate into the phenotype of most of the neurons that were lost during ischemia, in an attempt to regenerate lost circuits and recover lost functions.46
Discouragingly, one of the setbacks is their slim capacity to migrate into areas of the cortex where higher mental functions lie.46 What is more, after a couple of weeks 80% of these newly formed neurons die and actually just 0.2% of dead tissue is replaced.46 We hypothesize that if the percentage of incorporated renewed cells could be increased somehow, (e.g. neurotrophic, or angiogenic factors) restoration of neurological functions would be much greater as well.
Bone marrow stem cellsBone marrow stem cells (BMSC) are an array of different type of multipotent and pluripotent cells homed in the spongy tissue of almost all bones. Two basic lineages prevail: hematopoietic stem cells (HSC, PBSC if obtained peripherally) and mesenchymal stem cells (MSC). HCS give rise to all the type of blood cells and are typically CD34+, CD133+ and negative for all markers of differentiation or further lineage commitment (CD13−, CD71−, CD19−, CD61−).53 MSC lie on the stroma of the bone marrow, and contrary to HSC, they can differentiate into a broader variety of cell types, such as osteoblasts, chondrocytes, myocytes and adipocytes and even neurons.54 MSC are usually CD34-.55
BMSC actually have limited cellular differentiation ability in comparison to other type of stem cells; evidence suggests instead that the beneficial properties are due to immunomodulatory mechanisms, as they migrate to sites of inflammation (by the mechanisms explained before)56 and secrete many bioactive molecules.57 This is supported by the fact that PBSC are also used with efficacy in the autologous therapy of non-hematopoietic tissues like neurons,58 skeletal muscle59 and heart.60 In multiple sclerosis and amniotrophic lateral sclerosis for instance, immunomodulatory effects and improvements were observed just 24h after intrathecal delivery of MSC, which would be an irrational time frame for differentiation and rather backs up the hypothesis of a bystander effect instead.61 Furthermore, six months later, evidence of integration or even survival of these cells was very poor.62 In an animal model, CD34+ cells (HSC) were tracked by magnetic resonance, where they prove they migrate to lesion sites but just persisted for about 3 to 4 weeks.63
Even though there is a very low rate of transdifferentiation into neurons, there is still clinical recovery, motor evoked potential improvements, as well as reconstruction of the ischemic tissue.64 As stated before, the benefits of BMSC would be by enhancing endogenous neurogenesis rather than cellular lineage reprogramming. The mechanisms involved appear to be paracrine secretion of bioactive molecules and upgrade regulation of receptors that reinforce and augment the natural recovery processes implemented by the brain; subsequently increasing the number of new functional neurons derived from endogenous neuroblasts.
It has been proved that exogenous administration of brain-derived neurotrophic factor (BDNF) stimulates neuroegenesis,65 therefore, endogenous secretion of BDNF and similar trophic factors by stem cells would aid in such purposes. BMSC increases concentration of SDF1a as well as expression of the SDF-1 receptor, CXCR4 in the perischemic area.66 There is also promotion of basic fibroblast growth factor (bFGF) and other trophic factor like β-nerve growth factor (β-NGF)67 which would not only promote proliferation, but will reduce apoptosis as well.68
BMSC increase the number of oligodendrocyte progenitors and increase axonal density around the ischemic lesion, extending and orienting axons parallel to the boundary of the penumbra.69 They do this by reducing expression of axonal growth inhibitory proteins, such as reticulon and neurocan, enabling axonal and neurite outgrowth.70
MSC also share the properties of secreting many trophic factors (BDNF, SDF-1, NGF, bFGF, and VEGF) and promoting neurogenesis71 with the added benefit of a greater potential than regular HSC to transdifferentiate into neurons themselves.72,73 MSCs carry the benefit of being readily obtained from bone marrow and easily expanded by culture in vitro, though this involves a time frame of 4 to 5 weeks before being delivered back to patients.74
MSC are pretty safe. Because of their low major histocompatibility complex proteins they are considered immune privileged and cause no immunogenicity, neither acute or chronic.75 In a recent meta-analysis, there was no association between MSC and neoplastic potential, infection, embolism or zoonosis; in fact the only side effect was transient low-grade fever (OR: 16.82).76
