Ebola virus disease was first described in 1976 originating from the Ebola River in the Democratic Republic of Congo. Since then, Ebola virus has become an important public health threat in Africa, and now it is of great concern worldwide due to the recent outbreaks (9216 cases with 4555 deaths up to October 20th, 2014), and it is so far the largest and deadliest recorded in history. Five Ebola virus species have been identified (including Zaire, Sudan, Ivory Coast, Reston, and Bundibugyo Ebola virus), and four of them have proved to be highly pathogenic for both human and non-human primates, causing viral hemorrhagic fever with case fatality rates of up to 90%, for which no approved therapeutics or vaccines are currently available. Ebola virus infections are characterized by immune suppression and a systemic inflammatory response that causes impairment of the vascular, coagulation, and immune systems, leading to multiorgan failure and shock, and thus, in some ways, resembling septic shock. The major affected countries, Sierra Leone, Guinea, Liberia, and Nigeria, have been struggling to contain and to mitigate the outbreak. Gene sequencing of the 2014 virus (2014WA) outbreak has demonstrated 98% homology with the Zaire Ebola virus, with a 49% case fatality ratio across the affected countries. In this review the characteristics of the viruses, pathogenesis, diagnosis, treatment, and the cases reported in health care workers (HCW) are described, as well as a summary of outbreaks of the virus since its discovery, including these last two outbreaks in Africa.
Introduction
Critical challenges have resulted from infectious diseases, including the emergence and reemergence of old and new infectious diseases.1 Emerging pathogens can be into two categories: microbes newly introduced to humans from other species, and existing but previously rare human pathogens that rise rapidly in prevalence or pathogenicity.2 Their appearance is often attributed to human intrusion on animal habitats, environmental evolution and deforestation, changing socioeconomic conditions, increased global connectivity, and genetic changes in microorganisms.3 Ebola virus is an example of the second category because it was introduced in 1976,4,5 and possibly considered rare because we lack the tools to routinely detect it.3 Ebola, or Ebola virus disease (EVD), is a severe, often fatal disease affecting humans and nonhuman primates,6 outbreaks of which occur in Africa within 10° of the Equator affecting mostly the central part of the continent.7,8
Viral genome structure and organization
Ebola virus is a non-segmented negative stranded RNA virus, member of the Filoviridae family from the Mononegavirales order.9 The Ebola virus has an 80 nm diameter and its length can be up to 14 000 nm. The viral genome consists of seven genes: nucleoprotein, virion protein (VP) 35, VP40, glycoprotein, VP30, VP24, and RNA-dependent RNA polymerase (L).9,10 The glycoprotein is the only transmembrane surface protein and serves for viral attachment to host cells.10,11 The ribonucleoprotein complex assists replication, transcription,12 particle formation,13 and interferes14 and antagonizes interferon signaling.15
Ebola virus species
There are five identified Ebola virus species: Zaire, Sudan, Tai Forest, Bundibugyo and Reston. Zaire and Sudan Ebola viruses were first isolated in 1976 at the simultaneous outbreaks in southern Sudan and northern Zaire, now Democratic Republic of Congo (DRC).4,5 Initially the etiological pathogen was the same in both regions, but further analyses identified two species.16 The third Ebola virus specie, Tai Forest, was isolated from an infected ethnologist who did a necropsy on a chimpanzee in the Tai Forest reserve in Ivory Coast, Western Africa.17 The fourth Ebola virus specie, Bundibugyo, was identified in Uganda in 2007.18 The fifth specie, Reston, was identified on infected Cynomolgus monkeys (Macaca fascicularis) imported from the Philippines, and quarantined in Reston, VA, USA.19,20 Reston Ebola virus is not pathogenic to humans, only to non-human primates21 and pigs.22
Viral evolution and phylogeny
According to phylogenetic analysis, the Ebola viruses divides into five different branches. The branches of Zaire, Tai Forest, and Bundibugyo Ebola viruses are grouped together, suggesting a common ancestor. The Reston and Sudan Ebola viruses share similarly a common ancestor.23 The Zaire Ebola viruses are subdivided into five distinct lineages according to different outbreaks. Viruses isolated during 1976 to 1977 represent the first lineage. The second lineage is made of viruses isolated from 1994 to 1996. The virus isolated in Gabon during 2002 makes the third lineage. Viruses isolated during 2007 to 2008 represent the fourth lineage. The viruses from the 2014 West African outbreak make the fifth lineage.24 It is important to note that the Ebola virus from this outbreak out-groups the rest of the lineages, suggesting an introduction of the virus to the human population.25 In contrast, the Zaire Ebola virus from the DRC outbreak groups with the second lineage, viruses isolated during 1996 in Gabon.26 This fact confirms that both outbreaks are not related. These findings denote a constant evolution of these viruses as expected from the RNA viruses.
