To compare the effects of intravenous hyoscine butylbromide versus placebo on the duration of labor in term pregnancies.
Materials and methods86 patients were selected and randomly assigned to receive intravenous hyoscine butylbromide (20mg twice) (n=43) or an intravenous placebo (saline solution 10cc) (n=43). We evaluated maternal adverse effects, effects on neonatal Apgar score, cervical dilatation 1h after drug administration and the interval between the onset of labor and delivery.
ResultsNo significant differences were observed in the duration of phases of labor between the two groups. No significant differences were found between the groups in Apgar scores at 1 and 5min. No maternal adverse effects were observed or reported due to the use of hyoscine butylbromide or the placebo. On the first phase of primigravidas it shortens the first labor phase up to 159.1±84min vs. the control group (p=0.002).
ConclusionHyoscine butylbromide oxytocin shortens the duration of labor in term primigravida pregnancies. No side effects were reported.
Labor is a sequential physiological event. It integrates changes that will take place in the myometrium, decidua and cervix, which occur over a period of days, or even weeks. Biochemical changes appear before the onset of uterine contractions and cervical dilatation.1 Labor at term, supposes the liberation of inhibitor factors which affect pregnancy and the myometrium, which sets off an active process through uterine stimulators.2 Different substances intervene during labor, like prostaglandins (PG), estrogens and oxytocin, among others.
It was customary to follow the natural evolution of labor without the application of any method to accelerate it (passive handling of labor), either that or with the use of medications which accelerate it (active handling of labor).2
In the beginning of the 20th century, in 1906, the first uterotonic was discovered, oxytocin. It was not until 1911 when its implementation began to accelerate labor.2 Oxytocin is a cyclic nonapeptide obtained through chemical synthesis. Its synthetic form is identical to that of a hormone stored in the posterior hypophysis, it stimulates the smooth muscle of the uterine strongly toward the end of the pregnancy, during labor and immediately after childbirth. During this phase, oxytocin receptors in the myometrium increase. This is a fast-acting hormone, with a latent period of under a minute after intravenous application and between 2 and 4min after intramuscular injection.3 In the active phase of the first period of labor, oxytocin is commonly used, in patients who require it in order to cause uterine contractions and to regularize its frequency.4
In some countries, in addition to the above, other medications have been used to shorten labor, such as drotaverin and hyoscine N-butylbromide (BBH).4 This is part of a group of scopolamine derivates, which are muscarinic antagonists and have antispasmodic effects. It inhibits cholinergic activity in abdominal and pelvis parasympathetic lymph nodes, having an effect on the smooth muscle of the digestive tract, urinary, biliary, female sexual organ and especially over the uterine–cervical plexus,5 which explains its effect in cervical dilatation. However, this has not yet been clearly stated.6
This medication does not have effects over uterine contractibility; it does not go through the blood–brain barrier and its fixation to proteins is very low, with a very quick distribution; after intravenous administration the time of action is around 10min, with a peak from 20 to 60min and an average life ranging from 4 to 5h.7 Its main way of excretion is renal. No adverse effects during pregnancy or lactation have been proven; however, its use is recommended with caution under the first trimester. Side effects include: dry mouth, facial flushing, intermittent loss of accommodation reflexes, urinary retention and constipation.6
There have been few studies in which the use of BBH was evaluated to shorten labor. These have reported the reduction of labor time compared to the control group which received placebo,5 and one study compared its effectiveness versus oxytocin.8 The objective of our study was to compare the efficacy and safety of BBH versus placebo during labor.
Materials and methodsA randomized experimental study was designed in which patients older than 18 years were included, with a term pregnancy (37–42 weeks), independent from the parity with cephalic presentation, with a clinically adequate pelvis for labor, where there was no evidence of macrosomia (estimated fetal weight over 4000g) and who were in the first period of labor in the active phase (dilatation of 4cm or more) with regular uterine activity (3–4 contractions in 10min). We excluded all patients who needed to complete childbirth abdominally due to different causes.
Patients who were eligible for this study were chosen randomly to receive active treatment with BBH versus a placebo using a sampling of two proportions, in an infinite population, with a potency of 90% to detect the difference of 30% among the study groups; a statistically significant level of 0.05 was established. Over these premises a sample size of 40 patients per group was calculated.
The study included a total of 86 women who met the inclusion criteria and who went to the Emergency Service of the “Dr. José E. González” University Hospital for the resolution of their pregnancy between June 2009 and July 2010. All patients signed an informed consent.
The patients were randomly distributed into one of the two groups: the cases group, with 43 patients who were administered 20mg of BBH (diluted in 9ml of saline solution) intravenously on two occasions with an interval of 1h, and the control group with 43 patients who were administered a placebo (10ml of saline solution) at a similar dosage and interval. After every dose, fetal and maternal cardiac frequency monitoring was performed and patients were questioned about side effects. Labor progress was evaluated in a conventional manner, monitoring the time of every period of labor, in addition to the events occurring during its evolution (spontaneous rupture of membranes, analgesia, forceps application, etc.). Also weight at birth, Apgar and Capurro were evaluated. Maternal and fetal complications were evaluated (uterine atony, vaginal and perineal tears, etc.) after childbirth. Central tendency measurements were utilized and the duration of every period of labor was determined.