Angiogenesis also plays a critical role in functional recovery. As in neurogenesis, angiogenesis is induced by several growth factors present in the penumbra 3 to 4 days after a stroke.77 It is so relevant, that patients who have a high density of blood vessels after stroke survive longer than those who do not.78 Animal models with denser vascularization have a better functional outcome as well.79 This density is determined by the presence of vascular growth factors, for there is a correlation between greater concentration gradients of them and increased blood vessel neoformation.80 Interestingly, neurogenesis actually enhances symbiotically angiogenesis by secreting the same factors.81,82 Given that BMSC up-regulate expression and paracrine secretion of angiogenic growth factors such as vascular endothelial growth factor (VEGF) and its receptor (VEGFR2) as well as angiopoeitins 1 and 2 and their receptor (TIE-1 and TIE-2),83 it is hypothesized that they would magnify the beneficial properties of neurogenesis and angiogenesis along with improving clinical outcomes and survival rate.84
Deciding which is better among HSC, PBSC or MSC is still unachieved. The only human clinical trial comparing HSC and expanded MSC found out that patients had better clinical outcomes (Barthel Index [BI]) with HSC.85
Other type of stem cellsBesides BMSC and NSC, other type of stem cells may be used for stroke and other diseases. Pluripotent stem cells (e.g. ESC), with a wider transdifferentiation spectrum, have the theoretical advantage over multipotent cells in its use for regenerative medicine; however, the downside is the accompanying increased risk of developing malignancies as well.86 In addition to this ESC bear ethical, technical and legal issues regarding the use of human embryos.87
As promising as they might be, iPCS have not been approved yet to be used in any clinical trial involving humans as concerns involving tumorigenicity abound.88 iPSC display more genetic and epigenetic abnormalities than other type of stem cells.89 Actually, their capability of developing pluripotent malignancies, such as teratoma surpasses that of ESC.90
Stem cell therapy for strokeDesign of clinical trialThe first attempt to treat stroke using stem cells was more than 15 years ago.91 Hitherto there is still no optimum model for a clinical trial. With stroke being so diverse and many aspects of stem cell therapy still unexplored, many variables have to be thrown into the equation. We will discuss each of these variables separately.
Selection of patientStroke lesion sizes and locations are broadly heterogeneous in addition to normal neuroanatomical variations among individuals. Besides, depending on the etiology, stroke outcomes and prognosis vary hugely as well.9 Therefore, stroke outcomes are extraordinarily diverse among patients; even without intervention, most patients exhibit limited spontaneous recovery, though a subgroup will remain severely impaired.92 On the other hand, even with effective thrombolysis most patients will still have neurological deficits.93
So without a control or a placebo group, it is difficult to distinguish whether improvements are stem cell therapy-based or just the natural history of the disease. Ideally, and specially to address efficacy, inclusion criteria should be the most homogenously possible (in age, etiology and comorbidities) to avoid confounding biases, even if this comes at the cost of shortening the number of patients.94
Selecting patients with little to no predicted natural recovery may highlight the benefits of cell therapy, though this represents an obstacle given that most patients do not exhibit explicit recovery until 3–6 months after stroke, a time frame which limit most of the clinical trials that advocate for administration of stem cells much earlier.95 The expected recovery can be anticipated early (within days after stroke) by the use of specialized techniques of neuroimaging (e.g. fiber numbers asymmetry)96 and neurophysiological assessments (e.g. motor-evoked potentials),97 which would help us select patients with the worst prognoses to treat them in acute phases; though these are not yet used routinely.28
Double blinding enhances statistical power to the clinical trial, but this may not be fitting for the more invasive interventions, such as intrathecal or intracerebral approaches.