Historical outbreaks of Ebola viruses
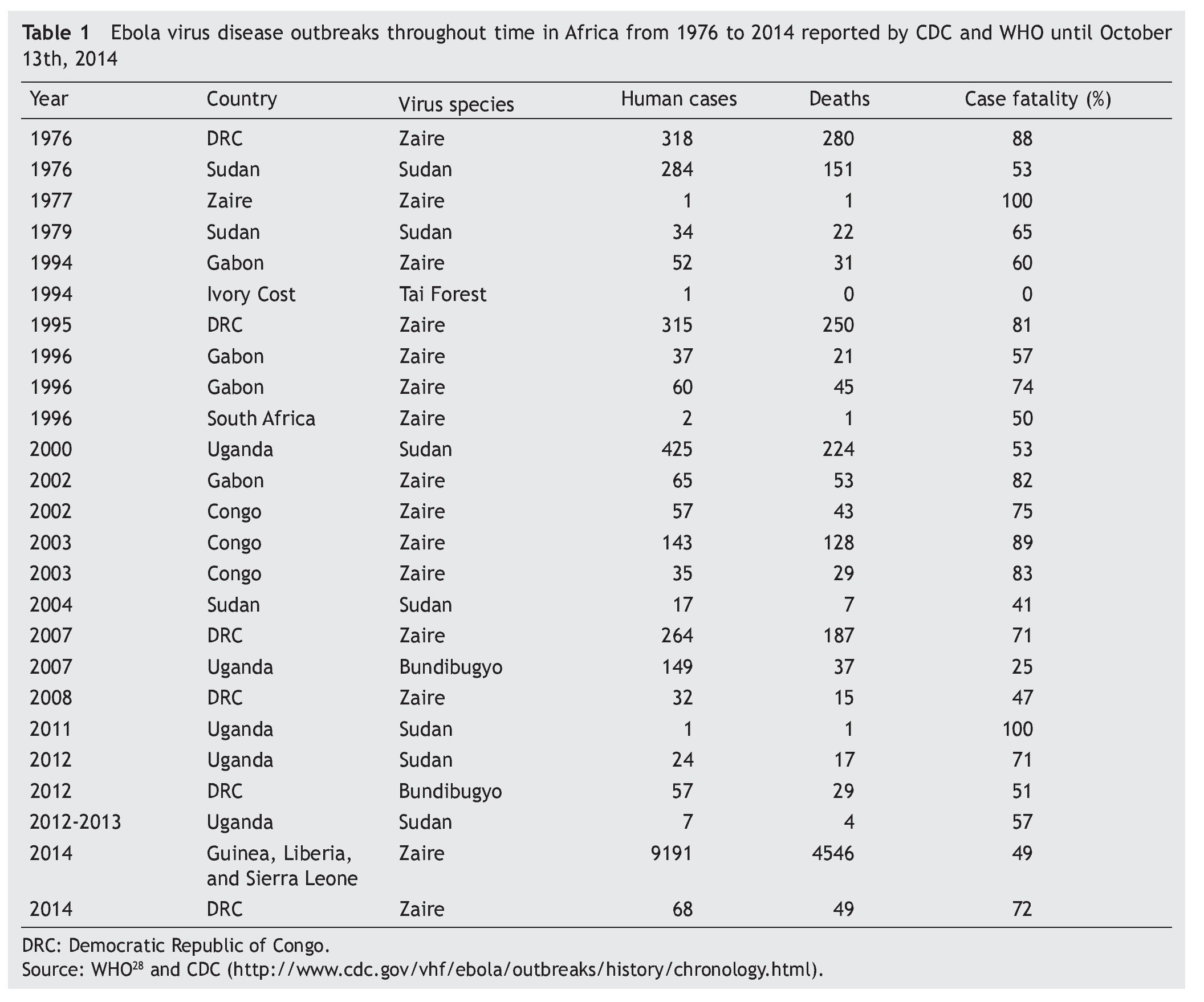
There have been multiple EVD outbreaks since 1976 which are listed chronologically in table 1.27 During the 70’s, DRC and Sudan were affected by Zaire and Sudan Ebola viruses causing 637 human cases and 454 deaths. There was a 15-year period where no EVD outbreaks were reported. In 1994 there was an outbreak in Gabon caused by Zaire Ebola virus. In the same year, the Tai Forest Ebola virus was discovered in Ivory Coast.17 For the following years, Zaire Ebola virus continued affecting DRC, Gabon and South Africa. Ebola outbreaks continued appearing between 2000 and 2004. Sudan Ebola virus affected Uganda and Sudan in 2000 and 2004, respectively. Zaire Ebola virus affected Gabon and Congo in three different outbreaks. The same virus specie was also responsible for the outbreaks in DRC in 2007, 2008 while Bundibugyo was introduced to Uganda in 2007.18 From 2011 to 2013, Sudan Ebola virus has affected Uganda. Bundibugyo Ebola virus continued causing outbreaks, but this time in DRC during 2012.27,28 The case fatality rate of each outbreak is different, even between same Ebola virus specie.
Current outbreaks in Africa
Although most previous EVD outbreaks occurred in Central Africa, the most recent outbreak started in West Africa. The 2014 West Africa outbreak is the largest ever observed, both by number of cases and geographical extension. Fortunately, the majority of the cases are concentrated in three countries in West Africa, with only a small number out of the area. Basically, there are two EVD outbreaks occurring simultaneously in Africa. The first one to appear was the outbreak in West Africa, in Guinea during December 2013, and was confirmed by the World Health Organization in March 2014. The second one occurred in western DRC during August 2014.28
The current outbreaks are both due to Zaire Ebola virus,25 but they are not related.26 The West African outbreak started in December 2013 in Guinea25 and spread into Liberia in March 2014, Sierra Leone in May, Nigeria in late July and Senegal in September.29 As of October 14, 2014, 9216 cases and 4555 deaths have been reported in seven affected countries (Guinea, Liberia, Nigeria, Senegal, Sierra Leone, Spain, and the United States of