The differences between groups were compared with χ2 or Fisher's exact test in the case of categorical variables and with the Student's t-test for continuous variables.
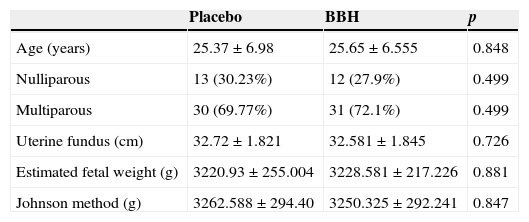
ResultsA total of 90 patients were included in the study. Of these, we discarded two patients from the case group and two from the control group, because it became necessary for them to have an abdominal birth. The cervical conditions of the placebo group upon entry were: dilation of 5.6±1.5cm, 73±11% effacement and Bishop Index of 8.8±1.2. And in the BBH group they were: dilation of 5.2±1.3cm, 72±11% effacement and Bishop Index of 8.6±1.2. Upon comparison of these variables, there was no statistically significant difference, which indicates that these two groups were homogenous (Table 1).
Comparison of the characteristics of both groups.
| Placebo | BBH | p | |
|---|---|---|---|
| Age (years) | 25.37±6.98 | 25.65±6.555 | 0.848 |
| Nulliparous | 13 (30.23%) | 12 (27.9%) | 0.499 |
| Multiparous | 30 (69.77%) | 31 (72.1%) | 0.499 |
| Uterine fundus (cm) | 32.72±1.821 | 32.581±1.845 | 0.726 |
| Estimated fetal weight (g) | 3220.93±255.004 | 3228.581±217.226 | 0.881 |
| Johnson method (g) | 3262.588±294.40 | 3250.325±292.241 | 0.847 |
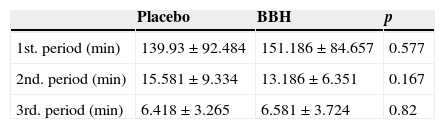
Regarding the results of the duration of labor, the first period of labor, evaluated in minutes, was 139.93±92.4 in the placebo group versus 151.186±84.6 in the BBH group (p=NS). The second period of labor had a duration of 15.5±9.33min in the first group and 13.18±6.35min in the second (p=NS). Finally, the third period registered a time of 6.4±3.2min in the placebo group and 6.5±3.7min in the BBH group. We were unable to find a statistically significant difference (Table 2).
Upon separating the groups by number of pregnancies, we found that the BBH group had a 159.1±84min time for the first phase of labor, with significant difference of p=0.002 compared to the control group (262.5±92min).
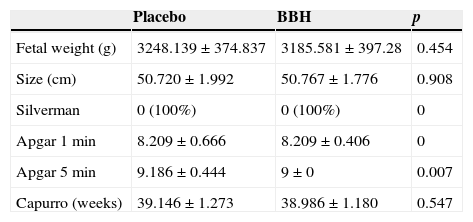
Concerning the perinatal results, we observed a fetal weight of 3248±374g, a length of 50.7±1.9cm and 39.14±1.2 Capurro weeks in the placebo group, and a fetal weight of 3185.5±397g, a length of 50.7±1.7cm and 38.9±1.1 Capurro weeks in the BBH group. We were unable to find a statistically significant difference. Concerning the condition of the babies immediately after birth, we reported an Apgar score at the first minute of 8.2±0.6 and 9.1±0.4 at 5min in the control group, and 8.2±0.4 at the first minute and 9±0 at 5min in the BBH group. We were unable to find a statistically significant difference (Table 3).
DiscussionPrevious studies performed with BBH have reported results which vary from no difference in cervical dilation time to decreases greater than 2h. Aggarwal et al.7 performed a study in which they found that in addition to a 35.6% diminishment in the perception of pain during labor due to the use of intravenous BBH, the first period of labor was reduced in a significant way, from 8h 16min in the control group, to 3h 46min in the BBH group. Samals et al. concluded that using intravenous BBH, at a dose of 20mg, was able to diminish the effort of labor by up to 32%.9
Other authors recently compared the use of this pharmaceutic to oxytocin without finding a statistically significant difference in the effort of labor,10 which is the reason the use of this medication should be considered as an option for primigravid patients which, for whatever reason, cannot receive oxytocin.
We can make a recommendation for the use of this pharmaceutic in patients with the results obtained by separately evaluating groups of primigravid and multigravid patients which received BBH vs. a placebo; with regard to the multiparous, there was no significant difference found between any of the groups.
Just like in other studies,11 our results show no adverse effects on the fetus, which were evaluated by the APGAR at 1 and 5min.
ConclusionsBased on the results obtained in our investigation, we can conclude that although we were not able to prove a statistically significant decrease in the effort of labor in both groups, we did demonstrate that when the groups of primigravidas and multigravidas are compared separately, the primigravida BBH group showed a statistically significant difference, with a p of 0.002.
Based on the previous studies, we can make a recommendation for the use of BBH in primigravid patients, also demonstrating that the administration of this product is safe during pregnancy, since there were no adverse effects presented during its administration, or among the babies in all the studied population.
Conflict of interestThe authors have no conflicts of interest to declare.
FundingNo financial support was provided.