DosageA consensus regarding dosage has not been met. Nonetheless, there is clear relation between more cells administered and better outcomes.67,98 Therefore, given the safety profile of autologous stem cells, efforts to recollect the highest number cells possible must be done. This of course would not apply for allogenic stem cells, where the risk of graft versus host disease and rejection are much greater with higher doses.99
Another matter regarding dosage concerns the use of granulocyte colony stimulating factor (GCS-F) either as an attempt to increase number of available stem cells for collection, or even as a mean of treatment itself. GCS-F, as an up-regulator of hematopoiesis has demonstrated to increase exponentially the number of PBSC and could theoretically work as if these have been exogenously administered (i.e. migrate to penumbra and enhance recovery).100 Safety of GCS-F has been established in hyperacute stages of stroke (24–48h after onset),101 which would carry an enormous advantage over stem cells, given that these would be difficult to have at hand that much early, especially in unstable patients. Although the trend is toward better outcomes,102 efficacy of GCS-F has not been thoroughly proven and is yet to be determined if they could be used as an alternative therapy alone or even as a coadjuvant of stem cell therapy.
Time of interventionIf any natural recovery is going to happen is not seen until around 3–6 months after the onset of stroke,103 and waiting that much could limit many of the immunomodulatory effects of stem cells. Besides most data points toward better functional outcomes if stem cells are administered much earlier.104 In one animal model, only the rats receiving BMSC intravenously 7 days after artery occlusion exhibited decreased ischemic lesion volume in contrast to those who received them at days 14 and 28. Interestingly tough, all three groups displayed clinically significant better functional outcomes compared to the placebo group, suggesting that 1 month after stroke might be a suitable time-frame.66,105