America).29 According to the World Health Organization Ebola Response Roadmap structure, Guinea, Liberia, and Sierra Leone are countries with widespread and intense transmission. Countries with an initial case or cases, or with localized transmission are: Nigeria, Senegal, Spain, and the United States of America.29 WHO officially declared the EVD outbreak over in Senegal due to a second EVD-negative sample from the single confirmed case in Senegal on September 5 (42 days ago).30 In this recent outbreak, by October 17, the case fatality rate was 49.4%, when all countries were included, and this rate was consistent among Guinea, Liberia and Sierra Leone.29 Nevertheless, the case fatality rate will vary as the human cases and deaths increase throughout time. The most recent account of cases as of October 20th, 2014 reported: 9216 total cases, 4218 laboratory-confirmed cases and 4555 total deaths. Concerning DRC outbreak, as of October 9, 2014 there have been 68 cases of EVD, 49 reported deaths and 72% case fatality rate.29
Pathogenesis
Information about the pathology and pathogenesis of Ebola virus infections in man is scarce. Yet, comprehensive studies have been done in animals. Guinea pigs and mice have been used to study Ebola hemorrhagic fever.31-33 Since isolates of Ebola virus obtained from primates do not typically produce severe disease in rodents on initial exposure, serial adaptation is needed to produce a uniformly lethal infection. However, the disease pathogenesis recorded in rodents is less accurate.34,35
Route of infection
Ebola virus enters the host through mucosal surfaces, breaks, and abrasions in the skin, or by parenteral introduction. Most human infections in outbreaks seem to occur by direct contact with infected patients or corpses.28,36,37 Infectious virus particles or viral RNA have been detected in semen, genital secretions,38,39 and in skin of infected patients;40 they have also been isolated from skin, body fluids, and nasal secretions of experimentally infected non-human primates.41,42
Laboratory exposure through needle sticks and blood have been reported.43 Butchering of a chimpanzee and bats for food were linked to outbreaks of Zaire Ebola virus in Gabon and DRC, respectively.44,45 The route of transmission of Ebola viruses seems to affect the disease course and outcome.