Nevertheless, patients with much more chronic stroke still exhibit improvements in neurological functions.106 Considering the importance of inflammatory chemotaxis for stem cell therapy, this hints to the hypothesis that stroke constitutes a state of very chronic state of inflammation. Therefore, independent of the time of administration, stem cells will always migrate to some degree to the areas of lesion or even to the scarring tissue. Evidence suggests that stem cells in early stages work as anti-inflammatory molecules and in chronic stages aid in endogenous recovery and neurorestoration.23
Administration time should also be decided depending on the feasibility of the route of administration as patients with hyperacute stroke (<72h) are usually neurologically unstable and cannot tolerate invasive procedures.107
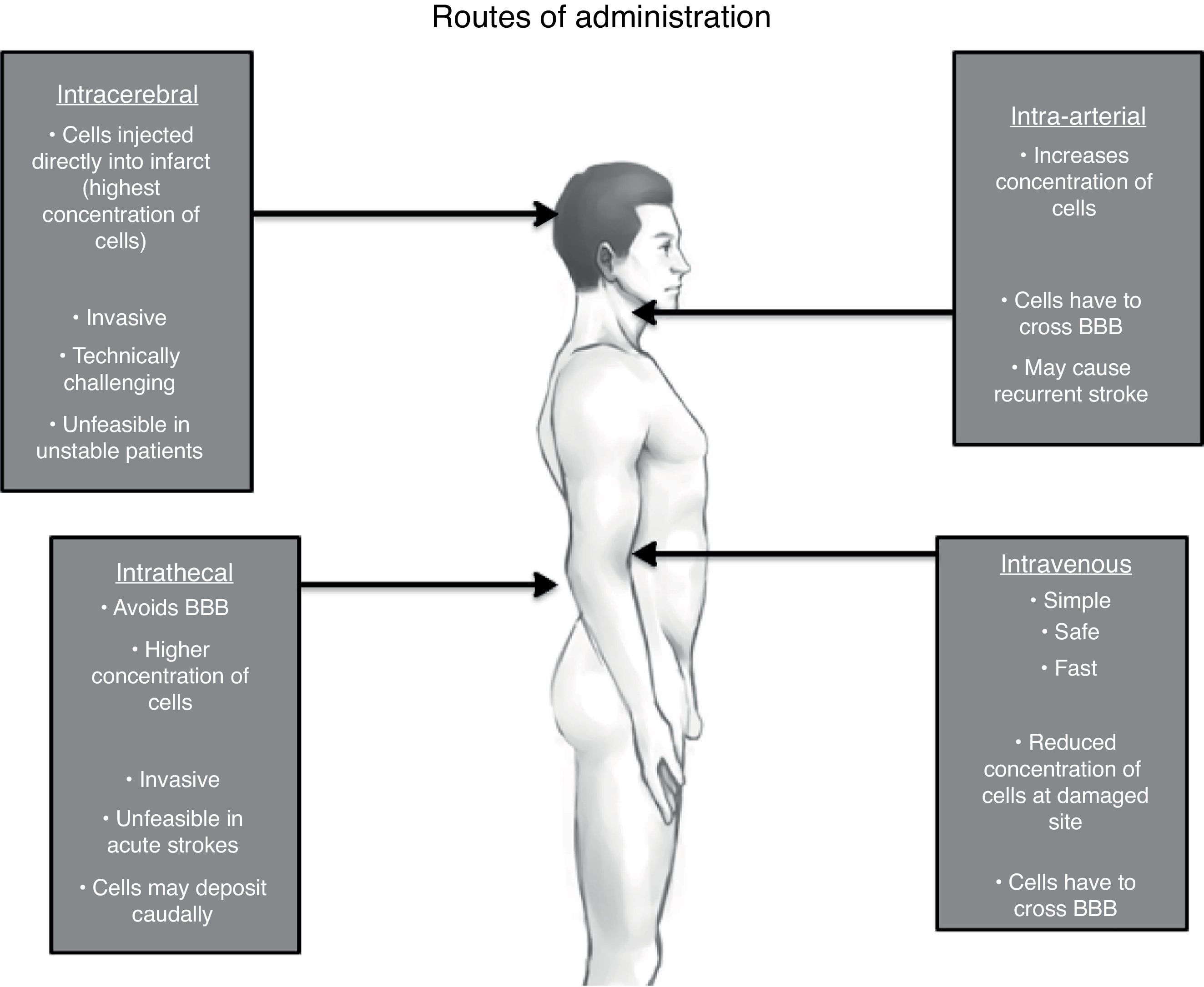
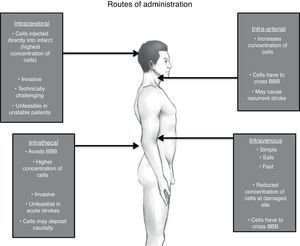
Route of administrationThe American Stroke Association in its recommendation for future stem cell research states that the safest and most effective route of cell delivery should be defined using preclinical trials.95 There are four major possible routes: intravenous (IV), intra-arterial (IA), intrathecal (IT) and intracerebral (IC).108,109 (See Fig. 1)
It is clear due to the many clinical trials, that the IV route is the safest and most feasible for administrating stem cells.74,85,110,111 Unfortunately, the most effective route is yet to be determined.
The basis for peripheral and non-invasive approaches (IV and IA) is the phenomenon of the selective permeability of the blood-brain barrier (BBB). After a brain insult, specially a hypoxic one, the tight junctions between the endothelial cells of the capillaries loosen up, therefore increasing its permeability and allowing income of many molecules including inflammatory cells.112 This breakdown may even persist for weeks or even months after the original insult, justifying stem cell therapies in chronic stages.113 Despite this, it remains unclear whether systemically infused stem cells are able to cross in a significant extent the BBB under both normal and pathological conditions.114
IA administration of stem cells theoretically increments the number of cells delivered to the lesion area in comparison to IV; yet stem cells through these route, especially in high doses, may cause recurrent stroke.115 On the other hand, another study that displayed safety of IA infusion in a window time of 3–7 days, though this was just made in 4 patients.116 Even though considered not as an invasive approach as others, safety of IA route must be reevaluated in larger clinical trials.