The mean incubation period for cases of Zaire Ebola virus infection due to injection is shorter than contact exposures.46 Also the case fatality rate was greater when the infection was acquired by injection.46
Target cells and tissues
Ebola virus has a broad cell tropism, infecting a wide range of cell types. Monocytes, macrophages, dendritic cells, endothelial cells, fibroblasts, hepatocytes, adrenal cortical cells, and several types of epithelial cells are capable of viral replication.40,41,47 Studies in non-human primates experimentally infected with Zaire Ebola virus suggest that monocytes, macrophages, and dendritic cells are the early and preferred replication sites of these viruses.48 These cells seem to have critical roles in dissemination of the virus as it spreads from the initial infection site to regional lymph nodes, probably through the lymphatic system, and to the liver and spleen through the blood.48,49 Monocytes, macrophages, and dendritic cells infected with Ebola virus migrate out of the spleen and lymph nodes to other tissues disseminating the infection.50,51
Ebola virus’ glycoprotein may be the primary determinant of vascular-cell injury, therefore infection of endothelial cells induces structural damage, which could contribute to the hemorrhagic diathesis.52 However histological analysis of autopsy tissue did not identify vascular lesions in early outbreaks and no vascular lesions in any subsequent studies have been reported.53
Liver and adrenal glands seem to be important targets for Ebola viruses. Various degrees of hepatocellular necrosis have been reported, but the lesions are not serious enough to explain the cause of death.10,41,47,53 The severe hepatocellular necrosis can explain the hemorrhagic tendencies because of the decreased synthesis of coagulation factors. As the adrenal cortex controls blood pressure homeostasis, impaired secretion of enzymes due to adrenal infection and necrosis leads to hypotension and sodium loss with hypovolemia.10,41
During Ebola virus infection, there is a lymphoid depletion and necrosis in patients with fatal disease and in non-human primates experimentally infected.51,54 Curiously, Ebola virus does not infect lymphocytes and the loss is due to lymphocyte apoptosis.55,56 The mechanism is not known, but it could involve the TNF-related apoptosis-inducing ligand (TRAIL) and Fas death receptor pathway.41,57