Another major pitfall of peripheral routes is the substantial loss of cells in other parts of the body.117 When injected IV, stem cells are distributed all around the body and are homed in other organs like the liver, kidney, lungs and spleen.118 Nonetheless, perhaps it isn’t strictly necessary for the cells to be homed in the penumbra area for them to perform their anti-inflammatory properties. Some types of stem cells may not even enter the central nervous system and may instead promote stroke recovery by acting on peripheral organs.28 Whether this contributes significantly to clinical improvements remains an interrogatory.
In one animal model of human umbilical cord blood-derived MSC,119 it was observed that there was a major migration and concentration of stem cells in the areas of hypoxia after IT administration compared to an IV approach, as well as a longer survival period of these giving a great advantage to this approach. Additionally, IT administration of stem cells for other neurological disorders has been found to be safe and well tolerated; with the main side effects being headache and transient low-grade fever.120–122 Nevertheless, some serious adverse effects have been described as well. Case reports of inflammatory hypertrophic cauda equine,123 demyelinating encephalomyelitis,124 and spinal myoclonus125 following intrathecal injection of stem cells (combination of ESC, MSC and HSC) for different diseases have been published. However, these interventions were performed in stem cell therapy clinics, with no more information given.
When implanted IC via stereotaxis, grafted MSC cells are visualized prominently just 24h after implantation and are homed almost exclusively in the affected site, positioning this route as the most effective in terms of cell concentration. Though as with animal models, evidence of these cells by neuroimaging (hypointensity on T2) became smaller gradually in the following 4 weeks until finally dissapearing.64 One must weight the risk and benefits when considering this invasive approach, particularly when using BMSC where benefits may just be temporal. Stereotaxis may be more justifiable if using exogenous NSC where there is a reasonable expectation of functional engraftment and a permanent incorporation to neural circuits.126 One must take into account that IC administration is unfeasible in acute and in unstable patients.
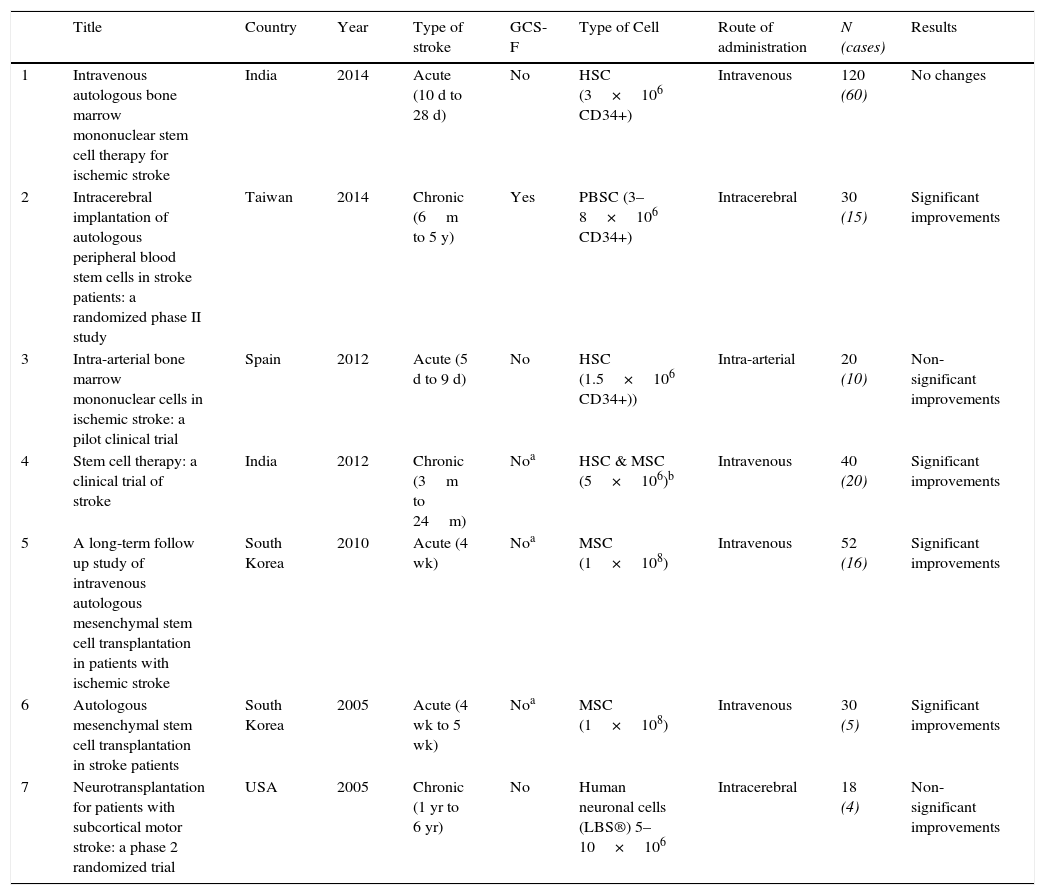
Randomized clinical trialsTo our knowledge, there are only 7 randomized clinical trials published that have used stem cell therapy for the treatment of acute stroke (Table 1).
Randomized clinical trials.