Host immune response
Ebola virus infection triggers the expression of several inflammatory mediators including interferon, interleukins 2, 6, 8, and 10, interferon-inducible protein 10, monocyte chemoattractant protein 1, regulated upon activation, normal T cell expressed and secreted (RANTES), TNFα, and reactive oxygen and nitrogen species.10,55,57-59 Virus-induced expression of these mediators produces an immunological imbalance that contributes to the progression of the disease. It was shown that a deregulated proinflammatory response resulted in fatal cases and well-regulated inflammatory responses were associated with recovery.58
Inhibition of the type I interferon response seems to be a key feature of filovirus pathogenesis.60,61 The Ebola virus VP35 works as a type I interferon antagonist15 by blocking activation of interferon regulatory factor 3 and possibly by preventing transcription of interferon β.62 Additionally, other studies suggest that expression of VP24 of the Ebola virus interferes with type I interferon signaling.14,63 Mutations in VP24 have been linked to adaptation of Zaire Ebola virus to produce lethal disease in mice64 and guineapigs.65
Clinical manifestations
The different species of Ebola virus seems to cause different clinical manifestations, but close observation of the diseases under good conditions has been rare. Generally the incubation period of EVD consists of 2-21 days (mean, 4-10).50 In contrast, the recent outbreak has a mean incubation period of 11.4 days and did not vary by country.66 The onset of symptoms is abrupt and starts with fever, chills, malaise and myalgia. The following symptoms indicate multisystem involvement and include: systemic (prostration); gastrointestinal (anorexia, nausea, vomiting, abdominal pain, diarrhea), respiratory (chest pain, dyspnea, cough, nasal discharge), vascular (conjunctival injection, postural hypotension, edema), and neurological (headache, confusion, coma) manifestations. Hemorrhagic manifestations arise during the peak of the illness and include petechiae, ecchymosis, uncontrolled oozing from venipuncture sites, mucosal hemorrhages, and