| Title | Country | Year | Type of stroke | GCS-F | Type of Cell | Route of administration | N (cases) | Results | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke | India | 2014 | Acute (10 d to 28 d) | No | HSC (3×106 CD34+) | Intravenous | 120 (60) | No changes |
| 2 | Intracerebral implantation of autologous peripheral blood stem cells in stroke patients: a randomized phase II study | Taiwan | 2014 | Chronic (6m to 5 y) | Yes | PBSC (3–8×106 CD34+) | Intracerebral | 30 (15) | Significant improvements |
| 3 | Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial | Spain | 2012 | Acute (5 d to 9 d) | No | HSC (1.5×106 CD34+)) | Intra-arterial | 20 (10) | Non-significant improvements |
| 4 | Stem cell therapy: a clinical trial of stroke | India | 2012 | Chronic (3m to 24m) | Noa | HSC & MSC (5×106)b | Intravenous | 40 (20) | Significant improvements |
| 5 | A long-term follow up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke | South Korea | 2010 | Acute (4 wk) | Noa | MSC (1×108) | Intravenous | 52 (16) | Significant improvements |
| 6 | Autologous mesenchymal stem cell transplantation in stroke patients | South Korea | 2005 | Acute (4 wk to 5 wk) | Noa | MSC (1×108) | Intravenous | 30 (5) | Significant improvements |
| 7 | Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial | USA | 2005 | Chronic (1 yr to 6 yr) | No | Human neuronal cells (LBS®) 5–10×106 | Intracerebral | 18 (4) | Non-significant improvements |
Abbreviations: GCS-F, Granulocyte colony stimulating factor; d, days; wk, weeks; m, months; yr, years; HSC, Hematopoietic stem cells; PBSC, Peripheral blood stem cells, MSC, Mesenchymal stem cells, LBS, Layton BioScience.
The first ever randomized clinical trial74 used ex vivo cultured autologous MSC and then infused them IV twice (weeks 4 and 8). All patients from the MSC group (n=5) had cerebral infarcts that involved the middle cerebral artery territory. They reported significant improvements in BI (3 and 6 months) and mRS (3 months). One year outcomes were not significant. The main limitations of this study are the small treatment group and a short follow-up period. A similar approach was used by the same authors but with a larger population (MSC group n=16 and control group n=36) and a longer follow-up (5 years).110 Significant improvement of the mRS were reported in the MSC group (p=0.046), in contrast with the control group (p=0.257). The mortality rate in the MSC group was lower than in the control group (Log rank: p=0.059), and there was no difference in comorbidites during the follow-up period. Notably, there was also a correlation between higher levels of the SDF-1a and clinical improvements, emphasizing the crucial role played by chemoattractants in this type of therapies.