post-mortem evidence of visceral hemorrhagic effusions.50 In the recent outbreak in West Africa, the most common symptoms were fever (87.1%), fatigue (76.4%), vomiting (67.6%), diarrhea (65.6%), anorexia (64.5%), headache (53.4%), and abdominal pain (44.3%).66 Specific hemorrhagic symptoms were rarely reported (<1-5.7%) but unexplained bleeding was reported in 18% of the cases.66 Regarding DRC outbreak, the most common symptoms were the same as the West African outbreak with almost similar proportions: fever (92%), fatigue (71%), vomiting (47%), diarrhea (68%), anorexia (39%), headache (45%), and abdominal pain (47%).26
Patients with fatal disease develop clinical signs early during infection and die typically between day 6 and 16, due to hypovolemic shock and multiorgan failure. Hemorrhages can be severe but are only present in fewer than half of patients. In non-fatal cases, patients have fever for several days and improve typically around day 6-11, about the time that the humoral antibody response is observed.10,38
Laboratory findings
Laboratory findings are less characteristic, but the following findings can be associated with EVD: early leucopenia (1000 cells/µL) with lymphopenia and subsequent neutrophilia, presence of atypical lymphocytes, thrombocytopenia (50,000-100,000 cells/µL), highly raised serum aminotransferase concentrations (aspartate aminotransferase exceeding alanine aminotransferase), hyperproteinemia, and proteinuria. Prothrombin and partial thromboplastin times are extended and fibrin split products are detectable.10
Diagnosis
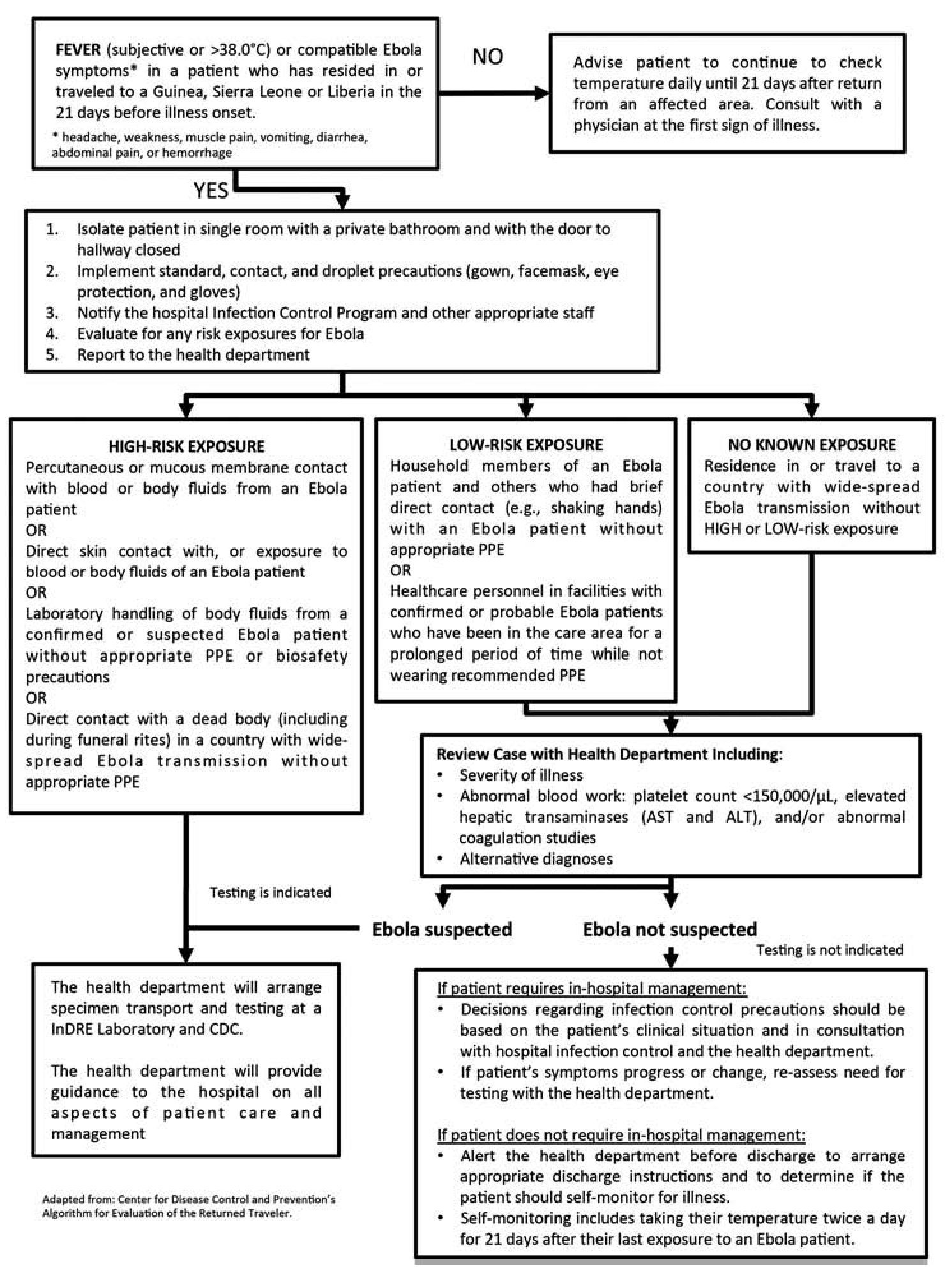
The diagnosis can be difficult in a recent infection, because early symptoms are non-specific to EVD and are often seen in patients with more common diseases such as malaria, typhoid fever and dengue fever. The algorithm shown on figure 1 could be helpful when evaluating the patient.67 The diagnostic confirmation can be made with the following procedures: antibody-capture enzyme-linked immunosorbent assay (ELISA),68,69 antigen-capture detection tests, serum neutralization test, reverse transcriptase polymerase chain reaction (RT-PCR) assay,70,71 electron microscopy,72,73 and virus isolation by cell culture.28,74
Figure 1 Algorithm for Evaluation of the Returned Traveler.