An IC approach has been used twice. Kondziolka et al. implanted stereotactically 5 or 10 million allogenic neuronal cells cultivated from human embryonic carcinoma-derived cells (LBS®) to 14 patients with chronic stroke.104 They demonstrated safety of the procedure, as no serious adverse effects occurred after a 5 year follow-up. Non-significant improvements were found, especially in those having an ischemic stroke. Regarding neuropsychological testing, marked improvement was seen,127 as well as improved F-fluorodeoxyglucose (FDG) uptake in hypoxic areas. The other clinical trial also used stereotaxis in 15 chronic stroke patients, implanting 3–8 million CD34+ cells after stimulated (with G-CSF) PBSC where recollected by apheresis.64 The treated group showed a significant improvement in the National Institute of Health Stroke Scale (NIHSS), European stroke scale (ESS) and ESS motor subscale (EMS). Further, there were reductions in fiber number asymmetry of the damaged corticospinal tracts as well as restoration of motor evoked potentials response, both correlating with better functional outcomes. These changes were not observed in the control group. Safety end-points were acknowledged.
The only IA clinical trial conducted showed functional improvements, though these were not significant.67 Ten patients were injected with 1.5×108 autologous HSC between 5 and 9 days after ischemic stroke. No serious adverse events, stroke recurrence (clinical or by image), nor tumor formation were observed during the follow-up period (6 months). Interestingly, there was a trend toward better clinical outcome when higher numbers of CD34+ cells were injected.
The most recent, and by far, the larger clinical trial performed (60 cases and 60 controls) infused intravenously 2.9×106 CD34+ obtained by HSC in subacute stroke patients (median of 18.5 days after stroke).111 Even though safety was met, no changes were observed as to functional improvements. This contrasts with results obtained by the same research group a few years back, where they used either HSC or MSC in chronic patients (n treated=20), and found statically significant improvements in BI, as well as increased number of cluster activation in motor cortex area, suggesting neuroplasticity.85
Follow-upA biological marker that can assess selectively improvements of neurological functions after an ischemic or hemorrhagic event is in great need,94 but that as it may, there is currently no validated marker for such purposes.128 Therefore, we must rely on other tools such as clinical scales and neuroimaging.
On this matter, more extensive, objective and specific neurological outcomes that measure beyond the classical NIHSS, BI, mRS, ESS, or EMS need to be developed and implemented for restorative treatments.129
Magnetic resonance imaging (MRI) is an invaluable resource to gauge more objectively improvements after stem cell therapy. The focus should not be on the reduction of stroke volume size or edema, as these do not translate directly into better functional outcomes, and should remain then as secondary endpoints.28 Rather, restructuring of white matter tracts, neurogenesis and angiogenesis can be better used as to monitor recovery, which can be evaluated through more sensitive MRI techniques such as anisotropy130,131 and magnetically labeled cells.132
Future & perspectiveThe feasibility and safety of stem cells in stroke patients have both been roundly confirmed. But in spite of the remarkable improvements observed in animal models, translation to clinical scenarios has not been achieved so far. The overall results of stem cell therapy for stroke have been inconclusive, at best. Yet, the tendency seems to lean toward better functional outcomes. Many unsolved issues remain regarding timing, dosage, type of cell, and route of administration. And until these are not addressed, conclusions concerning efficacy should not be given at all. Therefore, larger double-blind randomized clinical trials with homogenous selection criteria and domain-specific end points are strongly encouraged to clarify this matter. Certainly, a predictive marker of which patients would benefit the most from cell therapy would be of immense aid.
Given the magnitude of the physical, emotional and economic burden that stroke survivors have to endure, and its colossal impact on society as a whole, efforts to find the appropriate stem cell therapy for neurorestoration should not surcease but be encouraged.
FundingNo financial support was provided.
Conflict of interestThe authors have no conflict of interest to declare.
Disclosure statementThe authors have nothing to disclose.