Viral antigen/nucleic acid can be detected in blood from 3 up to 16 days post onset of symptoms.75 IgM antibodies can appear as early as 2 days post onset of symptoms and disappear between 30 and 168 days after infection. IgG-specific antibodies develop between days 6 and 18 after onset and persist for many years.75,76 All the diagnostic procedures should be done in BSL-4 laboratory, unless the samples are inactivated with gamma radiation by exposure to a cobalt-60 source77 or guanidinium isothiocyanate in case of nucleic acid extraction.78 These methods of inactivation allow the safe manipulation of material outside the containment laboratory.
Treatment
There is no specific treatment for EVD. Present strategies are mainly symptomatic and supportive.50 The goals of treatment are: volume repletion, maintenance of blood pressure (with vasopressors if needed), maintenance of oxygenation, pain control, nutritional support, treatment of secondary bacterial infections and preexisting conditions. Likewise, the treatment must provide supportive care for complications, such as hypovolemia, electrolyte abnormalities, refractory shock, hypoxemia, hemorrhage, septic shock, multiorgan failure, and disseminated intravascular coagulation.28,79,80
Progress has been made in new experimental approaches to post exposure prophylaxis and/or treatment that are effective in laboratory primates. There are several therapeutic candidates in study.81-83 Plasma, whole blood and other blood-derived components from convalescent patients have theoretical and anecdotal evidence that can improve survival in a small group of EVD cases.84,85 ZMapp, monoclonal antibodies against Ebola made in plants, has been already used in a few patients with EVD. However, the number of treated patients is too small to evaluate safety and efficacy.82,83,86 Other potential therapeutics under development include: T-750 (favipiravir) is a pyrazine carboxamide derivative that inhibits the viral RNA-dependent RNA polymerase or induce lethal mutagenesis by incorporating into the viral RNA.87 BCX-4430, a new nucleotide analogue, inhibits virus replication by inhibiting viral RNA polymerase function.88 RNA based drugs as small interfering RNAs and phosphomorpholino oligonucleotides may effectively prevent EVD in nonhuman primates by targeting the Zaire Ebola virus RNA polymerase L protein.89 Nematode-derived anticoagulation protein, rNAPc2, plays an important role in weakening coagulation and inflammation.90 Some of these have demonstrated safety in humans and efficacy in animal models, however clinical evaluation will be required to determine whether they are efficacious in EVD and whether they are safe at the doses required.82 In addition to these novel products, there are also several existing medicines that have been approved for treatment of other diseases and conditions but which may be re-purposed for EVD.82,91,92
Prevention
It is not always possible to identify patients with EVD early in the course of their illness because initial symptoms may be non-specific. For this reason, it is important that HCW at all levels carefully apply standard precautions on a consistent basis, with all patients in every practice and at all times.93 These include: hand hygiene; use of disposable medical examination gloves before contact with body fluids, mucous membrane, non-intact skin and contaminated items, and gown and eye protection before procedures and patient-care activities likely to involve contact with or projection of blood or body fluids. In addition, regular application of best practices for injection safety and safe handling and disposal of sharp instruments, safe cleaning and disinfection of the environment and of reusable equipment, and safe laundry and waste management should be a high priority in the health care facility.6,28,94
Suspected or confirmed cases should be placed in single isolation rooms with an adjoining dedicated toilet or latrine, showers, sink equipped with running water, soap and single-use towels, alcohol-based hand rub dispensers, stocks of personal protective equipment (PPE), stocks of medicines, good ventilation, screened windows, doors closed and restricted access.95,96 If isolation rooms are unavailable, cohort these patients in specific confined areas while rigorously keeping suspected and confirmed cases separate and ensure the items listed here for isolation rooms are readily available. Make sure that there is at least 1 meter distance between patient beds.95,96 Before entering care areas, every HCW should don personal protection equipment (PPE) according to the expected level of risk and following the steps recommended by WHO.94 The PPE include: gloves, an impermeable long-sleeve gown, boots/closed-toe shoes with overshoes, and a mask and eye protection for splashes. After exiting care areas, perform careful removal of PPE to avoid contamination of any area of the face (i.e. eyes, nose, or mouth) or non-intact skin.94,95
People with percutaneous or muco-cutaneous exposure to blood, body fluids, secretions, or excretions from a patient with suspected or confirmed EVD should immediately and safely stop any current tasks, leave the patient care area, and safely remove PPE. Immediately after leaving the patient care area, wash the affected skin surfaces or the percutaneous injury site with soap and water. Irrigate mucous membranes with copious amounts of water or an eyewash solution, and not with chlorine solutions or other disinfectants. The incident should be immediately reported to the local coordinator. Exposed persons should be medically evaluated including for other potential exposures and receive follow-up care, including fever monitoring twice daily for 21 days after the incident.95,97
Transmission of the virus to the HCW has been documented in most of the outbreaks and has been a sometimes exaggerated concern. A recent review of the human to human transmission shows that most of the cases in HCW occurred due to the lack of and/or the inappropriate use of the protective equipment and that with the appropriate use of the equipment transmission rarely happened.94-97 As October 20th, 2014 the CDC tightened the recommendations for prevention of transmission to the HCW including more careful protocols for dressing and undressing, including a monitor for the process94-97.
Vaccines
There are two promising candidate vaccines that have clinical-grade vials available for phase 1 pre-licensure clinical trials.98,99 The first vaccine (cAd3-ZEBOV) was developed by GlaxoSmithKline in collaboration with the US National Institute of Allergy and Infectious Diseases. It uses a chimpanzee-derived adenovirus vector with the Zaire Ebola virus GP gene inserted.100 The second (rVSV-ZEBOV) was developed by the Public Health Agency of Canada in Winnipeg. The vaccine uses an attenuated or weakened vesicular stomatitis virus expressing the different Ebola virus GP gene.101 Two phase 1 trials of the cAd3-ZEBOV started in September 2014 in USA and UK, and the first phase 1 trial of VSV-ZEBOV is due to start early in October in USA.82,99
Economical aspects in West Africa EVD outbreak
In Guinea, Sierra Leone and Liberia, where widespread and intense transmission of EVD is occurring, medical doctors are scarce and governmental health expenses are low.102-104 That can be translated to inadequate and insufficient health care. Health care infrastructure is inadequate, and health workers and essential supplies including personal protective equipment are scarce. Complicated patients could not be adequately treated and decease and the lack of sufficient and isolated beds can spread the disease to the rest of the community. These countries were already handling major health challenges, like malaria and other endemic diseases, and were not prepared for EVD. These countries’ borders are porous, and movement between them is constant. Traditional practices, such as bathing of corpses before burial, have facilitated transmission. Decades of conflict have left the populations distrustful of governing officials and authority figures such as health professionals.105,106 This contributed to the vandalizing of health clinics and even the “rescue” of sick patients from the clinics, spreading the disease. As a consequence, the outbreak could not be contained and spread from Guinea to Liberia, Sierra Leone, Nigeria and Senegal.29
Conclusions
Although the current epidemic of EVD in West Africa is unprecedented in scale, the clinical course of infection and the transmissibility of the virus are similar to those in previous EVD outbreaks.107 This epidemic is exceptionally large, not predominantly because of the biologic characteristics of the virus, but rather because of the attributes of the affected populations and because control efforts have been insufficient to halt the spread of infection. The HCW should be alert, well informed, and ready for action in case a patient arrives with EVD symptomatology.
Acknowledgements
We thank Sergio Lozano-Rodriguez, M.D. (UANL), for his assistance in reviewing the manuscript.
Received: October 2014;
Accepted: October 2014
*Correspondence author:
Servicio de Infectología,
Facultad de Medicina y Hospital Universitario “Dr. José Eleuterio González”,
Universidad Autónoma de Nuevo León,
Ave. Francisco I. Madero y Ave. Gonzalitos s/n, Col. Mitras Centro,
64460 Monterrey, Nuevo León, México.
E-mail address:javramos31@gmail.com (J. Ramos Jiménez).